Abstract
Age-related decline in microstructural integrity of certain white matter tracts may explain cognitive decline associated with normal aging. Whole brain tractography and a clustering segmentation in 48 healthy individuals across the adult lifespan were used to examine: interhemispheric (corpus callosum), intrahemispheric association (cingulum, uncinate, arcuate, inferior longitudinal, inferior occipitofrontal), and projection (corticospinal) fibers. Principal components analysis reduced cognitive tests into 6 meaningful factors: (1) memory and executive function; (2) visuomotor dexterity; (3) motor speed; (4) attention and working memory; (5) set-shifting/flexibility; and (6) visuospatial construction. Using theory-based structural equation modeling, relationships among age, white matter tract integrity, and cognitive performance were investigated. Parsimonious model fit demonstrated relationships where decline in white matter integrity may explain age-related decline in cognitive performance: inferior longitudinal fasciculus (ILF) with visuomotor dexterity; the inferior occipitofrontal fasciculus with visuospatial construction; and posterior fibers (i.e., splenium) of the corpus callosum with memory and executive function. Our findings suggest that decline in the microstructural integrity of white matter fibers can account for cognitive decline in normal aging.
Keywords: Aging, Anisotropy, Cognition, Diffusion tensor imaging, White matter
1. Introduction
In normal aging, a wide range of cognitive functions experience age-related decline (Hedden and Gabrieli, 2004). Improved understanding of the neurobiological substrates of cognitive decline in normal aging has been facilitated by the advent of diffusion tensor imaging (DTI). DTI is a clinical neuroimaging technique quite sensitive to alterations in brain white matter microstructure (Le Bihan, 2003), and provides an opportunity to study white matter in vivo in a manner not previously possible with conventional magnetic resonance imaging (MRI). DTI has emerged as an important tool in the study of neuropsychiatric and neurologic disorders (Ciccarelli et al., 2008; Kubicki et al., 2007), as well as in the study of healthy and pathologic aging (Sullivan and Pfefferbaum, 2006). In normal (or healthy) aging, DTI studies have begun to demonstrate that microstructural integrity of cerebral white matter exhibits age-related decline (Salat et al., 2005; Sullivan and Pfefferbaum, 2006; Sullivan et al., 2008). Thus, combining DTI approaches with examination of cognitive functions susceptible to age-related decline should help further elucidate mechanisms of cognitive decline in normal aging.
White matter changes in normal aging likely play an important role in contributing to age-related cognitive decline. Early volumetric MRI studies were equivocal in their assessment of white matter changes with age (Sullivan and Pfefferbaum, 2006). In contrast, DTI studies have shown widespread age-related declines in fractional anisotropy (FA) in white matter (Charlton et al., 2006; Salat et al., 2005; Sullivan and Pfefferbaum, 2003). FA measures the degree to which diffusion of water molecules is restricted by microstructural elements such as cell bodies, axons, myelin, and other constituents of cytoskeleton (Beaulieu, 2002); thus, reduced FA associated with normal aging may be related to a number of age-related changes in white matter demonstrated in postmortem studies of aging: change in the axon’s cytoskeleton, reduction in axon density (Sullivan et al., 2006), decline in number and length of myelinated fibers (Marner et al., 2003), breakdown in the myelin sheaths (Bartzokis et al., 2004; Bartzokis, 2004; Sullivan and Pfefferbaum, 2006), trapping of fluid between thin or lysed sheaths, or bulbous swelling of oligodendrocytes (Peters and Sethares, 2002; Peters et al., 2001; Sullivan et al., 2008). These studies support that DTI findings in normal aging align with histopathological findings in normal aging in white matter.
Age effects demonstrated with DTI are regionally diverse and typically show an anteroposterior gradient of age-related decline (Sullivan and Pfefferbaum, 2006; Sullivan et al., 2008). This gradient has been hypothesized by some investigators as underlying cognitive decline of frontally-based functions (Kochunov et al., 2007; Salat et al., 2005; Sullivan and Pfefferbaum, 2006). In quantifying age-related decline, most DTI studies have utilized region of interest or voxel-based morphometry approaches, where white matter integrity in focal brain regions were examined. For a review please see Sullivan and Pfefferbaum (2006).
More recently, investigators have used DTI tractography-based approaches in their examination of age-related decline of white matter fiber tracts (Stadlbauer et al., 2008; Sullivan et al., 2006, 2008; Zahr et al., 2009). White matter tracts serve as connections between brain regions, and likely play an important role in coordinating complex cognition. Information is required to transfer quickly between different brain regions and age-related damage to any part of these white matter connections could lead to changes in cognitive performance (Charlton et al., 2008; Mesulam, 2000). However, an important limitation of tractography is that the output is only a mathematical representation of underlying structure and may not always reflect brain anatomy (Sullivan et al., 2006). In particular, false positive and false negative results can be produced due to noise, partial volume effects, and complex fiber architecture within a voxel (Pierpaoli et al., 2001; Wakana et al., 2007). Nevertheless, several studies have shown that streamline tractography can produce anatomically faithful reconstructions of white matter fasciculi that agree with anatomic definitions based on postmortem studies (Catani and Thiebaut de Schotten, 2008; Catani et al., 2002; Jones, 2008; Mori and van Zijl, 2002; Schmahmann et al., 2007).
Few studies have combined tractography approaches with cognitive testing in normal aging. Comprehensive measurement of cognitive functions that may decline in normal aging requires administration of a battery of cognitive tests that include measures of processing speed, working memory (and executive function), episodic memory, mental flexibility (Hedden and Gabrieli, 2004), set shifting, attention, visuospatial performance, and visual processing (Davis et al., 2008), among others. To our knowledge, only 1 published tractography study has applied a relatively comprehensive cognitive battery (Zahr et al., 2009): in a sample of 12 younger and 12 older adults, performance in the working memory and problem-solving domains correlated with the microstructural integrity of the genu and the fornix and several fiber systems were also correlated with motor performance.
Structural equation modeling (SEM) in DTI (Charlton et al., 2008; Fonteijn et al., 2008) can provide a framework to examine the complex relationship among age, microstructural integrity of white matter fiber tracts, and cognitive performance. However, no published study to our knowledge has used diffusion tensor tractography and SEM to examine how age and microstructural integrity of white matter fiber tracts might influence cognition. Therefore, in this study, we used whole brain tractography and a novel clustering segmentation method, to measure microstructural integrity of white matter fiber tracts, and their relationship with cognitive performance in healthy individuals whose age-range spans the adult lifespan. We first investigated the relationship between age and specific white matter fiber tracts that may be susceptible to age-related decline, and for which we demonstrated high reliability of FA measurement, using our whole brain tractography, clustering segmentation approach (Voineskos et al., 2009). These tracts include intrahemispheric association fibers (cingulum bundle [CB], inferior longitudinal fasciculus [ILF], uncinate fasciculus [UF], arcuate fasciculus [AF], inferior occipitofrontal fasciculus [IFOF]), interhemispheric or commissural fibers of the corpus callosum (segmented into 5 subdivisions), and, for comparative purpose, projection fibers of the corticospinal tract (CST). Then, following principal components analysis of all cognitive tests chosen to index a wide range of cognitive functions susceptible to age-related decline, e.g., executive function, working memory, motor speed, visuospatial function, set shifting, etc. (Hedden and Gabrieli, 2004), we used SEM to examine the relationship of aging, white matter tract integrity, and cognitive performance. Based on the literature, we hypothesized that we would primarily observe associations between microstructural integrity of cortico-cortical white matter tracts (in particular those connecting to frontal cortical regions) with specific cognitive functions, primarily reflecting the frontally-based model of cognitive decline in normal aging.
2. Methods
2.1. Recruitment and characterization of study participants
This study was conducted from March 2007 to March 2009 in Toronto, Canada. Sixty-four individuals volunteered via registries and advertisement. All participants were assessed with the Edinburgh handedness inventory (Oldfield, 1971), interviewed by a psychiatrist, completed the Structured Clinical Interview for DSM-IV Disorders (First et al., 1995) and the Mini Mental Status examination (Folstein et al., 1975). All participants completed a urine toxicology screen. Fifty-three healthy participants met the inclusion criteria (age between 18 and 85; right-handedness) and none of the exclusion criteria (any history of a mental disorder; current substance abuse or a history of substance dependence, positive urine toxicology, a first degree relative with a history of psychotic mental disorder, a dementia, a history of head trauma with loss of consciousness, seizure, or another neurological disorder). Participants were characterized using the following instruments (Table 1): Wechsler Test for Adult Reading (WTAR); Hollingshead index (Hollingshead, 1975); Clinical Illness Rating Scale for Geriatrics (CIRS-G) (Miller et al., 1992); weight, height, and blood pressure. Three individuals did not complete all DTI and cognitive protocols and 2 individuals had artifacts on their DTI scan that prevented reliable analysis, leaving 48 participants for final analyses.
Table 1.
Participants’ characteristics
| Demographic | Mean ± SD | Range |
|---|---|---|
| Age (years) | 49 ± 17 | 22–81 |
| Education (years) | 15 ± 2 | 12–20 |
| Socioeconomic Status (4 factora) | 49 ± 11 | 27–66 |
| IQ (WTAR) | 118 ± 7 | 92–127 |
| MMSE | 29 ± 1 | 26–30 |
| BMI | 25 ± 5 | 19–36 |
| Systolic BP | 122 ± 12 | 105–153 |
| Diastolic BP | 75 ± 8 | 59–91 |
| CIRS-G (total score) | 2 ± 2 | 0–11 |
Key: BMI, body mass index; BP, blood pressure; CIRS-G, Cumulative Illness Rating Scale - Geriatrics; MMSE, Mini Mental State Examination; WTAR, Wechsler Test of Adult Reading.
Four factors are education, occupation, sex, and marital status.
2.2. Neuropsychological assessment
Participants underwent a battery of cognitive tests (Table 2) that was administered over approximately 1.5 hours. This battery assessed a wide range of cognitive domains, with a focus on domains that are most likely to be affected by age, primarily using tests validated in elderly populations: executive function, working memory, immediate memory, delayed or episodic memory, attention, set-shifting, response inhibition, mental flexibility, visuospatial construction, processing speed, fine visuomotor, and motor skills.
Table 2.
Cognitive battery of neuropsychological tests administered
| Neuropsychological tests | Domain | Test scores |
|---|---|---|
| Executive interview (EXIT) (Royall et al., 1992) | Executive function | High = poor performance |
| Finger tapping (Halstead, 1947) | Fine motor speed | Number of finger taps |
| Grooved pegboard (Matthews and Klove, 1964) | Fine visual-motor coordination | Time to completion |
| Letter fluency (Ruff et al., 1989) | Semantic memory | Total words for F+A+S |
| Letter-number span (Wechsler, 1997) | Working memory | Total numbers and letters |
| Letter cancellation test (Geldmacher et al., 2000) | Visuospatial attention/scanning | Time to completion |
| Trail-making test (A and B) (Reitan and Wolfson, 1985) | Flexibility | Ratio TrailsB/TrailsA |
| Stroop neuropsychological screening test (Trenerry, 1989) | Set shifting/response suppression | Ratio score |
| Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Gold et al., 1999; Hobart et al., 1999) | All RBANS scores are total scores | |
| List learning | Verbal memory/encoding | |
| Story memory | Verbal memory/encoding | |
| Figure copy | Visuospatial constructional | |
| Line orientation | Visuospatial/constructional | |
| Picture naming | Language | |
| Category fluency | Language | |
| Digit span | Attention | |
| Digit-symbol coding | Attention/visual processing and encoding | |
| List recognition | Delayed memory | |
| Story recall | Delayed memory | |
| Figure recall | Delayed memory/visuospatial |
Key: F+A+S, Total words starting with ‘F’, ‘A’, ‘S’.
2.3. Image acquisition
Diffusion weighted images were acquired using an 8-channel head coil on a 1.5 Tesla GE Echospeed system (General Electric Medical Systems, Milwaukee, WI), which permits maximum gradient amplitudes of 40 mT/m. A single shot spin echo planar sequence was used with diffusion gradients applied in 23 noncollinear directions and b = 1000 seconds/mm2. Two b = 0 images were obtained. Fifty-seven to 62 slices, with no gap, were acquired for whole brain coverage oblique to the axial plane. Slice thickness was 2.6 mm, and voxels were isotropic. The field of view was 330 mm and the size of the acquisition matrix was 128 × 128 mm, echo time was 85.5 ms, and repetition time was 15,000 ms. To improve the signal-to-noise ratio, the entire sequence was repeated 3 times. Inversion recovery prepped spoiled gradient recall images, fast spin T2 weighted images, and fluid attenuated inversion recovery images were also acquired.
2.4. Image analysis and tractography
Diffusion weighted images were then transferred to a workstation for analysis. The 3 repetitions were coregistered to the first b = 0 image in the first repetition using FSL (FMRIB Software Library, v. 4.0; www.fmrib.ox.ac.uk) to produce a new averaged image, with gradients reoriented according to the registration transformation. A final diffusion tensor was then estimated using a weighted least squares approach. Registration corrects eddy current distortions and subject motion, important artifacts that can affect the data, and averaging improves the signal-to-noise ratio. A brain “mask” was then generated. Points were seeded throughout each voxel of the brain.
Whole-brain tractography was performed with a deterministic (streamline) approach (Runge-Kutta order 2 tractography with a fixed step size of 0.5 mm). The 3 threshold parameters for tractography were Tseed, Tstop, and Tlength. Tseed and Tstop are anisotropy thresholds used to limit the tractography to white matter. The linear anisotropy measure CL (Westin et al., 2002) was used, where CL = (λ1 − λ2)/λ1 and, λ1 and λ2 are the 2 largest eigenvalues of the diffusion tensor sorted in descending order (where the goal of the anisotropy thresholds is to limit tractography to the white matter). Thresholds were based on the CL rather than on FA, because FA can be relatively high in regions of planar anisotropy which may indicate tract crossings or branching (Ennis and Kindlmann, 2006). Advantages of using CL as the tracking threshold have been previously demonstrated (Westin et al., 2002). Using CL thresholds will avoid seeding in planar regions that happen to have reasonably high FA. This is an advantage because in planar regions, the major eigenvector is unlikely to correspond to an actual axon direction, and rather it is some average of multiple tracts (Westin et al., 2002). Using CL for tractography also facilitates fiber clustering (below) because it reduces the likelihood of fiber jumping from 1 structure to another, a side-effect of streamline methods in planar partial-volume regions. So by somewhat reducing partial-volume tractography errors, it improves the ability of the clustering to separate different structures (O’Donnell and Westin, 2007). The Tlength threshold is used to eliminate very short fibers from being generated. The parameters chosen for this study were Tseed = 0.3, Tstop = 0.15, and Tlength = 20 (in mm). Tractography and creation of white matter fiber tracts was performed using 3D Slicer (open source software; www.slicer.org) and MATLAB 7.0 (www.mathworks.com).
As previously described by O’Donnell and co-workers (O’Donnell et al., 2006), pairwise fiber trajectory similarity was quantified by first computing a pairwise fiber distance. The mean closest point distance was employed, defined as the mean distance between pairs of closest points on 2 fibers. The directed distances between fibers “A” and “B” are converted to a symmetric pairwise fiber distance by taking the mean of the distance from A to B and from B to A. Each distance is then converted to an affinity measure suitable for spectral clustering via a Gaussian kernel (Wij) = e^ (−d2ij/σ2), a method employed in the clustering literature (Shi and Malik, 2000). The role of σ (σ = 60 mm used in the present study) is to define the size scale of the problem by setting the distance over which fibers can be considered similar (O’Donnell and Westin, 2007). A spectral embedding of fibers is then created based on the eigenvectors of the fiber affinity matrix. In our clustering application, we used the top 15 eigenvectors of the fiber similarity matrix to calculate the most important shape similarity information for each fiber. The use of the top 15 eigenvectors has been shown to produce excellent spatial and quantitative reliability of the selected white matter tracts (Voineskos et al., 2009), as well as good reproducibility (O’Donnell and Westin, 2007). The clustering algorithm used was k-way normalized cuts, as it produces clusters with high within-cluster similarity and low between-cluster similarity (Ng et al., 2002). In the k-way normalized cuts algorithm, “k” represents the number of clusters. In our reliability study (Voineskos et al., 2009) and in the present study white matter fibers were automatically segmented into 200 clusters. Following this step, groups of clusters were manually combined to comprise each neuroanatomical tract of interest by a trained operator (ANV) with neuroanatomical knowledge (a priori neuroanatomical knowledge is required for this step). Clusters of the same anatomical tract tend to have similar weights, thus facilitating selection (O’Donnell et al., 2006). Our white matter tract “clustering” segmentation method eliminates the need to manually place regions of interest (ROIs) to identify fiber bundles, following whole brain tractography (O’Donnell and Westin, 2007; O’Donnell et al., 2006), thus eliminating some forms of user bias inherent in ROI approaches. White matter tracts of interest can then be visualized in their correct anatomic location and selected to evaluate tract-specific diffusion parameters (Voineskos et al., 2009). In this study (Fig. 1), 10 association fibers (5 on each side): the left and right UF, left and right IFOF, left and right CB, left and right ILF, left and right AF, 2 projection fibers (left and right CST) were selected. Five clusters within the corpus callosum (CC) were selected: genu (CC1), premotor and supplementary motor projections (CC2), motor projections (CC3), sensory projections (CC4), and finally parietal, temporal and occipital projections (CC5). The CC was segmented using the clustering method, and selection of neuroanatomical subdivisions were made according to a previously demonstrated DTI-based topographical study of the corpus callosum (Hofer and Frahm, 2006), and confirmed in more recent studies (Voineskos et al., 2009; Wahl et al., 2007). Correct selection of all tracts was verified by superimposing clusters on both the FA and T1 images (Mori et al., 2005). Tract variability can be introduced when the operator selects additional cluster(s) to add to the main cluster initally selected for the neuroanatomic tract of interest. Overall, this approach is at least as reliable as the multiple ROI approach (Voineskos et al., 2009), and was consistently reliable for all white matter tracts in the present study. Two individuals, blind to participant information, performed the entire clustering procedure on 10 individuals: as reported elsewhere (Voineskos et al., 2009), reliability was demonstrated both spatially and quantitatively (i.e., both voxel overlap and scalar measures of the tensor showed high agreement). MATLAB (v. 7.0) was then used to calculate FA (Basser and Pierpaoli, 1996). Presented data represents the mean values along the selected tracts.
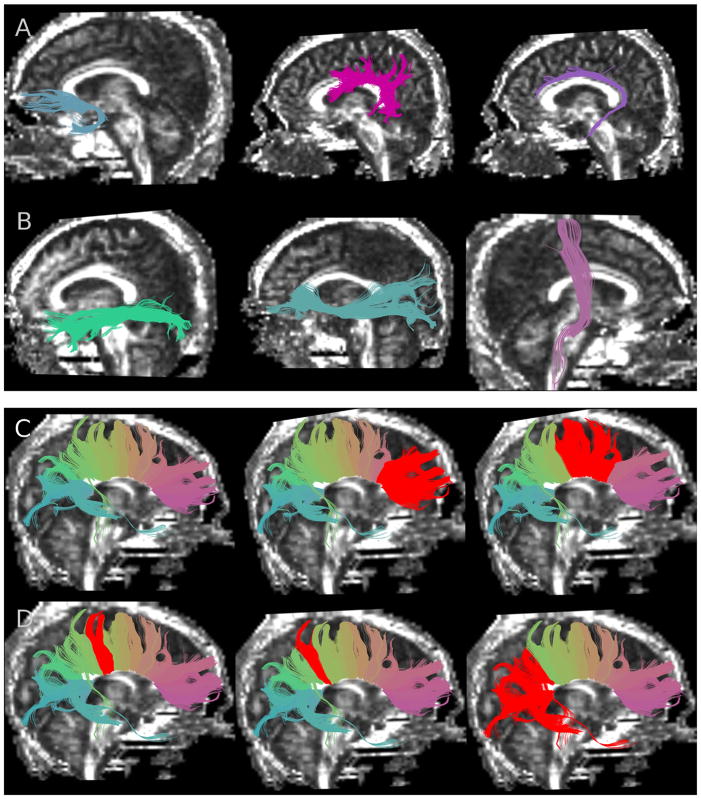
Fig. 1.
Segmented white matter fiber tracts superimposed on fractional anisotropy images. (A) From left to right: uncinate fasciculus, arcuate fasciculus, cingulum bundle; and (B) from left to right: inferior longitudinal fasciculus, inferior occipitofrontal fasciculus, corticospinal tract; (C) from left to right: corpus callosum (whole structure with fibers color-coded according to similarity of shape and location), genu of corpus callosum (CC1) selected in red, premotor and supplementary motor fibers of corpus callosum (CC2) selected in red; and (D) from left to right: motor fibers of corpus callosum (CC3) selected in red, sensory fibers of corpus callosum (CC4) selected in red, and parietal, temporal and occipital fibers of corpus callosum (CC5) selected in red.
2.5. Statistical analysis
Age-related decline of white matter is generally considered equivalent in men and women (Sullivan and Pfefferbaum, 2006; Sullivan et al., 2001); however, others have reported that gender differences in FA may be present (Hsu et al., 2008). Similarly there are reports suggesting the presence (Kubicki et al., 2002) and absence (Nestor et al., 2008) of hemispheric asymmetry with respect to bilateral tract FA. Thus, to test for effects of gender and hemisphere on all white matter tracts in the present study, a repeated measures analysis of covariance (ANCOVA) was performed for bilateral tracts with gender as the between group factor, hemisphere and tract as within group factors, and age as a covariate. A separate repeated measures ANCOVA for the corpus callosum was conducted with gender as the between group factor, corpus callosum subdivision as the within group factor, and age as a covariate. FA for tracts not showing hemispheric effects were averaged as follows: (left side FA + right side FA)/2, an approach previously used (Sullivan et al., 2008; Zahr et al., 2009). FA for each tract was then regressed against age.
Second, Pearson product moment correlations comparing cognitive performance with age were computed. Raw scores were converted to z-scores. The z-scores were then multiplied by (−1) for tests in which a high score reflected poor performance. Where available (namely for all tests of the Repeatable Battery for the Assessment of Neuropsychological Status) (RBANS), age-normed scores of each cognitive test were regressed against age to ensure that there was no stratification within the sample. Z-scores of all cognitive tests were then submitted to principal components analysis (PCA) using SPSS 15.0, Chicago, Illinois (PCA; orthogonal transformation varimax solution). Principal components were retained if eigenvalues were greater than 1, and factor scores were then calculated for each principal component for each participant for SEM.
Finally, SEM was used to simultaneously estimate relationships among age, white matter integrity, and the factor scores for the 6 retained cognitive principal components: memory and executive function; attention and working memory; set shifting/flexibility; visuomotor speed and dexterity; and visuosopatial construction. SEM was conducted using AMOS software, Chicago, Illinois (Arbuckle, 1999). A literature-derived model was built, emphasizing frontally-based white matter tracts and their relationship with age and executive function (O’Sullivan et al., 2005; Sullivan and Pfefferbaum, 2006; Sullivan et al., 2006; Zahr et al., 2009). Thus paths from the genu (CC1) to “memory and executive function”, and “attention and working memory”, and from the CB to “set shifting/flexibility” (O’Sullivan et al., 2005; Zahr et al., 2009) were drawn. A path from posterior fibers of the CC (CC5) to “memory and executive function” was also included based on previous reports (Ziegler et al., 2008). White matter tracts that might contribute to motor speed, namely premotor and supplementary motor fibers of CC (CC2), motor fibers of the CC (CC3) (Sullivan and Pfefferbaum, 2006), and UF (Zahr et al., 2009) were included. The ILF was hypothesized to contribute to ’visuomotor speed and dexterity’ based on a previous report that the ILF may subserve a “fast or direct stream” of visual processing (Catani et al., 2003), and that the ILF in a previous study demonstrated a potential relationship with a motor speed component that included the Grooved Pegboard task (Zahr et al., 2009), a task that is part of our “visuomotor speed and dexterity” component. Paths from both the IFOF, given its occipito-parieto-temporal-frontal connection (Karnath et al., 2009; Kier et al., 2004), and from CC5 (given its interhemispheric parietal, temporal, and occipital connections), to visuosopatial construction ability were also included. A path from the AF (hypothesized function related to language (Catani et al., 2007) and working memory (Aboitiz and Garcia, 1997)) was drawn to the “attention and working memory” component. The CST was not included because it was not hypothesized to be involved in any of the cognitive domains from the PCA. After the path coefficients were derived, the paths were thresholded to achieve a second, more parsimonious model, by eliminating paths with p values > 0.10 (a threshold of p = 0.10, rather than p = 0.05 was chosen to ensure that relevant paths were not eliminated due to low power). Path elimination was also monitored via successive improvement of the χ2 statistic, comparative fit index (CFI), and root mean square error of approximation (RMSEA). Good model fit can be reflected by a χ2 to degrees of freedom ratio of less than 2.0 (Ullman, 2001), a CFI of greater than .90 (Bentler, 1990), and an RMSEA of less than .06 (Hu and Bentler, 1999) or .05 (Schumacker and Lomax, 2004). The CFI and RMSEA are among the measures least affected by sample size (Fan and Thompson, 1999), and in fact, the CFI performs very well at all sample sizes (Bentler, 1990). As a robustness check, the final model fitting was repeated using bootstrapping. Ninety-five percent confidence intervals of the path parameters of the final model were estimated using this nonparametric resampling method. Two thousand replications were used in the bootstrapping (the maximum number possible using AMOS software) for all 48 participants.
3. Results
3.1. Age and DTI measures
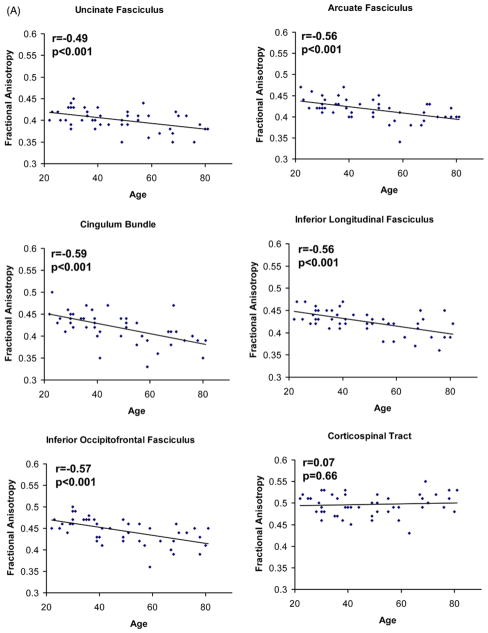
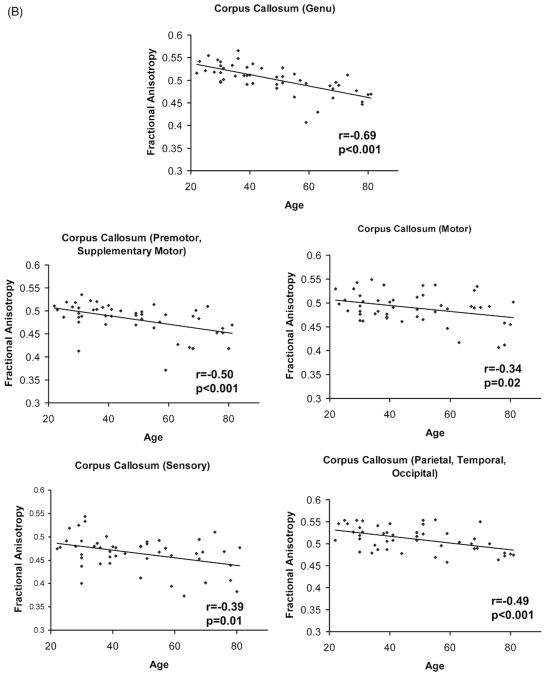
Following the repeated measures ANCOVA for bilateral tracts, no gender by tract (F(5,225) = 0.46, p = 0.80), hemisphere by tract (F(5,225) = 1.28, p = 0.27), or gender by hemisphere by tract interactions were found (F(5,225) = 0.71, p = 0.61). The repeated measures ANCOVA examining the effect of gender on subdivisions of the corpus callosum also revealed no gender by callosal subdivision interaction (F(4,180) = 1.42, p = 0.23). Therefore, a mean tract FA was calculated for all intrahemispheric (i.e., bilateral) tracts, and gender was not treated as a separate variable. All intrahemispheric tracts (UF, AF, CB, IFOF, ILF) displayed significant age-related decline except for the CST (Fig. 2). Within the corpus callosum, the genu callosal region (CC1) connecting left and right prefrontal cortex, experienced greater age-related decline than callosal regions connecting primary motor cortex (CC3) (CC1, r = −0.69 vs. CC3, r = −0.34, t45 = 3.9, p < 0.01) or primary sensory cortex (CC4) (CC1, r = −0.69 vs. CC4, r = −0.39; t45 = 3.6, p < 0.01), using a test of significance of the difference between dependent correlations from the same sample (Blalock, 1972) t = (rxy −rzy)* square root[{(n −3) (1 + rxz)}/{2(1 − rxy2 − rxz2 − rzy2 − + 2rxy* rxz* rzy)}], where “n” represents sample size, “x” represents age, “y” and “z” are FA values for 2 white matter tracts of interest, and “r” represents the Pearson product moment correlation of the 2 variables. The most posterior callosal subdivision (CC5) containing interhemispheric parietal, temporal, and occipital fibers also experienced significant age-related decline, but not to the same extent as the genu (CC1, r = −0.69 vs. CC5, r = −0.49; t45 = 3.4, p < 0.01).
Fig. 2.
Relationship between age and fractional anisotropy of white matter tracts: (A) ucinate fasciculus, arcuate fasciculus, cingulum bundle, inferior longitudinal fasciculus, inferior occipitofrontal fasciculus, and corticospinal tract; and (B) corpus callosum (genu), corpus callosum (premotor and supplementary motor), corpus callosum (motor), corpus callosum (sensory), and corpus callosum (parietal, temporal, and occipital).
3.2. Age and cognitive performance
Table 3 shows the relationship of age with scores on each cognitive test. There was no correlation of age with age-normed scores, suggesting that there was no age-related stratification in performance in our sample. The principal components analysis of the cognitive data yielded 6 factors with eigenvalues ≥1 (see Supplementary Table 1). The 6 factors (or principal components) were theoretically meaningful, collectively accounting for 73.9% of the variance in test scores. The 6 principal components were: (1) memory and executive function (λ = 6.9%, 32.8% of variance); (2) visuomotor speed and dexterity (λ = 3.1%, 15.0% of variance); (3) motor speed and coordination (λ = 1.6%, 7.4% of variance); (4) attention and working memory (λ = 1.4%, 6.7% of variance); (5) set shifting/flexibility (λ = 1.3%, 6.2% of variance); and (6) visuospatial construction (λ = 1.2%, 5.8% of variance).
Table 3.
Correlations between age and cognitive scores
| Test | Mean ± SD | Correlation with age | p Value |
|---|---|---|---|
| EXIT* | 2.8 ± 2.4 | .48 | 0.001 |
| Finger taps (DH) | 44.4 ± 10.6 | −.63 | <0.001 |
| Finger taps (NDH) | 39.6 ± 9.3 | −.59 | <0.001 |
| Grooved pegboard (DH)* | 72.3 ± 16.8 | .35 | 0.014 |
| Grooved pegboard (NDH)* | 82.7 ± 20.0 | .32 | 0.029 |
| Letter fluency | 14.7 ± 4.3 | −.22 | 0.13 |
| Letter number span | 16.2 ± 4.0 | −.42 | 0.003 |
| Letter cancellation* | 63.2 ± 24.8 | .43 | 0.002 |
| TrailsB/TrailsA* | 2.5 ± 1.0 | .34 | 0.019 |
| Stroop ratio* | 2.2 ± 0.5 | .44 | 0.002 |
| RBANS | |||
| List learning | 30.4 ± 4.4 | −.44 | 0.002 |
| Story memory | 19.7 ± 3.1 | −.29 | 0.045 |
| Figure copy | 17.6 ± 2.7 | −.19 | 0.19 |
| Line orientation | 17.6 ± 1.9 | −.50 | <0.001 |
| Picture naming | 9.5 ± 0.9 | −.24 | 0.10 |
| Category fluency | 23.0 ± 5.1 | −.40 | 0.005 |
| Digit span | 12.2 ± 2.6 | −.42 | 0.003 |
| Coding | 50.3 ± 12.9 | −.59 | <0.001 |
| List recall | 7.2 ± 2.2 | −.39 | 0.007 |
| Story recall | 10.3 ± 1.6 | −.38 | 0.009 |
| Figure recall | 12.5 ± 5.3 | −.50 | <0.001 |
Key: DH, dominant hand; NDH, nondominant hand; EXIT, executive interview; RBANS, repeatable battery for the assessment of neuropsychological status.
Tests for which higher scores indicate poorer performance.
3.3. Structural equation modeling
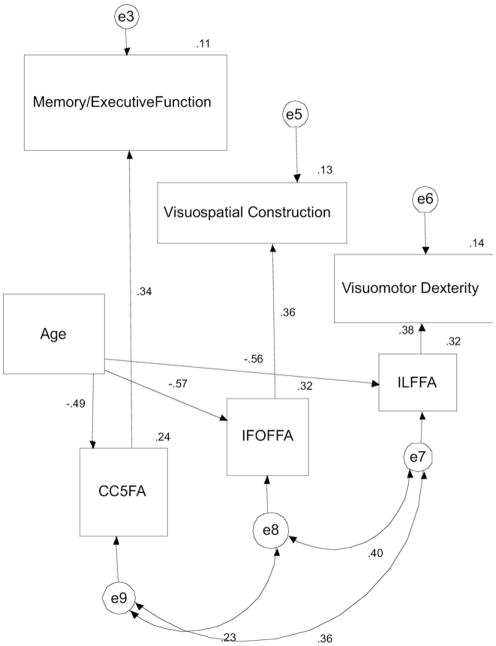
The original literature derived model did not provide a good fit (χ2 = 1880, df = 84, p < 0.001, CFI = .000, RMSEA = .679; see Supplementary Figure 1). To modify the model, each path that did not meet the p = 0.10 significance threshold was systematically removed. Thus, paths from CC1 to “memory and executive function” and “attention and working memory” were removed, from CC2 and CC3 to “motor speed”, from CB and AF to “attention and working memory”, from CB to “set shifting”, and from CC5 and ILF to “visuospatial construction” were also removed. Then, variables representing white matter tracts (CC1, CC2, CC3, CB, UF) that had no remaining relationships with any cognitive component were removed, and cognitive components (motor speed, working memory and attention, set shifting) that had no remaining relationship with any white matter tract were removed. Thus, the remaining tracts were the ILF, IFOF, and CC5. The remaining cognitive components were “memory and executive function”, “visuomotor dexterity”, and “visuospatial construction”. Finally, direct paths from age to “memory and executive function”, “visuomotor dexterity”, and “visuospatial construction” were removed, because these paths also did not meet the significance threshold. By removing these paths, a new statistically significant model was created where age and white matter tract integrity did determine cognitive performance. For this final model (Fig. 3), there was excellent model fit (χ2 = 5.0, df = 12, p = 0.96, CFI = 1.00, RMSEA = .000) (remainder of data listed shown in Fig. 3 and in Table 4). As explained, good model fit can be measured by a χ2 to degree of freedom ratio of <2 (here it is 0.42), a CFI of greater than 0.91 (here it is 1.0), and an RMSEA of <.05 (here it is .000) (Bentler, 1990; Schumacker and Lomax, 2004; Ullman, 2001). This new model contains age, white matter tracts (CC5, ILF, IFOF), and principal cognitive components (memory and executive function, visuomotor dexterity, and visuospatial construction). This model suggests that CC5 integrity broadly predicted memory and executive function, ILF integrity predicts performance on tasks requiring visuomotor dexterity, and IFOF integrity predicts performance on tasks requiring visuospatial construction. Finally, effects of age on these 3 cognitive components occurred via the 3 white matter tracts in the model.
Fig. 3.
Final parsimonious model illustrating relationships among age, white matter tract integrity, and cognitive performance. Curved 2-way arrows represent covariance terms between each variable. One-way arrows represent the impact of 1 variable on another. e3, e5, e6, e7, e8, e9 represent error variance terms associated with each variable. Abbreviations: CC5FA, fractional anisotropy of parietal, temporal, and occipital fibers of corpus callosum; IFOFFA, fractional anisotropy of inferior occipitofrontal fasciculus; ILFFA, fractional anisotropy of inferior longitudinal fasciculus.
Table 4.
Regression coefficients for final model, along with 95% confidence intervals (CI) and significance
| Paths | Standardized regression coefficient | p Value* | 95% CI | p Value* |
|---|---|---|---|---|
| Age → IFOF FA | −.57 | <0.001 | −.421 to −.707 | 0.001 |
| Age → ILF FA | −.56 | <0.001 | −.343 to −.735 | 0.001 |
| Age → CC5 FA | −.49 | <0.001 | −.236 to −.677 | 0.001 |
| IFOF FA → Visuospatial construction | .37 | 0.007 | .144–.548 | 0.001 |
| ILF FA → Visuomotor Dexterity | .38 | 0.005 | .001–.604 | 0.050 |
| CC5 FA → Memory/executive function | .34 | 0.014 | .082–.531 | 0.016 |
Key: CC5, occipital, parietal, and temporal fibers of corpus callosum; FA, fractional anisotropy; IFOF, inferior occipitofrontal fasciculus; ILF, inferior longitudinal fasciculus.
p Values of the 95% CI were derived by 2000 bootstrap replications.
In this model, all standardized regression weights were significant (see Table 4). The bootstrap confidence intervals (2000 replications, the maximum permissible with AMOS software) suggested a robust model. The 95th percentile confidence intervals derived by bootstrapping demonstrated a p value of ≤0.05 (see Table 4).
4. Discussion
This study demonstrated age-related decline in FA in all cortico-cortical white matter fiber tracts. An anteroposterior gradient of age related decline was evident in corpus callosum fibers. No gradient was evident in the intrahemispheric association fibers studied (CB, AF, UF, IFOF, and ILF). As expected, the projection fiber studied, the CST, demonstrated no age-related decline. Following PCA of a cognitive battery, and construction of an SEM based on existing theories of cognitive aging, each path was tested systematically to produce a final SEM. In our final model, age-related change in integrity of the CC5 subdivision (i.e., posterior projections/splenium) of the corpus callosum predicted “memory and executive function”, of the IFOF predicted “visuospatial construction” performance, and of the ILF predicted “visuomotor dexterity” performance.
Our finding for potential ILF function is novel. The role of the ILF in cognitive performance has been unknown, and direct approaches to characterize ILF function such as peri-operative stimulation have not been successful (Mandonnet et al., 2007). In our study, age-related disruption of the ILF predicted performance in tasks that require visuomotor dexterity and fast visual processing. Tests that loaded high on this cognitive factor include the grooved pegboard task, letter cancelation task, and the digit-symbol coding task. Using DTI, virtual in vivo dissection has provided important evidence for the ILF, where reconstructions closely matched those of early anatomical descriptions (Catani et al., 2002), and it was suggested that the ILF may subserve a “direct short-latency pathway” of visual processing, i.e., a fast stream of visual processing, whereby the parahippocampal gyrus (in the temporal lobe) and visual cortex (in the occipital lobe) communicate (Catani et al., 2003). Our results provide in vivo evidence that the ILF may subserve this “direct short-latency pathway” of visual processing. Evidence for ILF function in this direct pathway was suggested in a case report on a patient who had sustained a lesion restricted to this direct pathway and was unable to learn novel, nonverbalizable visual stimuli. The conclusion was that the direct pathway serves to prime medial temporal structures to facilitate the processing of visual information (Ross, 1980). Such a “priming” role could be critical, for instance, during the performance of the digit-symbol coding task. Performance on the digit-symbol coding task depends on the speed and accuracy of the task, whereby the participant must visually process the symbol, link the symbol to the digit, and then transcribe the digit. Although the digit-symbol associations key is always available for the participant to refer to during the task, the participant’s performance is likely to improve if the medial temporal structures are “primed” and active representation of the digit-symbol associations are readily available to be matched with direct information from the visual cortex.
Our SEM demonstrated a significant association of the IFOF with visuospatial construction ability (cognitive tasks loading high on this factor included the figure-copy task, and the figure-recall task). The inferior occipitofrontal fasciculus (IFOF) is a large association bundle of fibers connecting the occipital and frontal lobes, and also connects the frontal lobe with the posterior part of the parietal and temporal lobes (Kier et al., 2004). The IFOF is an ideal candidate to mediate interactions between visual, spatial, and executive functions considering its occipitoparietal and occipitofrontal connections. Performance on a complex visuospatial construction task is likely to depend on the involvement of more than 1 cognitive function. In particular, the abilities to process visual and spatial abilities combined with executive function are likely mediated by the IFOF’s connection to anterior frontal regions, from occipital and parietal regions, which may allow for better organization and planning of the “construction” part of the task. Furthermore, the fronto-temporal connection of the IFOF may play a role in figure-recall, which would also require the aforementioned visual and spatial abilities. Direct (i.e., invasive) investigations have demonstrated a potential language function for the IFOF via electrostimulation of this pathway intraoperatively, whereby semantic paraphasias were induced (Duffau et al., 2005). Nevertheless, studies where damage to the IFOF is known (Karnath et al., 2009; Urbanski et al., 2008), suggest a role for the IFOF in visuospatial function, and we have demonstrated such a relationship in our normal aging population.
Posterior fibers of the corpus callosum (i.e., occipital, parietal, temporal) were associated with the “memory and executive function” factor. Most notably, tests of episodic memory, loaded particularly high on this factor, such as Story Recall and List Recall. Considerable evidence from functional neuroimaging studies implicates temporoparietal areas in episodic memory (e.g., Buckner and Wheeler, 2001; Rugg et al., 2002). Furthermore, white matter integrity underlying temporal and posterior parietal areas, but not frontal areas, was associated with episodic memory performance in an elderly population (Ziegler et al., 2008). Diminishing integrity in occipital fibers of the corpus callosum may also be related to perceptual processing declines that may explain worsening executive function in elderly individuals (Davis et al., 2008), which aligns with our finding relating posterior callosal fibers to executive function. Other cognitive tasks that loaded on this factor of “memory and executive function” included the Stroop color-word task and the Letter Number Span task. Therefore, our findings add to previous findings, namely, association of integrity of the splenium of the corpus callosum with the Stroop task in a small elderly sample (Sullivan et al., 2006), association of integrity of the splenium with working memory performance in a chronic alcoholism population (Pfefferbaum et al., 2000), and association of white matter integrity of temporal and posterior parietal areas with episodic memory performance in an elderly population (Ziegler et al., 2008).
Surprisingly, we found that the genu of the corpus callosum, which connects left and right dorsolateral prefrontal cortex, did not contribute significantly to any 1 cognitive domain. Others have found that the genu predicts executive function test performance, particularly working memory (Zahr et al., 2009). Despite our negative findings here, the genu remains an important candidate in executive function processes given that it connects left and right dorsolateral prefrontal cortex, cortical regions with critical roles in executive function and working memory. Age related compensatory mechanisms may explain the lack of significance between genu microstructure and overall executive function performance found in our study. Some elderly subjects may recruit compensatory brain regions or networks, maintaining their level of executive function, that may compensate for age-related decline in a specific region or white matter tract. Such compensatory strategies are particularly relevant to frontal brain regions and the genu of the corpus callosum, and have been demonstrated in functional MRI studies, where different patterns of recruitment of brain regions predicted executive function performance in elderly individuals (Cabeza et al., 2002). Some have suggested that subtle compromise of the genu may actually enable bilateral engagement of the hemispheres, thus enabling compensatory mechanisms (Buckner, 2004) and influencing resource allocation in executive function processes (Banich, 1998; Reuter-Lorenz and Stanczak, 2000; Sullivan and Pfefferbaum, 2006). It is possible that in a more homogeneous sample (i.e., smaller age range), where age effects do not contribute to the variance in performance, such as in younger healthy adults, the genu of the corpus callosum may play a more prominent role in overall executive function.
Some limitations of this study should be considered. We did not study all white matter tracts in the brain. Such an approach might have elicited relationships between other white matter tracts and cognitive performance, thus accounting more comprehensively for widespread declines in cognitive function. However, we focused primarily on cortico-cortical white matter tracts for which we have previously shown high reliability, both spatially and quantitatively (Voineskos et al., 2009). Others have studied the fornix of the hippocampus (Fitzsimmons et al., 2009), a white matter tract that has been demonstrated to play a role in working memory function (Zahr et al., 2009). However, highly reliable segmentation of the fornix using streamline tractography can be a challenge with either clustering or region of interest segmentation methods (O’Donnell and Westin, 2007; Wakana et al., 2007), and thus it was not included in our study. Healthy elderly subjects often have white matter hyperintensities (Wen and Sachdev, 2004), generally not accounted for in DTI studies of healthy aging. In our sample, 7 subjects (all age 55 and greater, data not shown) had indications of white matter disease on fluid-attenuated inversion recovery images (FLAIR). Two had extensive confluent deep white matter hyperintensities (WMH), and 5 had either small- or medium-sized focal WMH in deep white matter. WMH may affect white matter microstructural integrity (FA), though a relationship between these measures is only just beginning to be understood (Zhan et al., 2009). It is also possible that relationships between white matter tract integrity and specific cognitive tests were present, but not detected. Reduction of individual cognitive tests into principal components was necessary to reduce the complexity of the data. A larger sample size might have provided us with more power for our model, which in turn might have permitted retention of more paths in our model, and further insight into the relationship of other white matter tracts with cognitive components in the model. A larger sample might have also given a narrower range of confidence intervals in bootstrapping procedures. However, the white matter tracts in our current model demonstrated strong association with the cognitive tasks included, and reasonable bootstrap estimates. Finally, our cross-sectional design is a limitation of our study. Cross-sectional studies examining aging are potentially confounded by cohort differences and might therefore overestimate age-related differences, particularly in cognitive decline. Yet others have suggested that cross-sectional studies where some demands were placed on participants (e.g., volunteering to come to a University Hospital for testing), as required in our study, might over-represent higher performing elderly adults (Hedden and Gabrieli, 2004). Given the design of our study, it is possible that our findings may not reflect the true evolution of white matter integrity or cognition with age. This would require a longitudinal study lasting several years and an analysis of individual trajectories of white matter integrity and cognition. However, in the absence of any such extended longitudinal DTI study of healthy aging (to our knowledge), our findings of an age effect on both white matter integrity and cognition support that the 2 processes are related.
In summary, we demonstrated significant age-related decline in the great majority of white matter tracts studied. Our findings emphasize the relationship between age-related decline in white matter integrity and cognitive function, and verify the functional ramifications of DTI metrics. We identified potential cognitive functions for 2 white matter tracts, the ILF and IFOF, consistent with their neuroanatomical connections, and provide supporting evidence for the role for posterior projections of the CC in memory and executive function. The investigation of functional ramifications of white matter measures remain an important area of inquiry that will likely continue to offer fascinating insight into relationships between brain structure and function. Future combinations of DTI and functional neuroimaging and/or neurophysiological tools in vivo may yield even more powerful conclusions about the mechanisms of age-related cognitive decline in the brain.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research Clinician scientist Award (ANV); APA/APIRE AstraZeneca Young Minds in Psychiatry Award (ANV); Canadian Institutes of Health Research Fellowship (TKR); National Institutes of Health (NIH) [R01, grant no. MH074794] (MES), [R01, grant number MH 50,740] (MES), NIH [1P50, grant no. MH08272] (MES), NIG/HS Nih [U54 grant no. GM072977-01] (MES), VA merit (MES), VA Schizophrenia Center Grant (MES), the Sandra A. Rotman Research Institute (BGP), and the Centre for Addiction and Mental Health.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging. 2010.02.009.
Footnotes
Disclosure statement
Dr. Bruce Pollock receives research support from the National Institute of Health. Within the past year he has been a member of the advisory board of Lundbeck, Canada, and has served 1 time as a consultant for Wyeth Pharmaceuticals (October 2008). He is currently a faculty member of the Lundbeck International Neuroscience Foundation (LINF).
Dr. Benoit Mulsant has in the past received research support or honoraria from AstraZeneca, Corcept, EISAI, Eli Lilly, Lundbeck, Forest, GlaxoSmithKline, Janssen, and Pfizer. Dr. Mulsant owns stock of less than $10,000 in value in Akzo-Nobel, Alkermes, AstraZeneca, Biogen Idec, Ceslsion, Elan, Eli Lilly, Forest, General Electric, and Orchestra Therapeutics.
The remaining authors have no financial disclosures to report.
The study was approved by the Review and Ethics Board of the Centre for Addiction and Mental Health (Toronto, Canada) and all participants provided informed, written consent.
References
- Aboitiz F, Garcia R. The anatomy of language revisited. Biol Res. 1997;30:171–183. [PubMed] [Google Scholar]
- Arbuckle J. Amos 4.01. Vol. 4. Smallwater Corporation; Chicago: 1999. [Google Scholar]
- Banich MT. The missing link: the role of interhemispheric interaction in attentional processing. Brain Cogn. 1998;36:128–157. doi: 10.1006/brcg.1997.0950. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. Author reply, 49–62. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Blalock H. Social Statistics. McGraw-Hill; New York: 1972. [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nat Rev Neurosci. 2001;2:624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci U S A. 2007;104:17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiol Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Catani M, Johansen-Berg H, Clark C, Thompson A. Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol. 2008;7:715–727. doi: 10.1016/S1474-4422(08)70163-7. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electro-stimulations. Brain. 2005;128:797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Ennis DB, Kindlmann G. Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med. 2006;55:136–146. doi: 10.1002/mrm.20741. [DOI] [PubMed] [Google Scholar]
- Fan XB, Thompson WL. Effects of sample size, estimation method, and model specification on structural equation modeling fit indexes. Struct Equation Model. 1999;6:56–83. [Google Scholar]
- First MBSR, Gibbon M, Williams JBW. Strucutured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), Version 2. Biometrics Research; New York: 1995. [Google Scholar]
- Fitzsimmons J, Kubicki M, Smith K, Bushell G, Estepar RS, Westin CF, Nestor PG, Niznikiewicz MA, Kikinis R, McCarley RW, Shenton ME. Diffusion tractography of the fornix in schizophrenia. Schizophr Res. 2009;107:39–46. doi: 10.1016/j.schres.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fonteijn HM, Norris DG, Verstraten FA. Exploring the Anatomical Basis of Effective Connectivity Models with DTI-Based Fiber Tractography. Int J Biomed Imaging. 2008;2008:423192. doi: 10.1155/2008/423192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldmacher DS, Fritsch T, Riedel TM. Effects of stimulus properties and age on randomarray letter cancellation tasks. Aging Neuropsychol Cogn. 2000;7:194–204. [Google Scholar]
- Gold JM, Queern C, Iannone VN, Buchanan RW. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia I: sensitivity, reliability, and validity. Am J Psychiatry. 1999;156:1944–1950. doi: 10.1176/ajp.156.12.1944. [DOI] [PubMed] [Google Scholar]
- Halstead W. Brain and Intelligence. University of Chicago Press; Chicago: 1947. [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hobart MP, Goldberg R, Bartko JJ, Gold JM. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia. II: convergent/discriminant validity and diagnostic group comparisons. Am J Psychiatry. 1999;156:1951–1957. doi: 10.1176/ajp.156.12.1951. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, Chiu HC, Chen WH. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage. 2008;39:566–577. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equation Model. 1999;6:1–55. [Google Scholar]
- Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44:936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Rorden C, Ticini LF. Damage to White Matter Fiber Tracts in Acute Spatial Neglect. Cereb Cortex. 2009;19:2331–2337. doi: 10.1093/cercor/bhn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Matthews CG, Klove H. Instruction Manual for the Adult Neuropsychology Test Battery. University of Wisconsin Medical School; Madison, WI: 1964. [Google Scholar]
- Mesulam M. Brain, mind, and the evolution of connectivity. Brain Cogn. 2000;42:4–6. doi: 10.1006/brcg.1999.1145. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: principles and strategies–a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Negae-Poetscher Lv, Zijl PC. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: a diffusion tensor imaging study. Neuropsychology. 2008;22:246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A, Jordan M, Weiss Y. On Spectral Clustering: Analysis and an Algorithm. MIT Press; Cambridge, MA: 2002. [Google Scholar]
- O’Donnell LJ, Westin CF. Automatic tractography segmentation using a high-dimensional white matter atlas. IEEE Trans Med Imaging. 2007;26:1562–1575. doi: 10.1109/TMI.2007.906785. [DOI] [PubMed] [Google Scholar]
- O’Donnell LJ, Kubicki M, Shenton ME, Dreusicke MH, Grimson WE, Westin CF. A method for clustering white matter fiber tracts. AJNR Am J Neuroradiol. 2006;27:1032–1036. [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan M, Barrick TR, Morris RG, Clark CA, Markus HS. Damage within a network of white matter regions underlies executive dysfunction in CADASIL. Neurology. 2005;65:1584–1590. doi: 10.1212/01.wnl.0000184480.07394.fb. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Killiany RJ. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J Comp Neurol. 2001;435:241–248. doi: 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Publishing; Tucson, AZ: 1985. [Google Scholar]
- Reuter-Lorenz PA, Stanczak L. Differential effects of aging on the functions of the corpus callosum. Dev Neuropsychol. 2000;18:113–137. doi: 10.1207/S15326942DN1801_7. [DOI] [PubMed] [Google Scholar]
- Ross ED. Sensory-specific and fractional disorders of recent memory in man. I. Isolated loss of visual recent memory. Arch Neurol. 1980;37:193–200. doi: 10.1001/archneur.1980.00500530031001. [DOI] [PubMed] [Google Scholar]
- Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Quayhagen M, Light RH. Selective Reminding Tests–A Normative Study of Verbal-Learning in Adults. J Clin Exp Neuropsychol. 1989;11:539–550. doi: 10.1080/01688638908400912. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schumacker RE, Lomax RG. A Beginner’s Guide to Structural Equation Modeling. Lawrence Erlbaum Associates; Mahwah, NJ: 2004. [Google Scholar]
- Shi J, Malik J. Normalized cuts and image segmentation. IEEE, Trans Patterns Anal Mach Intell. 2000;22:888–905. [Google Scholar]
- Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Quantitative diffusion tensor fiber tracking of age-related changes in the limbic system. Eur Radiol. 2008;18:130–137. doi: 10.1007/s00330-007-0733-8. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol. 2003;45:244–255. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci Biobehav Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: Relations to timed performance. Neurobiol Aging. 2008;31:464–481. doi: 10.1016/j.neurobiolaging.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Trenerry MR. Stroop Neuropsychological Screening Test Manual. Psychological Assessment Resources; Odessa, FL: 1989. [Google Scholar]
- Ullman JB. Structural equation modeling. In: Tabachnik BG, Fidell LS, editors. Using Multivariate Statistics. Allyn & Bacon; Need-ham Heights, MA: 2001. pp. 653–771. [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Catani M, Oppenheim C, Touze E, Chokron S, Meder JF, Levy R, Dubois B, Bartolomeo P. Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. J Neurol Neurosurg Psychiatry. 2008;79:598–601. doi: 10.1136/jnnp.2007.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, O’Donnell LJ, Lobaugh NJ, Markant D, Ameis SH, Niethammer M, Mulsant BH, Pollock BG, Kennedy JL, Westin CF, Shenton ME. Quantitative examination of a novel clustering method using magnetic resonance diffusion tensor tractography. Neuroimage. 2009;45:370–376. doi: 10.1016/j.neuroimage.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Memory Scale. Psychological Corporation, Harcourt Brace Jovanovich; San Antonio, TX: 1997. [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60 to 64-year-old individuals. Neuroimage. 2004;22:144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Westin CF, Maier SE, Mamata H, Nabavi A, Jolesz FA, Kikinis R. Processing and visualization for diffusion tensor MRI. Med Image Anal. 2002;6:93–108. doi: 10.1016/s1361-8415(02)00053-1. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: a quantitative fiber tracking study. Neuroimage. 2009;44:1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W, Zhang Y, Mueller SG, Lorenzen P, Hadjidemetriou S, Schuff N, Weiner MW. Characterization of white matter degeneration in elderly subjects by magnetic resonance diffusion and FLAIR imaging correlation. Neuroimage. 2009;47(Suppl 2):T58–T65. doi: 10.1016/j.neuroimage.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S. Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]