Abstract
Purpose
The advent of imaging software programs have proved to be useful for diagnosis, treatment planning, and outcome measurement, but precision of 3D surgical simulation still needs to be tested. This study was conducted to determine if the virtual surgery performed on 3D models constructed from Cone-beam CT (CBCT) can correctly simulate the actual surgical outcome and to validate the ability of this emerging technology to recreate the orthognathic surgery hard tissue movements in 3 translational and 3 rotational planes of space.
Methods
Construction of pre- and post-surgery 3D models from CBCTs of 14 patients who had combined maxillary advancement and mandibular setback surgery and 6 patients who had one-piece maxillary advancement surgery was performed. The post-surgery and virtually simulated surgery 3D models were registered at the cranial base to quantify differences between simulated and actual surgery models. Hotelling T-test were used to assess the differences between simulated and actual surgical outcomes.
Results
For all anatomic regions of interest, there was no statistically significant difference between the simulated and the actual surgical models. The right lateral ramus was the only region that showed a statistically significant, but small difference when comparing two- and one-jaw surgeries.
Conclusions
Virtual surgical methods were reliably reproduced, oral surgery residents could benefit from virtual surgical training, and computer simulation has the potential to increase predictability in the operating room.
INTRODUCTION
Le Fort osteotomy advancements and BSSO setbacks alone and in combination are performed for the correction of skeletal Class III deformities. The conventional treatment planning procedure for these orthognathic surgeries involves making plaster models of the teeth and dentoalveolus. The desired surgical outcome of the dentition is then determined. A lateral cephalometric radiograph is taken and traced to focus on areas of interest. A relocation plan is then performed. This is frequently performed using computer software. Hard tissue computer predictions from lateral cephalograms for orthognathic surgical procedures have been shown to provide accurate hard tissue prediction.1,2 They have also been shown to be a reproducible and a quick method of profile prediction that is useful for treatment planning and patient presentation.3 Current lateral cephalometric models have also been linked to soft tissues. This allows one to make surgical changes in the hard tissues that are then reflected in the soft tissues.4,5 The surgery is then performed on the cast as a mock surgery. From these mock surgery casts, dental splints are created for use during the surgery. The splints are placed on the relocated dentition during the surgery to confirm that the actual surgery matches the model. In this way, the dentition serves as a guide to confirm correct surgical repositioning of the skeletal structures. During preparation for orthognathic surgery, the accuracy of cephalometric tracings and model surgeries is extremely important. The intent is to reduce intra-operative complications and minimize actual surgical time.
This conventional process is satisfactory but it has a number of limitations. As can be seen above, it is a manual process with multiple steps. It is only a partial view of the actual surgery because the model surgery is not a true mock surgery. It is a repositioning of the dentition to the desired end result in order to make a splint. It does not involve simulated cuts, or even the necessary components of the craniofacial complex to make such cuts. The relation to the craniofacial complex is loosely made through estimation of the casts to the lateral cephalometric radiograph. The lateral cephalometric radiograph is a two-dimensional image of a three-dimensional object. This results in errors of superimposition, distortion, anatomy location, and projection. Vertical positioning of the maxilla is very difficult.6 It also requires that you estimate by hand on the cast movements that have six degrees of freedom. This introduces a great deal of inaccuracy.
With the advent of three-dimensional imaging came the possibility for improved diagnosis and treatment planning. Many software systems have been developed that hope to improve surgical treatment and outcomes.7 Virtual surgeries can be performed pre operatively.8 Craniofacial Surgery Planners use a patient’s individual preoperative 3-D cone beam CTs for making surgical and other predictions. Noguchi demonstrated that three-dimensional simulated surgical repositioning of bones is helpful for analyzing both bone and soft tissue movements.9
The future of cone beam technology to enhance surgical prediction and preparation is very promising. Recent advances in imaging technology have made the acquisition of three-dimensional images more cost effective and at a reduced radiation dose. This is particularly the case with cone beam CTs. With the proliferation of cone beam CT 3-D imaging technology, we have seen a concurrent expansion of imaging software programs. These software programs have proved to be useful for diagnosis10, treatment planning, and outcome measurement, but precision of 3D surgical simulation still needs to be tested. The CranioMaxilloFacial (CMF) Application software was developed and surgical navigation components have been validated at the M.E. Müller Institute for Surgical Technology and Biomechanics, University of Bern, Switzerland11 (under the funding of the Co-Me network, http://co-me.ch/). Using an existing dataset of pre and post-surgery CBCT images from the grant, “Influences on Stability following Orthognathic Surgery,” NIDCR DE005215, we compared virtual surgical outcomes with actual surgical outcomes by superimposing the two images. Our null hypothesis is: The mean surface distance of the simulated surgical models when superimposed on the actual cone beam CT of orthognathic surgical patients at UNC is 0.5 mm. The voxel size of the images is 0.5 mm, therefore, we anticipate the error in our image superimpositions to be no greater than 0.5 mm. Our aim is to determine if the virtual surgery performed on the Cone beam CT segmentations can correctly simulate the actual surgical outcome and to validate the ability of this emerging technology to recreate the orthognathic surgery hard tissue movements in 3 translational and 3 rotational planes of space.
METHODS
Fourteen patients who had combined maxillary advancement and mandibular setback surgery and six patients who had one-piece maxillary advancement surgery were selected (11 females and 9 males). Patients ranged in age from 14–35 years with a mean age of 21 years.
All subjects were taken from a consecutive prospectively collected sample that had one of the above mentioned surgeries on or after November 16, 2004, and consented to participate in an NIH funded project “Influences on Stability following Orthognathic Surgery.”(DE 005215)
Patients who had cleft lip and palate, asymmetries, and other craniofacial anomalies were excluded.
Rigid fixation was used in all the surgeries.
Image acquisition
New Tom 3G Cone Beam CTs (QR-NIM s.r.l., Verona, Italy) with the patient in supine position were obtained prior to surgery and approximately 4 to 6 weeks after surgery (at splint removal).
Image analysis procedures for simulation of surgery (Figure 1)
Figure 1.
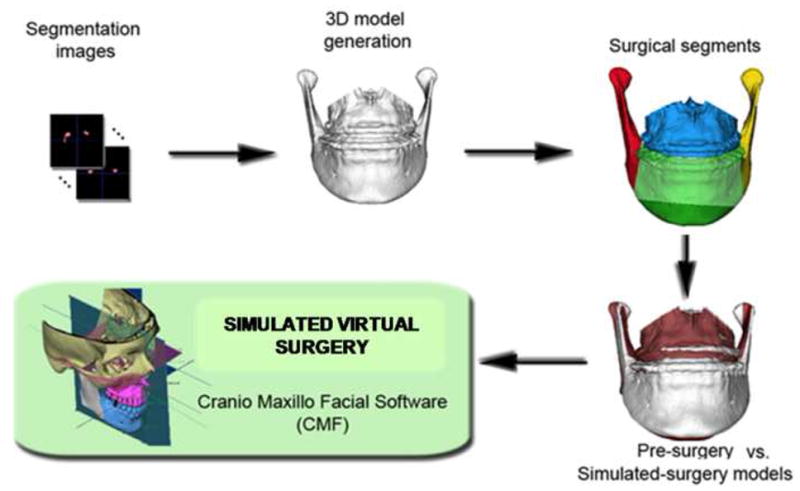
Sequence of image analysis procedures used for virtual surgical simulation: After segmentation of anatomic structures, i.e. outlining the shape of structures visible in the cross-sections of a CBCT volumetric dataset, the virtual cuts were performed. For each patient, simulated surgery outcomes were created, to compare to presurgery and actual surgery models. Virtual cuts matched clinical osteotomy segments that in this example were: chin, left ramus, right ramus, mandibular body and/or maxillary body. The virtual surgical segments were then displaced to determine if virtual surgery performed on the Cone beam CT surface models can correctly simulate the actual surgical outcome.
Construction of pre- and post-surgery 3D models from CBCT dataset
Segmentation involved outlining the shape of structures visible in the cross-sections of a volumetric dataset with the New Tom CBCT-3D images. Segmentation of anatomic structures was performed with ITK-SNAP.11 3D virtual models used in this aim were built from a set of ~ 300 axial cross-sectional slices for each image with the voxels reformatted for an isotropic of 0.5 × 0.5 × 0.5 mm. This resolution was used since higher spatial resolution with smaller slice thickness would have increased image file size and required greater computational power and user interaction time. After the segmentation with ITK-SNAP tool, a 3D graphical rendering of the volumetric object allowed navigation between voxels in the volumetric image and the 3-D graphics with zooming, rotating and panning.
Registration of pre- and post- surgery 3D models
The mutual-information approach registers one image to another, using a rigid registration to evaluate within subject changes. This task was performed using the registration pipeline within the Imagine Software developed at UNC.12,13 Our superimposition methods are fully automated, using voxel-wise rigid registration of the cranial base instead of the current standard landmark matching method, which is observer-dependent and highly variable. After masking the maxillary and mandibular structures, the registration transform was computed solely on the grey level intensities in the cranial base. Rotation and translation parameters were calculated and then applied to register 3D models.
Surgical simulation
Surgical simulation was performed with the CranioMaxilloFacial (CMF) application software (M.E. Müller Institute for Surgical Technology and Biomechanics, University of Bern, Switzerland). Simulation involved the following procedures:
Registration. The registered virtual 3D surface models of pre and post-surgery were converted from .gipl files to .iv files and then imported into CMF.
Simulation of osteotomies. Simulated surgeries were performed on the three-dimensional pre-surgery models by a single examiner. The cuts for a standard BSSO and Maxillary LeFort I Osteotomy were executed by placing points on the pre-surgery models at the area and in the orientation of the osteotomy cuts. The locations of surgical cuts were determined by the anatomic characteristics of each patient, such as thickness of the mandibular ramus, position of the mandibular canal and proximity to the roots of the second molars.
Simulation of surgical displacements. The post-surgical model was used as a surgical guide. This was done by changing the color and reducing the opacity of the post-surgery model which was superimposed with the pre-surgery model. The magnitude and direction of the simulated movements were then guided by the registered post-surgical model. Movements for each surgical piece were performed allowing six degrees of freedom (anterior-posterior, lateral, superior-inferior, yaw, pitch, and roll).
Quantification of differences between simulated and actual post-surgery models. We computed the surface distances between simulated and actual post-surgery models at specific anatomic regions (condyles, lateral mandibular rami, lateral mandibular corpi, anterior mandibular corpi, chin, lateral maxillary body, and anterior maxillary body).
Statistics
Student t tests were performed for all 11 regions of interest to test whether the virtual surgeries showed no greater difference than 0.5 mm when compared to the actual surgeries. Student t tests were also performed to test whether the measurements between two-jaw and one-jaw surgery patients were statistically significant. Hotelling T2 was used to test the differences in the amount of movement between one- and two-jaw surgery patients. Paired F tests were performed to evaluate the difference between right and left lateral rami in patients who received two-jaw surgeries. Student t tests were calculated to assess the reliability of the 5 patients who received a second virtual surgery.
RESULTS
The virtual surgical models were superimposed on the models of the actual surgical outcomes. This generated visual displays of magnitude, direction, and location of disagreement between models (Figure 2). For all statistical testing, a P value of P < 0.05 was considered statistically significant. The differences between the superimpositions of the simulated and actual surgery images are shown in Figure 3. The mean difference for the left lateral maxilla was 0.536 mm and the median was 0.515 mm. The mean and median differences were less than 0.5 mm for the superimpositions of all of the other regions of interest. The 0.5 mm difference was selected because 0.5 mm is the spatial resolution of the cone beam images. For each region of interest, power was calculated and a student t test was performed to test if the surface distances between the simulated and the actual surgical models were no greater than 0.5 mm. The results are listed in Table 1. For all 11 regions of interest, there was no statistically significant difference between the simulated and the actual surgical models. The power in the right lateral maxilla, left lateral maxilla, and chin was less then 0.80. The power was greater than 0.96 for all other regions of interest.
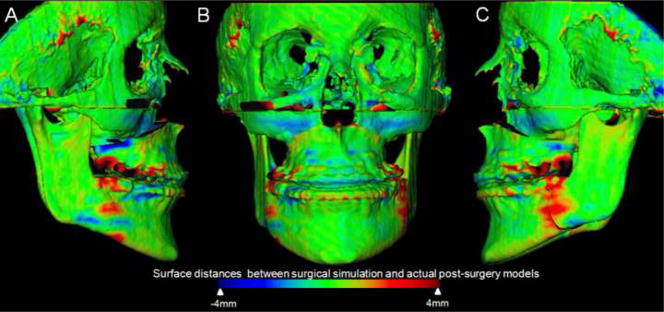
Figure 2.
Superimposition of virtual surgery models and post surgery models of a patient treated with maxillary advancement and mandibular setback. A, Right lateral view, B, Frontal view, and C, Left lateral view. Color maps demonstrate the location, direction, and magnitude of the differences between these models. Note that in the maxilla and mandible except for areas of surgical cuts the surface distances between simulated and actual surgery models are close to 0mm (green).
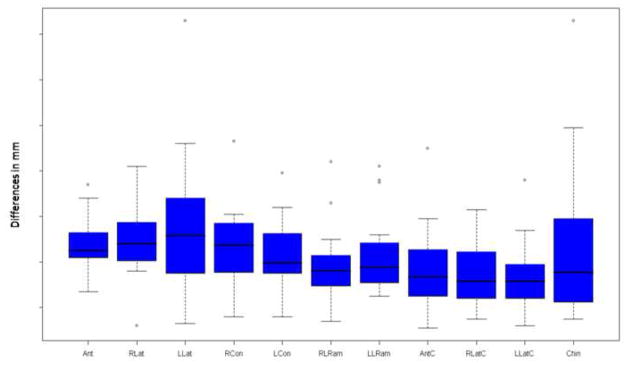
Figure 3.
The differences between virtual and actual post-surgery models are shown below. The x axis shows the 11 regions of interest and the y axis shows the difference in mm between the two images. All regions of interest except the left lateral maxilla showed a mean and median difference less than the 0.5 mm spatial resolution of the acquired image. (Ant = Anterior Maxilla, RLat = Right lateral maxilla, LLat = Left lateral maxilla, RCon = Right condyle, LCon = Left condyle, RLRam = Right lateral ramus, LLRam = Left lateral ramus, AntC = Anterior Corpus of the mandible, RLatC = Right lateral corpus of the mandible, LLatC = Left lateral corpus of the mandible, Chin = Chin)
Table 1.
Power was calculated. Student t tests were performed for each region of interest to test if the difference of the virtual surgical outcomes when superimposed on the actual surgical outcomes was less than the image spatial resolution of 0.5 mm.
| t Value | Probability t | Power | |
|---|---|---|---|
| Region of interest | |||
| Anterior Maxilla | −0.79167 | 0.561677 | 0.962 |
| Right Lateral Maxilla | −0.18841 | 0.14745 | 0.203 |
| Left Lateral Maxilla | 0.538988 | 0.403845 | 0.151 |
| Right Condyle | −0.8912 | 0.616033 | 0.986 |
| Left Condyle | −1.85496 | 0.920813 | 0.999 |
| Right lateral Ramus | −3.27984 | 0.99606 | 0.999 |
| Left Lateral Ramus | −1.81991 | 0.915435 | 0.999 |
| Anterior corpus | −3.29165 | 0.996163 | 0.999 |
| Right lateral corpus | −5.62111 | 0.99998 | 0.999 |
| Left lateral corpus | −5.27873 | 0.999957 | 0.999 |
| Chin | −0.45906 | 0.3486 | 0.631 |
P< .05 was used to determine statistical significance between the two images.
In comparing the two-jaw subjects with the maxillary advancement subjects, a student t test was performed. The results are listed in Table 2. The right lateral ramus was the only region of interest that showed a statistically significant difference when comparing the two-jaw and one-jaw surgeries. Mean translational and rotational displacements of the one- and two-jaw surgeries were also calculated. Hotelling T2 was then performed to test the differences between the two groups. For translational displacements, a value of 0.14928498 and an F value of 0.80 showed a Probability > F of 0.538. For rotational displacements, a value of 0.22166894 and an F value of 1.18 showed a Probability > F of 0.3477. There was no statistically significant difference between two-jaw and one-jaw surgeries when comparing translational and rotational displacements.
Table 2.
Student t tests were performed for each region of interest to test if there was a difference in the virtual surgical outcomes between one and two jaw surgery patients.
| t Value | Probability t | |
|---|---|---|
| Region of interest | ||
| Anterior Maxilla | −0.88331 | 0.388712 |
| Right Lateral Maxilla | −0.08929 | 0.929836 |
| Left Lateral Maxilla | −1.84928 | 0.080908 |
| Right Condyle | 0.351947 | 0.728965 |
| Left Condyle | −0.81534 | 0.425536 |
| Right lateral Ramus | 2.505062 | 0.022074* |
| Left Lateral Ramus | 0.638073 | 0.53146 |
| Anterior corpus | −1.20006 | 0.245671 |
| Right lateral corpus | 1.633646 | 0.119702 |
| Left lateral corpus | 0.605325 | 0.552519 |
| Chin | 0.100442 | 0.921104 |
P< .05 was used to determine statistical significance between the two images.
In two-jaw subjects there was very little translational variability in the right and left lateral rami as shown in Figure 4. The left lateral ramus showed greater rotational variability than the right lateral ramus as shown in Figure 5. The median for translational and rotational displacements in all groups was zero, but significant individual variability was manifest. Paired F tests were performed to test whether the right and left ramus displacements were significantly different. The F value for translational displacement was 3.2592593 and the probability > F was 0.0633288. The F value for rotational displacement was 1.024251 and the probability > F was 0.4192385. These tests did not demonstrate statistical significance between the right and left lateral rami displacements in two-jaw surgery patients.
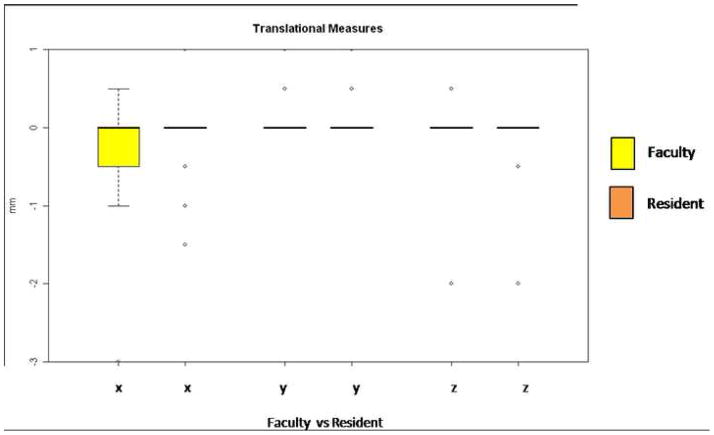
Figure 4.
Translational movements of the right and left lateral rami during mandibular setback surgery. The faculty member operated on the right side and is always shown in the left of the paired columns (yellow bloxplot; x, y, z coordinates). The resident operated on the left side and is always shown on the right of the paired columns (orange bloxplots; x, y, z coordinates). Directions of movement in mm: x coordinates, (+)left/(−)right movement; y coordinates (+)anterior/(−)posterior; and z coordinates (+)superior/(−)inferior movement.
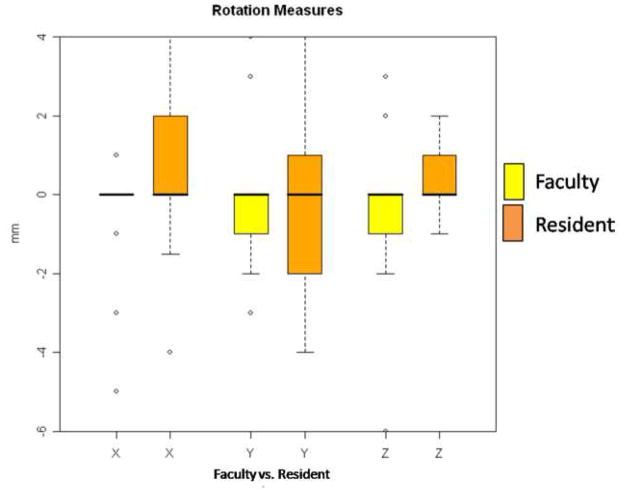
Figure 5.
Rotational movements of the right and left lateral rami during mandibular setback surgery. The faculty member operated on the right side and is always shown in the yellow of the paired columns. The resident operated on the left side and is always shown on the orange of the paired columns. Amount of rotation in degrees is shown: (+) signifies a clockwise rotation and (−) signifies a counterclockwise rotation. Column X: Axial plane or Pitch, Column Y: Sagittal plane or Yaw and Column Z: Coronal plane or Roll.
Five of the subjects were randomly selected to have the surgery repeated. The differences between the repeated surgical simulation and the actual surgical outcomes were recorded. These measurements were then compared to the initial differences in measurements for these patients. All of these measurements showed less than 0.4 mm difference between the initial surgical simulation and the repeated surgical simulation. This is less than the 0.5 mm spatial resolution of the cone beam images. Student t tests were performed and the results are shown in Table 3. There was no statistically significant difference between the initial and the repeated measurements for any of the regions of interest.
Table 3.
Five subjects received a second virtual surgery and measurements for each region of interest were recorded. Student t tests were performed for each region of interest to test the reliability of the repeated surgeries.
| t Value | Probability t | |
|---|---|---|
| Region of interest | ||
| Anterior Maxilla | 0.200548 | 0.850836 |
| Right Lateral Maxilla | 2.046469 | 0.110131 |
| Left Lateral Maxilla | 1.258634 | 0.276614 |
| Right Condyle | 0.043499 | 0.967389 |
| Left Condyle | 0.286611 | 0.788643 |
| Right lateral Ramus | 0.191565 | 0.857414 |
| Left Lateral Ramus | 1.152182 | 0.313421 |
| Anterior corpus | 1.617962 | 0.180981 |
| Right lateral corpus | −0.55405 | 0.60906 |
| Left lateral corpus | 0.202031 | 0.849752 |
| Chin | −0.18546 | 0.861896 |
P< .05 was used to determine statistical significance between the two images.
DISCUSSION
Differences between virtual and actual surgical outcomes were measured utilizing a voxel wise rigid registration of the cranial base. Previous studies have validated this method that has been shown to be more accurate than traditional landmark methods for three-dimensional superimpositions.11 The larger the number of points used for superimposition, the more accurate it becomes.14,15 Only two of the measured differences between pre and post-surgery models were greater than 1 mm. All differences were less than 2 mm. Differences of less than 2 mm have been shown to not be clinically significant.16–18
Pre-surgical predictions do not necessarily reflect the actual surgical outcomes that are produced. Surgery notes, although helpful, show variation between surgeons as to the estimated amount of movement. Furthermore, surgical notes do not reflect the necessary degree of precision we desire to accurately assess the validity and reliability of the virtual surgeries. Post-surgical models are the best measure of what movements were actually produced in the surgery. It is for this reason that we used the post-surgical models as a guide for positioning of the virtual surgical models. This limits our ability in this study to generalize our results because we cannot say that we were able to predict the surgical outcomes. Future studies can be used to predict surgical outcomes prior to surgery and assess whether surgical outcomes and segment movements are better controlled when computer assisted surgical simulation is performed prior to surgery. The techniques in this paper resulted in an evaluation of the methodology of the computer program itself, allowing assessment and visual display of the location, direction, and magnitude of agreement between virtual and actual surgery models. The difference between the actual surgical displacement values and the measured simulated values was smaller than the CBCT image spatial resolution of 0.5mm. Computer assisted surgical simulation allowed manipulation of the images in the necessary six degrees of freedom to accurately reproduce the actual surgical outcome.
Bi-maxillary surgery has been shown to be more difficult to predict than single jaw surgery.19–21 It has been suggested that this is due to the greater complexity of two-jaw surgeries. Our research indicates that for the hard tissue structures measured, there was no statistical difference between the one- and two-jaw surgery patients. The only exception was the statistically significant displacement of the right lateral ramus in two-jaw surgery patients. There was also no statistical difference in our population in the amount of translation or rotation that was performed in the maxillary body during the surgery. There was also no clinically significant difference between the two groups. Three-dimensional surgical planning allows us to overcome many of the limitations of conventional surgical planning. For example, an often cited difficulty of maxillary impaction surgery is posterior bone removal for vertical positioning of the maxilla. The unpredictability of the necessary bone removal can significantly alter surgical time. Our software allows us to visualize the hard tissue structures in the posterior maxilla and provide better operating room predictability. It allows the surgeon to have a better idea of how much bone removal will be necessary and then plan accordingly (Figure 6).
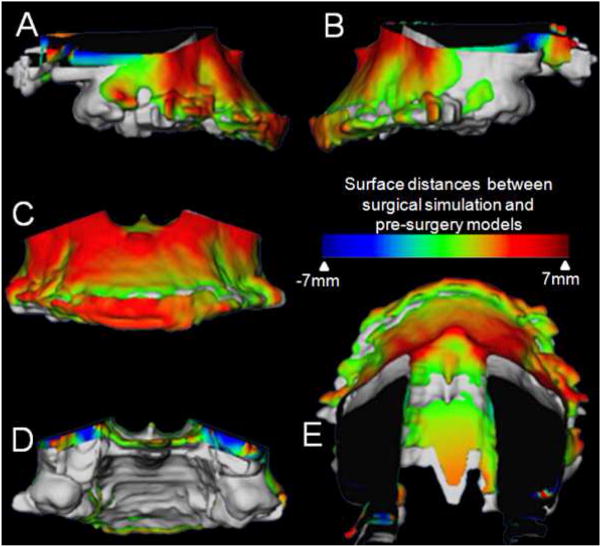
Figure 6.
Example of a maxillary impaction case in which surgical simulation helped plan areas and amount of bone removal for impaction. Superimposition of maxillary segment of virtual surgery models and pre-surgery models of patients treated with maxillary advancement and impaction. A, Right lateral view, B, Left lateral view, C, Frontal view, D, Posterior view and E, Superior view. The grey image is the pre-surgery model and the image with the color map is the post virtual (simulated) surgery image. Color maps demonstrate the location, direction, and magnitude of the differences between these models. Note the dark blue area in the posterior part of the maxilla indicating that 7mm of posterior bone removal will be necessary during the surgery.
We demonstrated greater variability in lateral ramus displacement on the left side performed by the surgical residents, while the surgeon performed the right side. However, the surgery residents’ displacement was not statistically significantly different from the attending faculty. Nor was it considered clinically significant. Increased displacement of the lateral ramus during surgery has the potential for decreased stability of the surgical outcome. It could be valuable to incorporate these emerging technologies into surgical training programs.22 We feel that there is potential for great benefit to residents by allowing them to perform surgical procedures in three dimensions prior to entering the operating room. This allows them to practice procedures as well as attempt different surgical scenarios. A systematic review of the literature by Gurusamy et al. demonstrated that virtual reality training for surgery residents resulted in increased accuracy, decreased operating time, and decreased error.23 This technology also allows potential for communication between colleagues and training over distances by sharing digital three-dimensional records.24 We see potential value in surgical resident training for surgical procedures to be supplemented through virtual surgical training.
There has been an explosion in recent years of commercially available programs for three-dimensional virtual surgery and visualization programs. The biggest drawback to these programs is the lack of validation of outcomes. It is desirable that craniofacial skeletal components, occlusion, and soft tissue outcomes are validated.25 This paper demonstrated that CMF application software can correctly simulate the actual surgical outcomes of craniofacial skeletal components of patients. However, the CT does not accurately render the teeth with the necessary precision for surgical simulation and splint fabrication.26,27 Three-dimensional laser scanning is a noninvasive way to accurately capture the occlusion that has been suggested by multiple groups.28–30 These images are then superimposed and merged on the cone beam images.31 Using three-dimensional printers, splints can be fabricated from the digital models.32 Soft tissue predictions also lack validation and are extremely difficult to accurately predict in three dimensions.6,33 Commercially available programs utilize spring deformation and morphing programs for soft tissue surgical predictions. This is not biomechanically accurate, nor has it been validated.34–37 The validation of soft tissue outcomes would greatly improve patient presentation and understanding of surgical outcomes.
Xia et al. demonstrated that computer aided surgical simulation (CASS) has lower material costs, as well as decreased patient and surgeon time. They foresee even greater time savings by outsourcing the surgical image processing to radiology technicians at imaging centers.38 Our research allowed us to demonstrate that the computer aided surgical simulations can accurately reproduce with six degrees of freedom the actual surgeries performed for Class III correction. This validation of the virtual surgery of hard tissue structures demonstrates the potential for comparable or better surgical outcomes. We see great benefit for this technology in the future as a tool that has been shown to reduce complication and increase predictability. It allows the surgeon to better predict possible surgical complications and adapt accordingly to mitigate potential difficulties.39,40,22,41–44 It has also been utilized to allow more complex surgeries to be successfully performed in a single procedure rather than the previous multiple staged surgeries.41 Future benefits also include the fabrication of stereolithographic models and surgical splints. These have the potential to greatly reduce intra-operative time, complications, and surgical surprises.41 The accuracy of computer assisted surgery has been shown to be within 1 mm when using a referencing splint.45 A number of these programs such as the CMF application software we tested are also equipped with a surgical navigation feature that allows the surgical simulations to be transferred to the operating room.36,43,44,46,47 Many, such as CMF, currently take the form of passive intra-operative orientation and tracking systems. In final form there is potential for robotic execution of specific steps autonomously.43 Therefore, we can anticipate the potential for faster, cheaper, and better outcomes through this emerging technology. This rapidly developing technology will have a significant impact on a surgeon’s future work.
CONCLUSIONS
Three-dimensional diagnosis and treatment planning has great potential for future benefit to patients and surgeons. The validation of these rapidly emerging technologies is paramount. It is particularly valuable to validate craniofacial skeletal components, the occlusion, and soft tissues.
Our virtual surgical methods were reliably reproduced.
Oral surgery residents could benefit from virtual surgical training.
The virtual surgery accurately recreated all surgical movements in 3 rotational and 3 translational planes of space.
One- and two-jaw virtual surgeries were equally valid and accurate.
Preoperative simulation can allow for increased predictability in the operating room.
Future validation of occlusal and soft tissue components would be very beneficial.
Acknowledgments
Supported by NIDCR DE017727, DE005215 and DE018962.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loh S, Yow M. Computer prediction of hard tissue profiles in orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 2002;17(4):342–7. [PubMed] [Google Scholar]
- 2.Loh S, Heng JK, Ward-Booth P, Winchester L, McDonald F. A radiographic analysis of computer prediction in conjunction with orthognathic surgery. Int J Oral Maxillofac Surg. 2001;30(4):259–63. doi: 10.1054/ijom.2001.0089. [DOI] [PubMed] [Google Scholar]
- 3.Mankad B, Cisneros GJ, Freeman K, Eisig SB. Prediction accuracy of soft tissue profile in orthognathic surgery. Int J Adult Orthodon Orthognath Surg. 1999;14(1):19–26. [PubMed] [Google Scholar]
- 4.Pektas ZO, Kircelli BH, Cilasun U, Uckan S. The accuracy of computer-assisted surgical planning in soft tissue prediction following orthognathic surgery. Int J Med Robot. 2007;3:64–71. doi: 10.1002/rcs.127. [DOI] [PubMed] [Google Scholar]
- 5.Smith JD, Thomas PM, Proffit WR. A comparison of current prediction imaging programs. Am J Orthod Dentofacial Orthop. 2004;125(5):527–36. doi: 10.1016/S0889540604001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones RM, Khambay BS, McHugh S, Ayoub AF. The validity of a computer-assisted simulation system for orthognathic surgery (CASSOS) for planning the surgical correction of class III skeletal deformities: single-jaw versus bimaxillary surgery. Int J Oral Maxillofac Surg. 2007;36(10):900–8. doi: 10.1016/j.ijom.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Maki K, Inou N, Takanishi A, Miller AJ. Computer-assisted simulations in orthodontic diagnosis and the application of a new cone beam X-ray computed tomography. Orthod Craniofac Res. 6 Suppl 1:95–101. doi: 10.1034/j.1600-0544.2003.241.x. discussion 179–82, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Meehan M, Teschner M, Girod S. Three-dimensional simulation and prediction of craniofacial surgery. Orthod Craniofac Res. 2003;6 Suppl 1:102–7. doi: 10.1034/j.1600-0544.2003.242.x. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi N, Goto M. Computer simulation system for orthognathic surgery. Orthod Craniofac Res. 2003;6 Suppl 1:176–8. doi: 10.1034/j.1600-0544.2003.254.x. [DOI] [PubMed] [Google Scholar]
- 10.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Chapuis J, Schramm A, Pappas I, Hallermann W, Schwenzer-Zimmerer K, Langlotz F, Caversaccio MA. New system for computer-aided preoperative planning and intraoperative navigation during corrective jaw surgery. IEEE Trans Inf Technol Biomed. 2007 May;11(3):274–287. doi: 10.1109/titb.2006.884372. [DOI] [PubMed] [Google Scholar]
- 12.Cevidanes LH, Bailey LJ, Tucker GR, Jr, Styner MA, Mol A, Phillips CL, et al. Superimposition of 3D cone-beam CT models of orthognathic surgery patients. Dentomaxillofac Radiol. 2005;34(6):369–75. doi: 10.1259/dmfr/17102411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cevidanes LH, Bailey LJ, Tucker SF, Styner MA, Mol A, Phillips CL, et al. Three-dimensional cone-beam computed tomography for assessment of mandibular changes after orthognathic surgery. Am J Orthod Dentofacial Orthop. 2007;131(1):44–50. doi: 10.1016/j.ajodo.2005.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gliddon MJ, Xia JJ, Gateno J, Wong HT, Lasky RE, Teichgraeber JF, et al. The accuracy of cephalometric tracing superimposition. J Oral Maxillofac Surg. 2006;64(2):194–202. doi: 10.1016/j.joms.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Xia JJ. Accuracy of the computer-aided surgical simulation (CASS) system in the treatment of patients with complex craniomaxillofacial deformity: A pilot study. J Oral Maxillofac Surg. 2007;65(2):248–54. doi: 10.1016/j.joms.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Donatsky O, Bjorn-Jorgensen J, Holmqvist-Larsen M, Hillerup S. Computerized cephalometric evaluation of orthognathic surgical precision and stability in relation to maxillary superior repositioning combined with mandibular advancement or setback. J Oral Maxillofac Surg. 55(10):1071–9. doi: 10.1016/s0278-2391(97)90282-2. discussion 1079–80, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Ong TK, Banks RJ, Hildreth AJ. Surgical accuracy in Le Fort I maxillary osteotomies. Br J Oral Maxillofac Sur. 2001;39(2):96–102. doi: 10.1054/bjom.2000.0577. [DOI] [PubMed] [Google Scholar]
- 18.Tng TT, Chan TC, Hagg U, Cooke MS. Validity of cephalometric landmarks. An experimental study on human skulls. Eur J Orthod. 1994;16(2):110–20. doi: 10.1093/ejo/16.2.110. [DOI] [PubMed] [Google Scholar]
- 19.Koh CH, Chew MT. Predictability of soft tissue profile changes following bimaxillary surgery in skeletal class III Chinese patients. J Oral Maxillofac Surg. 2004;62(12):1505–9. doi: 10.1016/j.joms.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Eckhardt CE, Cunningham SJ. How predictable is orthognathic surgery? Eur J Orthod. 2004;26(3):303–9. doi: 10.1093/ejo/26.3.303. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson R, Sarver DM. The predictability of maxillary repositioning in LeFort I orthognathic surgery. Am J Orthod Dentofacial Orthop. 2002;122(2):142–54. doi: 10.1067/mod.2002.125576. [DOI] [PubMed] [Google Scholar]
- 22.Kaban LB. Biomedical technology revolution: opportunities and challenges for oral and maxillofacial surgeons. Int J Oral Maxillofac Surg. 2002;31(1):1–12. doi: 10.1054/ijom.2001.0187. [DOI] [PubMed] [Google Scholar]
- 23.Gurusamy K, Aggarwal R, Palanivelu L, Davidson BR. Systematic review of randomized controlled trials on the effectiveness of virtual reality training for laparoscopic surgery. Br J Sur. 2008;95(9):1088–97. doi: 10.1002/bjs.6344. [DOI] [PubMed] [Google Scholar]
- 24.Hajeer MY, Millett DT, Ayoub AF, Siebert JP. Applications of 3D imaging in orthodontics: part I. J Orthod. 2004;31(1):62–70. doi: 10.1179/146531204225011346. [DOI] [PubMed] [Google Scholar]
- 25.Lane C. Completing the 3-dimensional picture. Am J Orthod Dentofacial Orthop. 2008;133(4):612–20. doi: 10.1016/j.ajodo.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Santler G. The Graz hemisphere splint: a new precise, non-invasive method of replacing the dental arch of 3D-models by plaster models. J Craniomaxillofac Surg. 1998;26(3):169–73. doi: 10.1016/s1010-5182(98)80008-5. [DOI] [PubMed] [Google Scholar]
- 27.Swennen GR, Barth EL, Eulzer C, Schutyser F. The use of a new 3D splint and double CT scan procedure to obtain an accurate anatomic virtual augmented model of the skull. Int J Oral Maxillofac Surg. 2007;36(2):146–52. doi: 10.1016/j.ijom.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Hajeer MY, Millett DT, Ayoub AF, Siebert JP. Applications of 3D imaging in orthodontics: part II. J Orthod. 2004;31(2):154–62. doi: 10.1179/146531204225020472. [DOI] [PubMed] [Google Scholar]
- 29.Kusnoto B, Evans CA. Reliability of a 3D surface laser scanner for orthodontic applications. Am J Orthod Dentofacial Orthop. 2002;122(4):342–8. doi: 10.1067/mod.2002.128219. [DOI] [PubMed] [Google Scholar]
- 30.Tsuji M, Noguchi N, Shigematsu M, Yamashita Y, Ihara K, Shikimori M, et al. A new navigation system based on cephalograms and dental casts for oral and maxillofacial surgery. Int J Oral Maxillofac Surg. 2006;35(9):828–36. doi: 10.1016/j.ijom.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Uechi J, Okayama M, Shibata T, Muguruma T, Hayashi K, Endo K, et al. A novel method for the 3-dimensional simulation of orthognathic surgery by using a multimodal image-fusion technique. Am J Orthod Dentofacial Orthop. 2006;130(6):786–98. doi: 10.1016/j.ajodo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Metzger MC, Hohlweg-Majert B, Schwarz U, Teschner M, Hammer B, Schmelzeisen R. Manufacturing splints for orthognathic surgery using a three-dimensional printer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(2):e1–7. doi: 10.1016/j.tripleo.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 33.Plooij JM, Swennen GR, Rangel FA, Maal TJ, Schutyser FA, Bronkhorst EM, et al. Evaluation of reproducibility and reliability of 3D soft tissue analysis using 3D stereophotogrammetry. Int J Oral Maxillofac Surg. 2009;38(3):267–73. doi: 10.1016/j.ijom.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Holberg C, Schwenzer K, Rudzki-Janson I. Three-dimensional soft tissue prediction using finite elements. Part I: Implementation of a new procedure. J Orofac Orthop. 2005;66(2):110–21. doi: 10.1007/s00056-005-0421-8. [DOI] [PubMed] [Google Scholar]
- 35.Marchetti C, Bianchi A, Bassi M, Gori R, Lamberti C, Sarti A. Mathematical modeling and numerical simulation in maxillofacial virtual surgery. J Craniofac Surg. 2007;18(4):826–32. doi: 10.1097/scs.0b013e318068434b. [DOI] [PubMed] [Google Scholar]
- 36.Mobarak KA, Krogstad O, Espeland L, Lyberg T. Factors influencing the predictability of soft tissue profile changes following mandibular setback surgery. Angle Orthod. 2001;71(3):216–27. doi: 10.1043/0003-3219(2001)071<0216:FITPOS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Mollemans W, Schutyser F, Nadjmi N, Maes F, Suetens P. Predicting soft tissue deformations for a maxillofacial surgery planning system: from computational strategies to a complete clinical validation. Med Image Anal. 2007;11(3):282–301. doi: 10.1016/j.media.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Xia JJ, Phillips CV, Gateno J, Teichgraeber JF, Christensen AM, Gliddon MJ, et al. Cost-effectiveness analysis for computer-aided surgical simulation in complex cranio-maxillofacial surgery. J Oral Maxillofac Surg. 2006;64(12):1780–4. doi: 10.1016/j.joms.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 39.Xia J, Samman N, Yeung RW, Shen SG, Wang D, Ip HH, et al. Three-dimensional virtual reality surgical planning and simulation workbench for orthognathic surgery. Int J Adult Orthod Orthognath Surg. 2000;15(4):265–82. [PubMed] [Google Scholar]
- 40.Gateno J, Teichgraeber JF, Xia JJ. Three-dimensional surgical planning for maxillary and midface distraction osteogenesis. J Craniofac Surg. 2003;14(6):833–9. doi: 10.1097/00001665-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Gateno J, Xia JJ, Teichgraeber JF, Christensen AM, Lemoine JJ, Liebschner MA, et al. Clinical feasibility of computer-aided surgical simulation (CASS) in the treatment of complex cranio-maxillofacial deformities. J Oral Maxillofac Surg. 2007;65(4):728–34. doi: 10.1016/j.joms.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Xia J, Ip HH, Samman N, Wang D, Kot CS, Yeung RW, et al. Computer-assisted three-dimensional surgical planning and simulation: 3D virtual osteotomy. Int J Oral Maxillofac Surg. 2000;29(1):11–7. [PubMed] [Google Scholar]
- 43.Troulis MJ, Everett P, Seldin EB, Kikinis R, Kaban LB. Development of a three-dimensional treatment planning system based on computed tomographic data. Int J Oral Maxillofac Surg. 2002;31(4):349–57. doi: 10.1054/ijom.2002.0278. [DOI] [PubMed] [Google Scholar]
- 44.Hassfeld S, Muhling J. Computer assisted oral and maxillofacial surgery--a review and an assessment of technology. Int J Oral Maxillofac Surg. 2001;30(1):2–13. doi: 10.1054/ijom.2000.0024. [DOI] [PubMed] [Google Scholar]
- 45.Gellrich N. Computer-assisted secondary reconstruction of unilateral posttraumatic orbital deformity. Plastic Reconst Surg. 2002;11;110(6):1417–1429. doi: 10.1097/01.PRS.0000029807.35391.E5. [DOI] [PubMed] [Google Scholar]
- 46.Hohlweg-Majert B, Schon R, Schmelzeisen R, Gellrich NC, Schramm A. Navigational maxillofacial surgery using virtual models. World J Surg. 2005;29(12):1530–8. doi: 10.1007/s00268-005-0091-0. [DOI] [PubMed] [Google Scholar]
- 47.Mischkowski RA, Zinser MJ, Ritter L, Neugebauer J, Keeve E, Zoller JE. Intraoperative navigation in the maxillofacial area based on 3D imaging obtained by a cone-beam device. Int J Oral Maxillofac Surg. 2007;36(8):687–94. doi: 10.1016/j.ijom.2007.04.001. [DOI] [PubMed] [Google Scholar]