Abstract
Magnetic resonance imaging (MRI) was performed in cocaine dependent subjects to determine the structural changes in brain compared to non-drug using controls. Cocaine dependent subjects and controls were carefully screened to rule out brain pathology of undetermined origin. Magnetic resonance images were analyzed using tensor-based morphometry (TBM) and voxel-based morphometry (VBM) without and with modulation to adjust for volume changes during normalization. For TBM analysis, unbiased atlases were generated using two different inverse consistent and diffeomorphic nonlinear registration techniques. Two different control groups were used for generating unbiased atlases. Independent of the nonlinear registration technique and normal cohorts used for creating the unbiased atlases, our analysis failed to detect any statistically significant effect of cocaine on brain volumes. These results show that cocaine dependent subjects do not show differences in regional brain volumes compared to non-drug using controls.
Keywords: cocaine, MRI, brain morphometry, tensor-based morphometry, Jacobian determinant, unbiased atlas, nonlinear registration
1 INTRODUCTION
Cocaine dependence leads to a number of medical and psychosocial complications and is a major public health problem. Based on results from the National Survey on Drug Use and Health: National Findings (http://oas.samhsa.gov/nsduh/2k7nsduh/2k7Results.cfm#TOC), in 2007 there were 2.1 million cocaine users aged 12 or older, comprising 0.8 percent of the population. These estimates are similar to the number and rate in 2006 (2.4 million or 1.0 percent). In 2007, 0.4 percent of youths aged 12 to17 used cocaine. The total societal cost of cocaine abuse (including health, lost productivity, and criminal justice costs) in 1995 was estimated to be 45 billion dollars (Cartwright, 2000).
Although much is known about the neurological underpinnings of cocaine abuse, more information about the long-term consequences of the disorder are becoming apparent only recently. Multiple lines of evidence, mainly based on neuroimaging, strongly indicate that chronic cocaine dependence is associated with both structural and functional changes in the brain. Perhaps the first reported cerebral atrophy on computerized tomography is by Pascual-Leone et al as early as 1991 (Pascual-Leone et al., 1991). Magnetic resonance imaging (MRI) is increasingly used to understand the cocaine-induced neuropathological changes because of MRI's ability to provide excellent anatomical details and its multi-modal nature (see for example, (Fowler et al., 2007)). A number of studies have used structural MRI to examine brain structure in cocaine users. These studies frequently find differences in grey or white matter volumes but there have been a number of inconsistencies in results across studies. Bartzokis et al., (2000) compared cocaine dependent, amphetamine dependent, and healthy control subjects on brain volume and found a reduction in temporal but not frontal grey matter. Using voxel based morphometry (VBM), Franklin et al., (2002), also found reduction in temporal grey matter concentration in cocaine users, however, unlike Bartzokis, Franklin also showed a reduction in grey matter concentration in orbitofrontal, anterior cingulate, and insular corticies. Fein et al., (2002) found only a reduction in prefrontal grey matter volumes in cocaine users, without any differences between cocaine users and controls in parietal, temporal or occipital regions. Tanabe et al., (2009), used VBM with correction for multiple comparisons to study a mixed group of cocaine, alcohol, amphetamine, cannabis, and opiate abusing subjects, finding a reduction in bilateral medial orbital frontal cortex.
White matter volume has also been found to be reduced in cocaine users, with a lack of normal age-related changes (Bartzokis et al., 2002). However, there have also been inconsistent findings on white matter volumes in cocaine users. Matochik et al., (2003) did not find any differences between cocaine users and controls in white matter density, but did find reduced grey matter density in the prefrontal cortex. Schlaepfer et al., (2006) studied a mixed group of cocaine, heroin, and cannabis, but not alcohol abusing subjects and found reduction in frontal white-matter volumes but no difference in grey matter volume between groups. Sim et al., (2007) found no significant difference in grey or white matter volume between cocaine users and controls, but did find differences in fractional anisotropy (FA) on diffusion tensor imaging (DTI) between groups. Neuroimaging studies also have shown inconsistent results regarding cocaine use and deep GM structural volumes. For example, Jacobsen et al. (2001) have shown that normalized caudate and putamen volumes were larger in the cocaine-dependent subjects. Another study examined amygdala and hippocampal volume in cocaine users, finding a reduction in amygdala volumes in cocaine users and no difference in hippocampal volumes (Makris et al., 2004). In summary, a number of studies have examined grey and white matter volumes in cocaine users and controls, with substantial inconsistencies in results.
Much of the earlier work on MRI morphometry was based on manual or semiautomatic segmentation. However, more recently, voxel-based morphometry (VBM) has been introduced for unbiased and automatic estimation of tissue volume changes (Ashburner and Friston, 2000). In VBM, all individual anatomical images are spatially normalized to a common stereotactic space, which are then segmented into different tissue classes followed by smoothing of each tissue map. This technique allows group analysis in an automated way without the need to define a priori the structures of interest.
Voxel-based morphometry requires tissue segmentation, followed by spatial smoothing for obtaining normal distribution of tissues. The spatial smoothing results in image blurring that prevents accurate and high-resolution estimation of local atrophies. The recently introduced “Optimized VBM” (Davatzikos et al., 2001; Good et al., 2001) overcomes some of the problems associated with the original VBM. In the optimized VBM, the spatially normalized GM voxel intensities are modulated by the local expansion/contraction determined by the deformation field that aligns each brain to the template. Consequently, the pre- and post-registered images contain the same number of of GM voxels and may prevent accurate determination of volume changes.
Tensor-based morphometry (TBM) is a more recent voxel based technique for estimating changes in brain structures (Ashburner and Friston, 1999) and has been shown to provide methodological improvements over VBM. The Jacobian determinant is one of the major TBM metrics that can directly measure tissue growth and atrophy (Chung et al., 2008). The main advantage of TBM over VBM is that the former can be applied directly on the JDs of deformation fields without the need for tissue segmentation or spatial smoothing (Hua et al., 2008a; Kim et al., 2008). In TBM, the regional structural differences are identified from the high resolution deformation fields that align individual images to a common anatomical template or atlas (see, for example, (Ashburner and Friston, 2000)). TBM has been used to assess volumes of WM in first-episode schizophrenia (Whitford et al., 2007) and monitor the progress of atrophy in semantic dementia (Brambati et al., 2009; Brambati et al., 2007). More recently, TBM has been used to detect regional gray matter atrophy in multiple sclerosis patients with minimal clinical disability (Tao et al., 2009a). TBM is also used to map systematic anatomic differences between different patient groups using cross-sectional data (Hua et al., 2008b).
Construction of a proper template is critical for voxel based techniques such as VBM and TBM. A desirable template is the one that requires minimum transformation to match the individual brain. Customized construction of the template is based on the population that matches closely with the population of interest (target population) (Lepore et al., 2007). The custom template should preserve the features across the population for successful high-resolution image normalization. Such a template would reduce blurring so that individual structures can easily be delineated. Custom templates thus help reduce the bias (Joshi et al., 2004; Kim et al., 2008; Lepore et al., 2007).
The quality of nonlinear registration plays a critical role in the construction of proper template and group analysis. The registration has to be inverse consistent, i.e. the image ordering should not affect the registration results (Christensen and Johnson, 2001). Lack of inverse consistency introduces bias (Beg and Khan, 2007). The nonlinear registration has to be diffeomorphic for preventing tearing and folding artifacts and preserving the image topology (Avants and Gee, 2004; Avants et al., 2005; Joshi et al., 2004). Many registration techniques used in the literature do not satisfy these conditions.
In this study, we have applied TBM for determining the atrophy of various brain structures in cocaine-dependent subjects. Since VBM is more commonly used in determining volume differences in drug abuse, we have also used this technique in this study. VBM analysis was performed without and with modulation to adjust for volume changes during normalization (Good et al., 2001). In the TBM analysis, two types of nonlinear registration algorithms (Avants et al., 2008; Tao et al., 2009a; Tao et al., 2009b) which are inverse consistent and diffeomorphic were used to generate three different unbiased templates based on two different groups of normal subjects. The statistical analysis for investigating various group differences was performed with the Statistical Parametric Mapping 8 (SPM8) software package (Friston et al., 1994). In the TBM based analysis, the regional volume changes in various brain structures were calculated as the average logarithm of JD maps of the deformation fields.
2 METHODS
2.1 Subjects
Thirty-four cocaine dependent and 36 non-drug using controls were recruited. All subjects completed a structured psychiatric interview (SCID) (First et al., 1996), medical history and physical examination, blood chemistry, and complete blood count. They were evaluated for clinically significant brain abnormalities by FLAIR (fluid attenuation by inversion recovery) scans on MRI. FLAIR scans were reviewed by a board certified radiologist blind to subject group. Cocaine dependent subjects and controls were carefully screened to rule out brain pathology of undetermined origin. In addition, female subjects underwent urine pregnancy test.
For cocaine dependent subjects inclusion criteria were the Diagnostic and Statistical Manual Fourth Edition (DSM-IV) (American Psychiatric Association, 1994) diagnosis of current cocaine dependence. For healthy control subjects, inclusion criteria were the absence of current or lifetime history of any DSM-IV substance or psychiatric disorders. For all subjects, exclusion criteria were: 1) non-psychiatric medical illness judged to be significant by study physician (such as diabetes or hypertension) or non-psychiatric medical illness which could affect the brain (such as prior head trauma or history of seizures); 2) current use of any medications that could affect the brain (other than drugs of abuse in the cocaine dependent group); 3) a positive pregnancy test; 4) positive breath alcohol test on the day of scanning; and 5) any abnormality on FLAIR scans judged to be clinically significant by the study Radiologist. In addition, healthy controls were excluded who had a positive urine drug screen for any drugs of abuse. Quantity of drug use within the cocaine dependent subjects was measured using the KMSK (Kellogg et al., 2003) and the ASI (McLellan et al., 1984).
Prior to any analysis, we have excluded subjects with visible pathology as determined by a board certified radiologist. The criteria used for exclusion of subjects were: 1) any tumors or brain mass, 2) white matter hyperintensities of sufficient severity to be probably related to vasculitis, vasculopathy, or demyelinating process such as multiple sclerosis, 3) evidence of prior stroke or traumatic brain injury 4) any cerebral atrophy in controls and severe atrophy in cocaine users 5) any significant congenital abnormalities, such as Virchow-Robin spaces. The rationale for these exclusions was to minimize any source of group differences related to non-specific pathology that was clearly unrelated to cocaine use. Since mild cerebral atrophy could have been due to cocaine use, these cocaine subjects were not excluded. Specific reasons for excluding subjects were as follows: Seven control subjects were excluded because of: 1) mild cerebral atrophy (5 cases), 2) presence of demyelenating process such as multiple sclerosis (1 case), and 3) vasculitis, vasculopathy, or demyelinating process such as multiple sclerosis (1 case).
Five cocaine subjects were excluded because of 1) prominent and extensive Virchow-Robin spaces (1 case), 2) microvascular disease (1 case), 3) microvascular disease, possibly vasculopathy or demyelinating process (1 case), 4) deep white matter atrophy plus mild diffuse cerebral cortical atrophy (1 case), and 5) encephalomalacia, gliosis, and pial siderosis most likely related to previous traumatic injury plus mild deep white matter atrophy (1 case). Three of the cocaine subjects whio were included in the analysis had mild cerebral atrophy and none of the included control subjects had mild cerebral atrophy.
Following these exclusions, twenty nine cocaine dependent and 29 non-drug using controls were included in this study (see Table 1). The age difference between the controls and cocaine subjects is statistically significant, with controls being younger, by seven years on the average.
Table 1.
Demographics by group. KMSK is the Kreek/McHugh/Schluger/Kellogg scale (Kellog et al., 2003).
| Variable | Cocaine dependent subjects |
Non-drug using controls |
|---|---|---|
| Age | 41.03 ± 9.14* | 34.27 ± 10.19 |
|
| ||
| Gender | 21 M, 8 F | 21 M 8 F |
|
| ||
| KMSK Cocaine | ||
|
| ||
| Frequency | 6.0 ± 1.36 | |
|
| ||
| Duration | 2.0 ± 0.89 | |
|
| ||
| Amount | 4.0 ± 1.83 | |
|
| ||
| Total | 12.0 ± 2.78 | |
|
| ||
| Addiction Severity Index | ||
| Years of Cocaine Use | 12.66 ± 7.73 | |
significant difference between groups p < 0.05.
2.2 Magnetic Resonance Imaging
All MRI scans were performed on a 3T Philips Intera scanner with a quasar gradient system (Philips Medical Systems, Best, Netherlands) and eight channel head coil. Images were acquired from the whole brain (from foramen magnum to vertex). Following the tri-pilot scan for prescribing the geometry for scanning, FLAIR images for identifying any incidental pathology were acquired in the transverse plane with TR = 11,414 ms, TE = 120 ms, flip angle = 90°, inversion delay (TI) = 2800 ms, field of view (FOV) = 240 mm × 190 mm, acquisition matrix = 252 × 162, reconstructed matrix = 256 × 256, in-plane resolution = 0.938 mm × 0.938 mm, 46 slices, slice thickness = 3.0 mm with no interslice gap, number of averages = 2. 3D-SPGR (Turbo Field Echo) images for morphometric measurements were acquired in the transverse plane with TR = 9.91 ms, TE = 4.60 ms, flip angle = 8°, FOV = 240 mm × 240 mm, acquisition and reconstructed matrix = 256 × 256, in-plane resolution = 0.938 mm × 0.938 mm, number of slices = 132, slice thickness = 1.0 mm, turbo factor = 201. A SENSE factor of 2 was used for all the scans.
2.3 Unbiased Atlases
All 3D-SPGR brain images acquired on controls and cocaine users were stripped of extameningeal tissues (brain extraction or skull stripping) using Brain extraction tool (BET) (Smith, 2002) available as part of MRIcro (http://www.sph.sc.edu/comd/rorden/mricro.html). A module (Ashburner, 2002) available in SPM2 was used for intensity non-uniformity correction.
The quality of atlas plays a critical role in the determination of the JD. In order to ensure the robustness of the results, we determined the JD using two different normal data sets and two different nonlinear registration techniques as described below. This resulted in three unbiased atlases as described here:
Twenty two 3D-SPGR, T1-weighted images obtained on normal subjects (recruited in this study) were used to generate an unbiased atlas using symmetric nonlinear registration with mutual information (SNRMI) described by Tao et al. (2009a).
Twenty normal T1-weighted images (age matched with the cocaine users) obtained from the OASIS database (http://www.oasis-brains.org/) were used to generate an unbiased atlas using SNRMI.
Ten 3D-SPGR T1-weighted images obtained on normal subjects were used to obtain an unbiased atlas using symmetric nonlinear registration with cross-correlation, SyN algorithm, proposed by Avants et al. (2008). Based on the suggestion regarding the required number of images for creating unbiased atlas with SyN algorithm by its authors, we have used ten image volumes from our normal controls.
Symmetric Nonlinear Registration with Mutual Information (SNRMI)
All the images were initially co-aligned with an atlas in the Montreal Neurological Institute (MNI) space (Collins et al., 1995; Mazziotta et al., 2001) which was obtained from the International Consortium for Brain Mapping (ICBM, http://www.loni.ucla.edu/ICBM/), using a twelve parameters affine registration algorithm. Following the application of affine transformation, the transformed images were aligned with the MNI atlas using the nonlinear registration algorithm (Tao et al., 2009a). The symmetric cost function for the nonlinear registration was constructed to assure inverse consistency required for morphometric measurement. Mutual information (MI) and dense MI flow were used as similarity measures and the external force for driving the registration process and accounting for the intensity variations in different image volumes. Alternative minimization method was implemented to minimize different terms in the cost function to avoid divergence. Displacement fields were updated through composition scheme to achieve diffeomorphic mapping. The geometric average of the transformation fields of these co-registered images was obtained by averaging the transformations defined on the grid points of the MNI template. Due to the diffeomorphism of the registration method, the JD of the geometric average transformation are positive for all voxels (Tao et al., 2009a). The unbiased template was obtained by mapping the averaged image in the MNI space through inversion of the geometric average transformation (Tao et al., 2009a).
2.4 Symmetric Nonlinear Registration with Cross-correlation (SyN)
Nonlinear registration algorithm SyN (Avants et al., 2008) (http://www.picsl.upenn.edu/ANTS/) was used to generate the unbiased atlas. SyN is a bi-directional diffeomorphic algorithm for maximizing the cross-correlation within the space of diffeomorphic maps using multiresolution Gaussian smoothing of the velocity field and transformation symmetry as regularization for minimizing the cost function in order to achieve inverse consistency and guarantee the positive JDs for diffeomorphism. The details of the algorithm can be found elsewhere (Avants et al., 2008). The SyN method available with ANTS software (http://www.picsl.upenn.edu/ANTS/) includes both affine and nonlinear registration. The application of the software generates affine transformation matrix and deformation fields which were then used to create the deformed images and the JDs.
Tensor-Based Morphometry Analysis
Following the creation of unbiased atlases, all images from controls and cocaine subjects were co-aligned with the unbiased atlases using the same nonlinear registration algorithm that was used for creating the individual atlas. All the images acquired on cocaine and normal controls were registered with the three unbiased atlases using twelve parameters affine registration prior to the application of symmetric nonlinear registration algorithms described above.
TBM analysis was performed between controls and cocaine users to identify and measure any volumetric differences in brain structures. The JDs of the stripped brain images were normalized by setting their mean to 1.0 to compensate for the differences in brain sizes included in the study. For analyzing the structural differences in the brain, logarithmic transformations of the JDs were used due to its symmetry (Tao et al., 2009b). The JDs were obtained for each brain in the space of unbiased atlas. This analysis was done with respect to all the three atlases.
Voxel-Based Morphometry Analysis
Since many of the published studies on drug abuse have employed VBM analysis for determining changes in regional brain volumes, we have also analyzed our data using this technique. The segmentation, smoothing, and statistical tools of SPM8 (Statistical Parameter Mapping) were used for VBM analysis to investigate changes in GM volumes. All the images from both groups were segmented into gray/white matter classes. Each of gray matter/white matter segmented images were normalized. We performed the analysis without and with modulation to adjust for volume changes during normalization. Smoothing with an isotropic Gaussian kernel of 8 mm × 8 mm × 8 mm FWHM was applied to gray matter/white matter segmented, normalized, and modulated images.
Statistical Analysis
For TBM, the analysis was performed on the JDs. For the VBM the analysis was performed on segmented and smoothed data. Analysis of covariance (ANCOVA) using SPM8 was applied to automatically identify volume differences between the control and cocaine-dependent subjects. The analysis was performed in two ways: first, without using any covariates, and second, age and gender were included as covariates. These analyses were performed with respect to each unbiased atlas which resulted in six analyses. Following each ANOVA analysis, t-statistics with Family-wise error (FWE) of 0.05 were computed voxel-by-voxel throughout the whole brain. The intrinsic smoothness of the residual field in each of these analyses as computed by SPM8 was greater than 3 voxels full-width-at-half-maximum and thus met criteria for valid Random Field theory FWE correction (Petersson et al., 1999).
Analysis of covariance (ANCOVA) using SPM8 was applied to smoothed gray matter images to locate volume difference between cocaine-dependent subjects and controls. The analysis was performed with and without age and gender as covariates. Following each ANCOVA analysis, t-statistics with Family-wise error (FWE) corrected P less than 0.05 were computed voxel-by-voxel for the whole brain.
2.5 Pearson Correlation
In order to determine the volume changes in regional brain structures, a template brain with 56 labeled gray matter (GM) structures was used (ICBM; http://www.loni.ucla.edu/ICBM/). The labeled image was co-aligned with the unbiased atlases using respective nonlinear registration algorithms, and the mean JD within each structure was computed. Pearson's correlation coefficients between brain structures and clinical evaluation metrics, KMSK values (frequency, duration, amount, and total), were evaluated with respect to each unbiased atlas. The mean JD of a given structure is used to obtain the correlation between that structure and KMSK measures. The computations for Pearson correlation coefficients along with corresponding p-values were carried out using MATLAB 7.8 version.
Figure 1 shows the schematic diagram demonstrating the steps involved in the present study starting from the generation of unbiased atlas to statistical analysis.
Figure 1.
Schematic diagram of the procedures involved in the present study. Number of input images depends on the method used (see text). Nonlinear registration in this diagram represents either SNRMI or SyN algorithm.
3 RESULTS
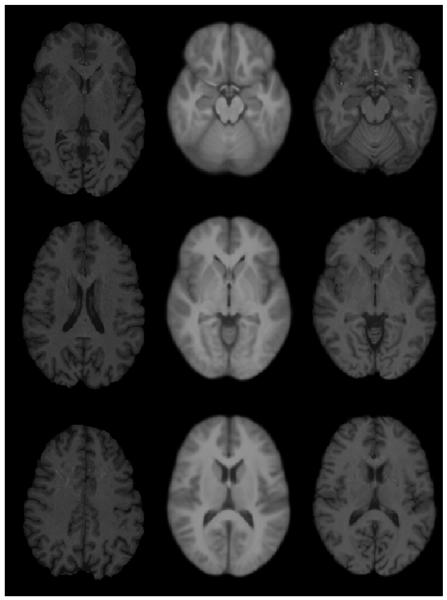
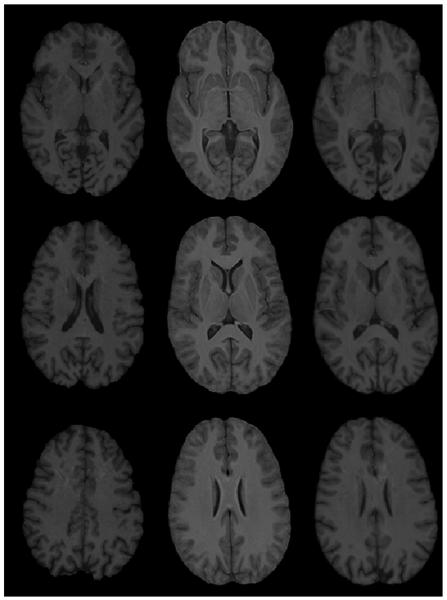
Figure 2 shows original images, unbiased atlas, and registered images with unbiased atlas at three different levels. In this figure, the unbiased atlas was obtained with the application of SNRMI using 22 normal images. Similarly, Figs. 2 and 3 show examples of registration based on SNMRI algorithm using 20 normal images from OASIS database and SyN algorithm using 10 images. The excellent quality of atlas (as judged by the sharpness and contrast and quantitative metrics described by Tao et al., 2009b) and performance of both the registration algorithms can be appreciated from these figures. In summary, all the three unbiased atlases generated with our normal volunteers and OASIS database along with both the nonlinear registration algorithms are observed to be of high quality. Similarly, both the nonlinear registration algorithms (SNMRI and Syn) provided excellent image registration.
Figure 2.
An example of SNRMI algorithm. Left column: Original image; middle column: unbiased atlas obtained with 22 normal brain images; and last column: registered images with unbiased atlas.
Figure 3.
Another example of SNRMI algorithm. Left column: Original image; middle column: unbiased atlas obtained with 20 normal brain images from OASIS database; and last column: registered images with unbiased atlas.
Neither TBM nor VBM (with or without modulation) results indicated volume differences in any of the GM structures and the whole brain WM between normal controls and cocaine users. This was observed to be true across all the three atlases used in the TBM analyses. In addition, there were no voxels that were significantly different between groups (FWE-corrected voxel P < 0.05) in any of the voxel-wise analyses throughout the whole brain.
Our exploratory analysis also did not indicate significant correlations between the volumes of any of the brain structures (including total WM) and any of the KMSK measures. This was true whether or not gender and age were included as covariates and across all the three atlases.
4 DISCUSSION
The major objective of these studies was to investigate the effect of chronic cocaine use on regional volumes in brain. We believe that these are the first studies on cocaine users that have employed TBM analysis, combined with nonlinear registration that is symmetric, inverse consistent, and diffeomorphic. Image registration is critical for group comparison. In addition, we have employed unbiased atlases for group comparison. In order to be confident of our results, we have employed two types of sophisticated image registration techniques that are shown to be robust and generated three unbiased atlases based on different groups of normal controls. Independent of the registration techniques and the control population for generating the unbiased atlases, we failed to detect any cocaine-related volume differences in any of the brain structures. In addition, we failed to observe any effect of different KMSK scores on various brain structures. This is somewhat different from those of many published reports that have demonstrated, as discussed in the “Introduction”, that cocaine affects the volumes of different brain structures.
In view of our inability to detect any brain volumetric changes in the cocaine users using TBM and since majority of the published data is based on VBM, we have also analyzed our data using VBM. However, we failed to detect any volumetric changes even with the VBM analysis. As discussed in the introduction, the published literature reports about the changes in structural volumes are not always consistent. For example, Jacobsen et al. (2001) have reported increased striatal volume in cocaine users, while, Tanabe et al. (2009) did not find regions of significantly increased GM in cocaine-dependent subjects compared with control subjects. Lim et al. (2008) used MANOVA to test for group differences between cocaine-dependent subjects and normal controls in four volumes: inferior frontal WM and GM and superior frontal WM and GM. Their overall analysis did not reveal significant group differences among these four volumes, but indicated strong trend. However, examination of the univariate effects showed no evidence of group differences in the superior frontal WM and GM regions and the cocaine dependent subjects had smaller inferior frontal GM volumes than controls.
In this study, we used SPM8 voxel-level FWE-corrected inference instead of spatial-extent inference (i.e., cluster-level) because of the fact that cluster-level inference leads to many false positives in VBM due to the non-stationary smoothness of the random field, and thus is not recommended for VBM (Ashburner and Friston, 2000). However, previous VBM studies, with the exception of a few studies (Sim et al., 2007; Tanabe et al., 2009) in cocaine users have not reported the results of FWE-corrected voxel-level inference, and thus it is difficult to compare our results with many of previous studies.
Other possible reasons for differences in the results between our studies and the published literature could include differences in the cohorts and other demographic factors. But, we believe that the main differences arise from the analysis methods that were employed in these different studies. As indicated above, our analysis is based on TBM and more sophisticated registration techniques for generating unbiased atlases. It is also worth noting that we observed the same results using three different atlases generated from two different control populations. Another factor that could have led to different findings in this study compared to previous studies was the exclusion of subjects (controls and cocaine users) who had any abnormalities on FLAIR scans judged to be clinically significant by the study radiologist. The majority of previous studies did not exclude subjects in this manner. This exclusion was done in order to control for gross brain injury that could be related to a number of causes other than cocaine use.
Change in brain volume is a nonspecific indicator of underlying pathology. Brain volume is affected by demyelination, neuronal/axonal loss, and gliosis, among others. Demyelination and neuronal/axonal loss result in atrophy while gliosis increases tissue volume. Advanced MRI techniques such as diffusion tensor imaging (DTI) and magnetic resonance spectroscopy have the potential to detect demyelination and neuronal/axonal loss.
Changes in the DTI measures of white matter have been reported by Lim et al. (2002, 2008) and Moeller et al. (2005, 2007). For example, Lim et al. (2002, 2008) reported decreased fractional anisotropy (FA) in the inferior frontal WM, internal capsule, and splenium and the body of corpus callosum. The decreased FA values in these white matter regions could reflect either demyelination or compromised or loss of axonal integrity. Moeller et al. (2005) have reported changes in FA only in the anterior part of the corpus callosum. Their more detailed DTI analysis indicates that this change reflects altered myelin without any concomitant compromised axonal integrity (Moeller et al., 2007). Using a 3T scanner and smaller slice thickness (3 mm) for reduced partial volume averaging, Ma et al. (2009) based on DTI, have reported that the isthmus of corpus callosum also exhibits evidence of altered myelin. Thus all these studies appear to indicate that only a few white matter structures are affected at microscopic level in cocaine-dependent subjects.
MRS studies of cocaine-dependent subjects have been reported in the literature, with an emphasis on n-acetylaspartate (NAA), considered to be a putative marker of neurons/axons. The published results on altered NAA levels in cocaine-dependent subjects are inconsistent. For example, Li et al. (1999) reported a 17% reduction in the NAA levels in the left thalamic region in the chronic cocaine users compared to controls. However, they did not observe and reduction in NAA in the left basal ganglia region. The same authors also reported no differences in the levels of creatine (Cr) which is considered to be a marker of gliosis (Narayana, 2005). More recently Cowan et al. (2009) examined the Brodmann's areas 18, 21, and 45 in cocaine-dependent subjects at 3T. In these studies, statistically significant association between the degree of lifetime cocaine use and NAA or myo-inositol (mI) was not observed in any of the three Brodmann's areas. Smith et al. (2001) examined the effect of prenatal cocaine exposure in children in frontal WM and striatum. They observed significant increase in Cr in the frontal WM, but NAA levels did not differ significantly compared to controls. Using multi-slice MRS imaging, O'Nell et al. (2001) reported a lack of association between cocaine use and the NAA levels in the cortical and subcortical GM throughout the brain. They have also reported an increase in Cr in the parietal WM in cocaine-dependent subjects. These various reports appear to be inconsistent about the cocaine-induced changes in NAA and suggest only a modest, if any, reduction in NAA, indicating that neuronal/axonal injury is not a major pathological component in cocaine-dependence. These studies also appear to suggest the presence of gliosis, as suggested by increased Cr, in a number of regions in the brain.
Based on the brief discussion above, both DTI and MRS studies suggest relatively limited damage to myelin and neuronal/axonal injury and some gliosis in cocaine dependence. While demyelination and neuronal/axonal loss could result in reduced volume, gliosis has the opposite effect. Thus our failure to observe any volumetric changes in various brain structures in cocaine-dependent subjects appears to be consistent with the DTI and MRS results.
In summary, the published literature on MRI-based volumetric studies in cocaine users is very inconsistent. It is also interesting that more recent studies (Sim et al., 2007; Lim et al., 2008; Tanabe et al., 2009) did not detect significant changes in the volumes in cocaine users. This raises questions about the existence of true volume differences between these two groups. We tried to point out some of the technical issues that could possibly explain the inconsistencies. Both DTI and MRS studies suggest relatively limited damage to myelin and neuronal/axonal injury and some gliosis in cocaine dependence. While demyelination and neuronal/axonal loss could result in reduced volume, gliosis has the opposite effect. Thus our failure to observe any volumetric changes in various brain structures in cocaine-dependent subjects may not be surprising.
5 LIMITATIONS
The main limitation of this study is the relatively small sample size. For example, Hua et al. (Hua et al 2008b), using the TBM analysis, based on 40 Alzheimer disease (AD) patients, were able to barely differentiate between AD and mild cognitive impaired (MCI) subjects. However, significant group differences were observed when this number was increased to 676. However, it should be pointed out that in our study the number of subjects in each group is similar or larger than in the previously published studies that reported differences in brain volumes between cocaine users and controls (Bartzokis et al., 2000; Franklin et al., 2002; Jacobsen et al., 2001; Makris et al., 2004; Matochik et al., 2003; Schlaepfer et al., 2006),. Another limitation of this study is the heterogeneity in the types of concomitant substances of abuse that different subjects within the cocaine group have used, since it is possible that different substances may have caused volumetric changes in different directions, thus obscuring changes purely due to cocaine.
In this study the controls and cocaine users are not age-matched. However, there was no group difference detected even when age was used as a covariate.
While TBM provides substantial methodological improvement over VBM in detecting volume changes (Kim et al., 2008), it may not detect changes over very small structures, at least in cross-sectional studies. Also, we have combined all the white matter structures into a single structure because we were unable to find a standard image with white matter structures parcellated.
Figure 4.
An example of SyN algorithm. Left column: Original image; middle column: unbiased atlas obtained with 10 normal brain images; and last column: registered images with unbiased atlas.
ACKNOWLEDGEMENTS
The TBM and nonlinear registration techniques that are employed in the current studies are developed as a part of the project supported by NIBIB/NIH grant # EB02095 (PAN). The MRI scanner was partially funded by NCRR/NIH grant # S10 RR19186-01(PAN). Recruitment, clinical assessment, and MRI scans are supported by NIDA/NIH grant # P50-DA0092629 (FGM). Assistance of Mr. Vipulkumar Patel in scanning the patients is gratefully acknowledged.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIBIB or NIDA or the NIH.
REFERENCES
- Ashburner J. Another MRI bias correction approach; 8th International Conference on Functional Mapping of the Human Brain; Japan. 25-28 September.2002. [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. Neuroimage. 2004;23(Suppl 1):S139–150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants B, Grossman M, Gee JC. The correlation of cognitive decline with frontotemporal dementia induced annualized gray matter loss using diffeomorphic morphometry. Alzheimer Dis Assoc Disord. 2005;19(Suppl 1):S25–28. doi: 10.1097/01.wad.0000183083.14939.82. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry. 2002;51:605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000;98:93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Beg MF, Khan A. Symmetric data attachment terms for large deformation image registration. IEEE Trans Med Imaging. 2007;26:1179–1189. doi: 10.1109/TMI.2007.898813. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, Miller BL, Ashburner J, Gorno-Tempini ML. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging. 2009;30:103–111. doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Renda NC, Rankin KP, Rosen HJ, Seeley WW, Ashburner J, Weiner MW, Miller BL, Gorno-Tempini ML. A tensor based morphometry study of longitudinal gray matter contraction in FTD. Neuroimage. 2007;35:998–1003. doi: 10.1016/j.neuroimage.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright WS. Cocaine medications, cocaine consumption and societal costs. Pharmacoeconomics. 2000;18:405–413. doi: 10.2165/00019053-200018040-00008. [DOI] [PubMed] [Google Scholar]
- Christensen GE, Johnson HJ. Consistent image registration. IEEE Trans Med Imaging. 2001;20:568–582. doi: 10.1109/42.932742. [DOI] [PubMed] [Google Scholar]
- Chung MK, Dalton KM, Davidson RJ. Tensor-based cortical surface morphometry via weighted spherical harmonic representation. IEEE Trans Med Imaging. 2008;27:1143–1151. doi: 10.1109/TMI.2008.918338. [DOI] [PubMed] [Google Scholar]
- Collins L, Holmes C, Peters T, Evans A. Automatic 3-D model-based neuroanatomical segmentation. Human Brain Mapping. 1995;3:190–208. [Google Scholar]
- Cowan RL, Joers JM, Dietrich MS. N-acetylaspartate (NAA) correlates inversely with cannabis use in a frontal language processing region of neocortex in MDMA (Ecstasy) polydrug users: a 3 T magnetic resonance spectroscopy study. Pharmacol Biochem Behav. 2009;92:105–110. doi: 10.1016/j.pbb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–1369. doi: 10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fowler JS, Volkow ND, Kassed CA, Chang L. Imaging the addicted human brain. Sci Pract Perspect. 2007;3:4–16. doi: 10.1151/spp07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes C, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1994;2:189–210. [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hua X, Leow AD, Parikshak N, Lee S, Chiang MC, Toga AW, Jack CR, Jr., Weiner MW, Thompson PM. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer's disease: an MRI study of 676 AD, MCI, and normal subjects. Neuroimage. 2008a;43:458–469. doi: 10.1016/j.neuroimage.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Leow AD, Lee S, Klunder AD, Toga AW, Lepore N, Chou YY, Brun C, Chiang MC, Barysheva M, Jack CR, Jr., Bernstein MA, Britson PJ, Ward CP, Whitwell JL, Borowski B, Fleisher AS, Fox NC, Boyes RG, Barnes J, Harvey D, Kornak J, Schuff N, Boreta L, Alexander GE, Weiner MW, Thompson PM, Alzheimer's Disease Neuroimaging, I. 3D characterization of brain atrophy in Alzheimer's disease and mild cognitive impairment using tensor-based morphometry. Neuroimage. 2008b;41:19–34. doi: 10.1016/j.neuroimage.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH. Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry. 2001;158:486–489. doi: 10.1176/appi.ajp.158.3.486. [DOI] [PubMed] [Google Scholar]
- Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage. 2004;23(Suppl 1):S151–160. doi: 10.1016/j.neuroimage.2004.07.068. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kim J, Avants B, Patel S, Whyte J, Coslett BH, Pluta J, Detre JA, Gee JC. Structural consequences of diffuse traumatic brain injury: a large deformation tensor-based morphometry study. Neuroimage. 2008;39:1014–1026. doi: 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore N, Brun C, Pennec X, Chou YY, Lopez OL, Aizenstein HJ, Becker JT, Toga AW, Thompson PM. Mean template for tensor-based morphometry using deformation tensors. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2007;10:826–833. doi: 10.1007/978-3-540-75759-7_100. [DOI] [PubMed] [Google Scholar]
- Li SJ, Wang Y, Pankiewicz J, Stein EA. Neurochemical adaptation to cocaine abuse: reduction of N-acetyl aspartate in thalamus of human cocaine abusers. Biol Psychiatry. 1999;45:1481–1487. doi: 10.1016/s0006-3223(98)00230-3. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, Kramer LA, Moeller FG. Diffusion tensor imaging in cocaine dependence: Regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2009.05.020. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS, Jr., Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, O'Brien CP, Barr HL, Evans F. The addiction severity index in three different populations. NIDA Res Monogr. 1984;55:217–223. [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, Swann AC, Narayana PA. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Narayana PA. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J Neuroimaging. 2005;15(4 Suppl):46S–57S. doi: 10.1177/1051228405284200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Dhuna A, Anderson DC. Cerebral atrophy in habitual cocaine abusers: a planimetric CT study. Neurology. 1991;41:34–38. doi: 10.1212/wnl.41.1.34. [DOI] [PubMed] [Google Scholar]
- Petersson KM, Nichols TE, Poline JB, Holmes AP. Statistical limitations in functional neuroimaging. I. Non-inferential methods and statistical models. Philos Trans R Soc Lond B Biol Sci. 1999;354:1239–1260. doi: 10.1098/rstb.1999.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer TE, Lancaster E, Heidbreder R, Strain EC, Kosel M, Fisch HU, Pearlson GD. Decreased frontal white-matter volume in chronic substance abuse. Int J Neuropsychopharmacol. 2006;9:147–153. doi: 10.1017/S1461145705005705. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Gilbride K, Kuo J, Poland RE, Walot I, Ernst T. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107:227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, Satz P, Myers H. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1993;5:419–427. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao G, Datta S, He R, Nelson F, Wolinsky JS, Narayana PA. Deep gray matter atrophy in multiple sclerosis: a tensor based morphometry. J Neurol Sci. 2009a;282:39–46. doi: 10.1016/j.jns.2008.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao G, He R, Datta S, Narayana PA. Symmetric inverse consistent nonlinear registration driven by mutual information. Comput Methods Programs Biomed. 2009b;95:105–115. doi: 10.1016/j.cmpb.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Grieve SM, Farrow TF, Gomes L, Brennan J, Harris AW, Gordon E, Williams LM. Volumetric white matter abnormalities in first-episode schizophrenia: a longitudinal, tensor-based morphometry study. Am J Psychiatry. 2007;164:1082–1089. doi: 10.1176/ajp.2007.164.7.1082. [DOI] [PubMed] [Google Scholar]