Abstract
Background
Spinal cord ischemia and paralysis are devastating perioperative complications that can accompany open or endovascular repair surgery for aortic aneurysms. Here, we report on the development of a new mouse model of spinal cord ischemia with delayed paralysis induced by cross-clamping the descending aorta.
Methods
Transient aortic occlusion was produced in mice by cross clamping the descending aorta through a lateral thoracotomy. To establish an optimal surgical procedure with limited mortality, variable cross-clamp times and core temperatures were tested between experiments.
Results
The onset of paresis or paralysis and postsurgical mortality varied as a function of cross-clamp time and core temperature that was maintained during the period of cross-clamp. Using optimal surgical parameters (7.5 min cross-clamp duration @ 33°C core temperature), the onset of paralysis is delayed 24–36 h postreperfusion and > 95% of mice survive through 9 weeks postsurgery. These mice are further stratified into two groups, with 70% (n = 19/27) of mice developing severe hindlimb paralysis and the remaining mice showing mild, though still permanent, behavioral deficits.
Conclusion
This new model should prove useful as a preclinical tool for screening neuroprotective therapeutics and for defining the basic biological mechanisms that cause delayed paralysis and neurodegeneration after transient spinal cord ischemia.
Introduction
Spinal cord ischemia with paralysis is a devastating perioperative complication of surgical repair of aortic aneurysms1. Paralysis can be immediate but is often delayed, with the reported incidence (0–40%) varying between centers and as a function of the surgery and Crawford classification. Recent reviews focusing on high-volume centers indicate that 5%-11% of cases are accompanied by delayed post-operative paresis or paralysis2–4.
To understand the mechanisms responsible for ischemic spinal cord injury (ISCI), a number of experimental models have been developed5–9. Rat, rabbit, dog, sheep and pig models are used regularly; however, none are ideal for testing pre-clinical therapies or understanding mechanisms of injury. They are either too expensive (e.g., pig, dogs), are associated with high incidence of morbidity and mortality, or lack reproducibility. In 2000, a mouse model of ISCI was developed to take advantage of a growing number of gene knock-in and knock-out mice10. Indeed, with the mouse genome defined, it is now possible to study the effects of spinal ischemia on specific genes and molecular signaling pathways. Although it has been ~10 yr since the first ISCI mouse model was described, it has been used sparingly, probably because the surgical approach is difficult and post-surgical mortality is high10. For animals that do survive, survival is limited to <1 week and paralysis is usually immediate10–13. Our goal was to create a new clinically-relevant model of mouse ISCI in which these various outcomes could be controlled more consistently.
Here, we describe a simple and reproducible pre-clinical model of murine ISCI caused by transient cross-clamp of the descending aorta through a lateral thoracotomy. Using a battery of physiological, behavioral and anatomical assays, we show that this ISCI model causes reproducible intraspinal inflammation and neuropathology accompanied by profound neurological impairment. The onset and magnitude of paralysis varies as a function of aortic cross-clamp time and the intraoperative core temperature that was maintained during the period of ischemia. By changing these parameters, mice can be consistently stratified into two groups, i.e., mice that develop immediate and severe paralysis and those in which paralysis is delayed in onset, thereby mimicking the phenomenon that occurs in a subset of humans undergoing similar surgery. This new model will facilitate the screening of neuroprotective therapeutics and will help reveal basic mechanisms of postischemia/reperfusion pathology and functional impairment caused by aortic cross-clamp.
Methods and Materials
Mice
Adult C57BL/6 mice (males and females, 7–12 weeks; 17–22g) were purchased from Harlan (Indianapolis, IN) or Jackson Laboratories (Bar Harbor, ME). All procedures were performed using aseptic technique and all mice were housed in HEPA-filtered Bio-clean units. All procedures were approved by and performed in accordance with The Ohio State University’s Institutional Lab Animal Care and Use Committee, Cleveland, Ohio.
Anesthesia and surgical preparation
For all surgical procedures, mice were maintained at pre-defined core temperatures (33, 35, or 36°C) on a temperature-controlled surgical platform (World Precision Instruments, Sarasota, FL). Mice were anesthetized by inhalation of 3% isofluorane driven by 100% O2 for induction, then maintained at 2% isofluorane (100mL/minute O2). Ventilation was achieved using an endotracheal cannula (see below) and a mouse ventilator [Hugo Sachs-Harvard Apparatus Minivent (Hollinston, MA): tidal/stroke volume = 250 µL; rate = 230 ventilations/min]. For endotracheal intubation, mice were anesthetized and a ventral midline incision was made between the ears and extending slightly past the anterior-aspect of the pinna. The underlying musculature and submaxillary glands were retracted laterally to expose the larynx and trachea and a lubricated mouse tracheal intubation cannula was inserted into the trachea (Hugo Sachs VK32). The throat incision was closed with surgical glue and a single suture. Prior to thoracotomy, mice were injected subcutaneously with heparin (200IU/kg), xylazine (0.1 mg/mouse in 0.02 mL) and gentomycin (0.1 mg/mouse in 0.1 mL).
Spinal cord ischemia was induced by transient aortic cross-clamp at the level of either the aortic arch or descending aorta, as described below. Numbers of animals in the different experimental groups are provided in table 1.
Table 1.
Summary of Surgical Parameters and Group Sizes
| Cross-clamp at: | Temperature | Cross-clamp time (min) | n |
|---|---|---|---|
| Aortic Arch, LSA | 35–36°C | 8–11 | 14 |
| Descending Aorta | 35°C | 3–7.5 | 11 |
| Descending Aorta | 35°C | 8–11 | 8 |
| Descending Aorta | 33°C | 3–7 | 11 |
| Descending Aorta | 33°C | 7.5 | 29 |
| Descending Aorta | 33°C | 8–10 | 3 |
| Descending Aorta | 33°C | 7.5 (acute time points) | 24 |
| Descending Aorta | 33°C | 7.5 (MAP and blood gases) | 6 |
Abbreviations: Left subclavian artery (LSA), mean arterial pressure (MAP)
Transient cross-clamp at the aortic arch and left subclavian artery
Initial studies used the methods described by Lang-Lazdunski et al10. Briefly, this method uses an anterior sternotomy with transient aortic occlusion produced by aneurysm clips placed at the aortic arch (between the left common carotid and left subclavian arteries) and on the left subclavian artery (at its origin). Core temperature was maintained at 35–36°C.
Transient cross-clamp at the descending aorta
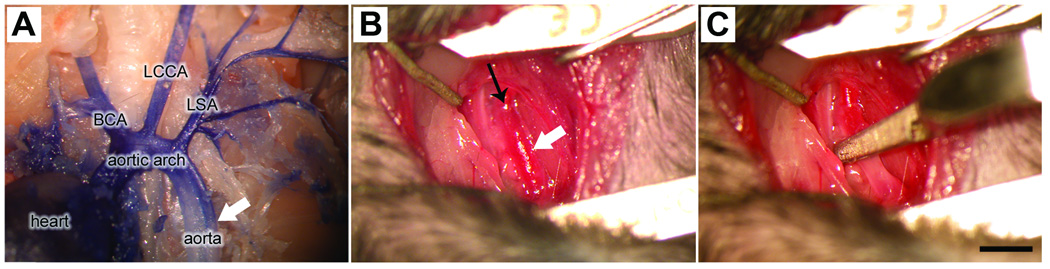
Intubated mice were placed on their right side (horizontal lateral position) and the left forelimb was positioned laterally beneath the mandible and secured to the surgical platform. A small transverse (dorsal to ventral) incision was made below the left forelimb and shoulder to expose the underlying rib cage. Using scissors, muscle between the 2nd and 3rd rib was cut, exposing the lateral pleura. Retractors were used to open the incision and lateral hooks were used to completely expose the inferior vena cava, thymus, left atrium and descending aorta. Forceps were used to blunt dissect ~2 mm of the descending aorta beginning 1 mm distal to the left subclavian artery. To ensure completeness of the cross-clamp, the aorta was elevated slightly using a stainless steel hook and then a small aneurysm clip was placed across the vessel (fig. 1A–C). Aortic cross-clamp was maintained for 3–11 minutes, after which the clip was removed and surgical sponges were used to absorb fluid overlying the surgical site. The rib cage, muscle and overlying skin were closed using 6–0 polypropylene and 5–0 dermalon/nylon sutures, respectively.
Figure 1.
Post-surgical care
After reperfusion, mice spontaneously recovered from anesthesia on the surgical platform while maintaining their core temperature. After ~15 min, mice were extubated and placed into an oxygen supplemented intensive care unit (oxygen flow rate ~1 L/min) where they were maintained at ambient room temperature for 4–5 days. Bladders were manually expressed 2×/daily for the duration of the experiment. Mice also were given 1.0–1.5 mL lactated ringers (subcutaneously) 2×/day and prophylactic antibiotics 1×/day through the first 7 days postsurgery. Some mice developed seizures 12–48 h postsurgery (see Results). To quiet seizure-like activity, mice were injected intraperitoneally with ketamine and xylazine diluted ~33% of a normal anesthetic dose14–16.
Behavioral Evaluation
Bilateral hindlimb function was monitored daily (in some cases up to 3×/day) for the first week, then 1×/week thereafter using the Basso Mouse Locomotor Rating Scale. The Basso Mouse Scale is a 10-point scale (0–9) that uses operational definitions to quantify the magnitude and rate recovery of hind limb movements, forelimb-hindlimb coordination and trunk stability in spinal cord injured mice17.
Hemodynamic monitoring and measurements of blood gases
Small superficial incisions were made over the left carotid and left femoral arteries in a small cohort (n=6) of intubated mice. To cannulate the femoral artery, the artery was isolated by blunt dissection and proximal blood flow was briefly interrupted by placing a small aneurysm clip proximal to the surgery site, then a small transverse incision was made on the artery using fine Vannas-style vascular scissors (Fine Science Tools, Foster City, CA). The cannula, heat-stretched polyethylene-10 tubing (0.2 mm outer diameter) with the tip cut to a bevel, was threaded through the incision into the proximal artery and secured with a single silk suture. The carotid artery was cannulated by threading a 0.3 mm-diameter cannula 5–6 mm towards the heart through a guide hole made using a 26-guage needle. The cannula was then secured using a silk suture. Both cannulas were attached to a 2-channel blood pressure/HR monitor (Columbus Instruments Physiomex, Columbus, OH).
Tissue harvest and processing
At different times postaortic cross-clamp, mice were anesthetized with ketamine/xylazine then transcardially perfused with 25 ml 0.1 M phosphate-buffered saline, followed by 100 ml 4% paraformaldehyde in phosphate-buffered saline. Spinal cords were removed and post-fixed for 2 h and then incubated in 0.2 M phosphate buffer for 18 h at 4°C. The next day, tissues were placed in 30% sucrose in 0.2 M phosphate buffer and stored for 48–72 h. Sucrose-infiltrated tissues spanning the low-cervical to sacral cord were blocked every 1.5 mm, embedded in Tissue Tek OCT compound (Sakura Finetechnical Co., Torrance, CA) and then were rapidly frozen on powdered dry ice. From these blocks, 10 µm cross sections were cut on a cryostat and collected onto SuperfrostPlus slides (Fisher Scientific, Pittsburgh, PA). Tissues were collected as 20 sets of serial sections (200 µm between adjacent sections) then were stored at −20°C.
Histology and immunohistochemistry
Adjacent sets of tissue sections were used for histological and immunohistochemical analyses. Sections were stained with a cocktail of anti-mouse 200-kDa neurofilament (chicken antineurofilament heavy chain) and biotinylated anti-human HuC/HuD with and without eriochrome cyanine (to visualize myelin), as described previously18,19. When primary antibodies were derived from mice, nonspecific staining was blocked using a cocktail of bovine serum albumin and complete horse and mouse serum for 1 h at room temperature. After blocking solution was removed, sections were overlaid with preconjugated primary and secondary antibody cocktail (e.g., containing mouse anti-X plus biotinlyated horse antimouse immunoglobulin G diluted in blocking solution) for 18 h at room temperature or 4°C. A list of primary antibodies and their final concentrations follows: chicken antimouse neurofilament heavy chain (axons/dendrites, 1 µg/ml; Aves Labs, Tigard, OR), biotinylated mouse anti-human HuC/HuD (neuronal somas, 1 µg/ml, Invitrogen, Carlsbad, CA), rat anti-mouse CD11b (macrophages/ microglia, clone MAC-1, 1 µg/ml, Abcam, Cambridge, MA), rabbit anticow glial fibrillary acidic protein (glial-fibrillary acidic protein: astrocytes, antiserum, 1:20,000 dilution, Dako, Carpinteria, CA).
Quantitative lesion analysis
Spinal cord lesion volumes at 7d postischemia were analyzed using Cavalieri’s method as described previously18,20. Images of antineurofilamant-heavy+HuC/D double–stained sections were digitized using a Zeiss Axioplan II Imaging microscope (Carl Zeiss, Thornwood, NY) and an MCID 6.0 Elite system (InterFocus Imaging, Cambridge, United Kingdom). Low-power digitized sections were printed at 30× magnification and areas of total tissue, spared gray matter and lesion were manually circumscribed. Spared gray matter was defined as tissue containing normal gray matter cytoarchitecture with visibly healthy neuron profiles present at a normal density. Frank lesion was defined as necrotic tissue or tissue lacking normal cytoarchitecture with few or no neuronal somas. White matter pathology (diminished density of transversely-oriented axon profiles) was not included in the lesion volume analysis. Uniform point grids were placed randomly onto print-outs of digitized images and points falling within each area of interest were tallied. Reference areas for each section (e.g., tissue area) and total tissue volume were estimated in the same manner. Point tallies were converted into volume estimates using the formula: volume = T × a/p × 11∑i−1Pi, where T equals the slice spacing, a/p equals the calculated area per point, and 11∑i−1Pi equals the sum of the points sampled. Areas per section for each region were calculated using the same formula with omission of the T multiplier.
Statistical analyses
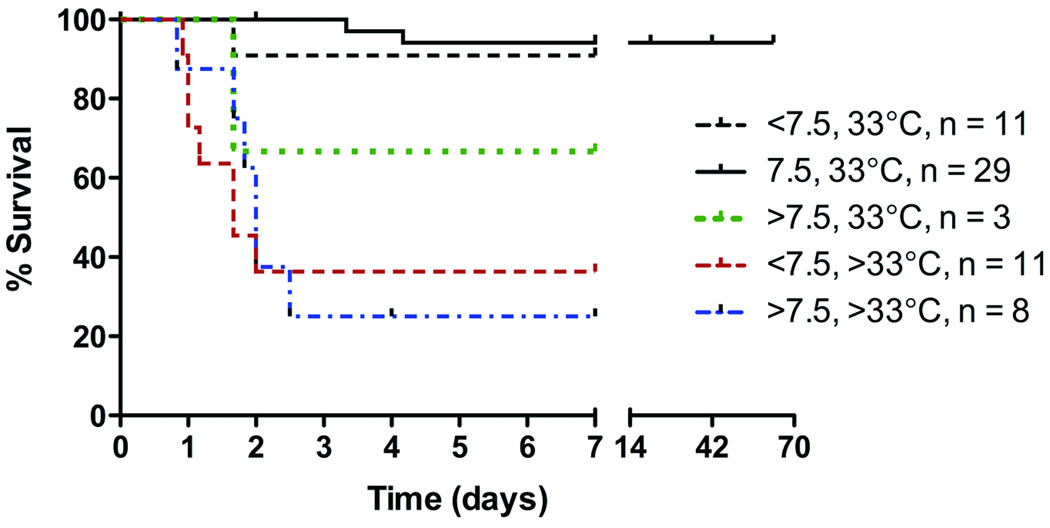
Statistical analyses were performed using GraphPad Prism 5.0, La Jolla CA. In all cases, P-values less than 0.05 were considered significant, using 2-tailed statistical comparisons. Kaplan-Meier survival curves (fig. 2 and Supplemental Digital Content 1, fig. 1) were compared using Log-rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests (Supplemental Digital Content 1, fig. 1 only). Behavioral scores (Basso Mouse Scale) are presented as mean +/− standard error of the mean (SEM). Lesion volumes at 7 days after ischemia/reperfusion were analyzed in one representative cohort using one-way ANOVA with Tukey’s multiple comparison test.
Figure 2.
Results
Transient cross clamp of the descending thoracic aorta is a simplified surgical approach that decreases mortality
To test therapeutics and define the biological mechanisms underlying paralysis caused by spinal cord ischemia subsequent to aortic cross clamp, a model system is needed in which experimental subjects can survive for weeks or months postsurgery. Our initial goal was to use the mouse model developed by Lang-Lazdunski et al10, then maintain mice for a minimum of 7 days postsurgery. To perform the Lang-Lazdunski model, mice receive an anterior sternotomy with transient (9–11 min) cross clamp at the aortic arch and the left subclavian artery while maintaining core temperature at 35–36°C.
In our hands, this procedure caused immediate hindlimb paralysis in 80% of cases (n = 12/15) and most mice (n =13/15) died 1 h to 4 days after ischemia-reperfusion (mean survival time = 39 h; Supplemental Digital Content 1, fig. 1 -- Kaplan-Meier survival curves of mice held at 35°C during aortic cross clamp at either the aortic arch/left subclavian artery [LSA] or descending aorta). Mice that died or were preemptively euthanized showed labored breathing and seizure-like activity. Seizure-like activities occurred in 67% of mice (n = 10 of 15), usually by 9 h postsurgery (range 1–40 h). Only n = 2/15 mice survived to our goal of 7 days postsurgery.
To ensure complete cessation of blood flow at the level of the cross clamp, one must be able to isolate the aorta and LSA from surrounding tissues then place the aneurysm clip across the entire circumference of the vessels. This is difficult to do in the mouse using the ventral approach described by Lang-Lazdunski. Also, because the vagus nerve passes near the LSA and is difficult to separate from the vessel, it can become inadvertently damaged during LSA cross clamp. This may explain the high mortality rate using the ventral approach. In an effort to increase survival, we modified our surgical approach such that a left lateral thoracotomy was used to position a single aneurysm clip on the thoracic aorta distal to the LSA (see fig. 1). The lateral thoracotomy facilitates access to the descending aorta, making it easier to isolate the vessel and ensure complete occlusion. Moreover, this approach is clinically relevant as it is often used to (surgically) repair descending thoracic and thoracoabdominal aortic aneurysms in humans.
Using the lateral approach for aortic cross-clamp (35°C), mice developed immediate paralysis (n = 5 of 8) with a marginal, albeit statistically significant, increase in survival (mean survival = 68 h postsurgery; p = 0.0250 vs. cross clamp at aortic arch/LSA; see Supplemental Digital Content 1, fig. 1). Unfortunately, only n = 1 of the 8 mice survived to our target of 7 days postocclusion. Still, because of the improved survival and the relative ease of cross-clamping the descending aorta via the lateral surgical approach, all subsequent studies used this surgical approach.
Intraoperative core temperature and duration of aortic cross clamp affect postischemia/reperfusion morbidity and mortality
To meet the goal of improving survival to at least 7 days, we experimented with changing intraoperative core temperature and duration of cross-clamp (table 1). In general, the survival and health of the animals varied as a function of core temperature and aortic cross-clamp duration (fig. 2; see also Supplemental Digital Content 1, fig. 2, which depicts survival of all animals as a function of cross clamp time and intraoperative core temperature). When core temperature was maintained at 35°C, survival was increased slightly by reducing cross-clamp times to < 7.5 min. In contrast, >88% of mice survived to the target survival time of 7 days (n = 38/43) when core temperature was maintained at 33°C. Generally, this improved survival was independent of cross clamp time; however, > 93% (n = 27/29) of mice survived ≥ 7 days (up to 63 days; see fig. 2 and Supplemental Digital Content 1, fig. 2) when cross-clamp was applied for 7.5 minutes at 33°C. Approximately 50% of these mice still developed seizure-like activity, but the frequency and intensity of these events was noticeably less than what was observed in mice subjected to longer cross clamp times at higher intraoperative core temperatures.
Transient occlusion at the descending thoracic aorta predictably alters mean arterial blood pressure and acid-base balance
To verify that the optimal protocol (7.5 min of aortic cross clamp with core temperature held at 33°C) affected hemodynamic and physiologic parameters as predicted, n = 6 mice were instrumented with indwelling carotid and femoral cannulas. Prior to aortic cross clamp, mean arterial pressure was 30–38 mmHg in the carotid artery and 24–34 mmHg in the femoral artery (see Supplemental Digital Content 1, fig. 3, which contains graphs depicting changes in mean arterial pressures caused by aortic cross-clamp as measured in the carotid and femoral arteries in 3 separate mice). During the period of cross-clamp, mean arterial pressure in the femoral artery dropped to ~10 mmHg. Once the aneurysm clip was removed, femoral mean arterial pressure returned to baseline. Conversely, carotid mean arterial pressure (proximal to the site of occlusion) increased to 60–80 mmHg during cross clamp then returned to baseline after clip removal.
Before cross clamp, pO2, pCO2, [HCO3], base deficit (beecf), pH and lactate levels were normal in femoral arterial blood (table 2). Transient (7.5 min) aortic cross clamp (with ischemia) predictably disrupted these physiologic parameters, causing hypercapnia and acid/base imbalances (increased base deficit and decreased HCO3) with acidosis (decreased pH and increased lactate).
Table 2.
Physiologic Parameters and Blood Gases Pre- and Postaortic Cross-Clamp
| pH | pO2 (mmHg) |
pCO2 (mmHg) |
Beecf (mmol/L) |
HCO3 (mmol/L) |
Lactate (mmol/L) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre- CC |
10 m reper |
Pre- CC |
10 m reper |
Pre- CC |
10 m reper |
Pre- CC |
10 m reper |
Pre- CC |
10 m reper |
Pre- CC |
10 m reper |
|
| MOUSE 1 | 7.345 | 7.003 | 463 | 150 | 34.7 | 36.2 | −7 | −22 | 19 | 9 | 4.7 | 10.85 |
| MOUSE 2 | 7.452 | 7.007 | 333 | 148 | 28.5 | 34.7 | −4 | −22 | 19.9 | 8.7 | 5.05 | 11.04 |
| MOUSE 3 | 7.374 | 7.053 | 219 | 97 | 27.9 | 28 | −9 | −23 | 16.3 | 7.8 | 6.86 | 13.2 |
Abbreviations: cross-clamp (CC), millimolar (mmol), minutes (m), reperfusion (reper)
The onset and severity of paralysis varies as a function of intraoperative core temperature and aortic cross-clamp time
Supplemental Digital Content 1, figure 2 provides a summary of the relationship between intraoperative core temperature, aortic cross-clamp time, placement of cross-clamp (e.g., aortic arch vs. descending aorta), survival and paralysis. Below is a descriptive account of these data.
35°C
Survival was poor in this group (see Supplemental Digital Content 1, figs. 1– 2), making a detailed analysis of locomotor behavior impossible. Only the onset and severity of functional deficits were recorded. Of those mice subjected to 8–11 min of cross clamp at 35°C (arch+LSA or descending aorta) that survived for a minimum of 4 h postsurgery, 72% (n = 18/25) were paralyzed immediately after recovery from anesthesia, showing flaccid hindlimbs without the ability to weight-support or step. Of the remaining seven mice, two developed paralysis within 24 h of reperfusion and the other five initially maintained the ability to step. These latter mice subsequently died before a detailed behavioral assessment could be completed.
33°C
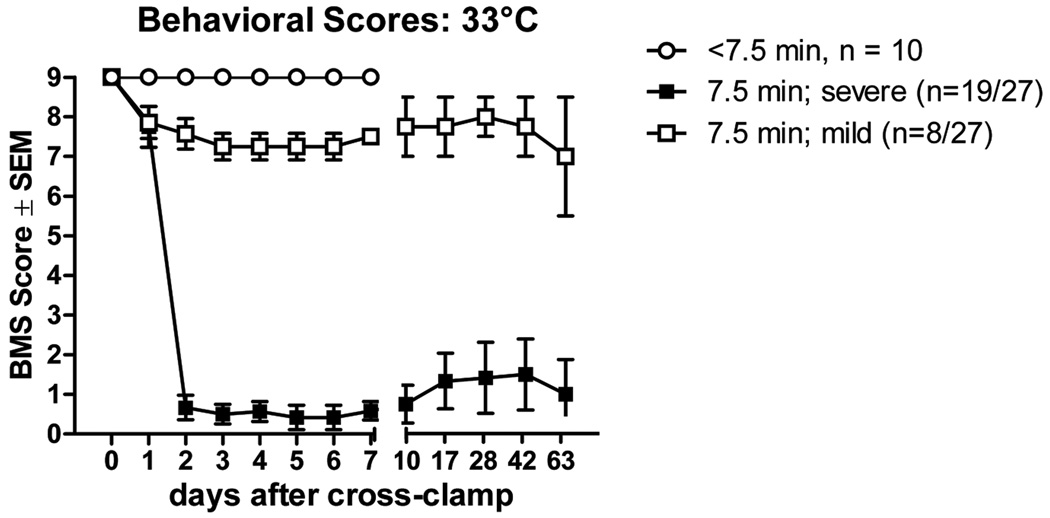
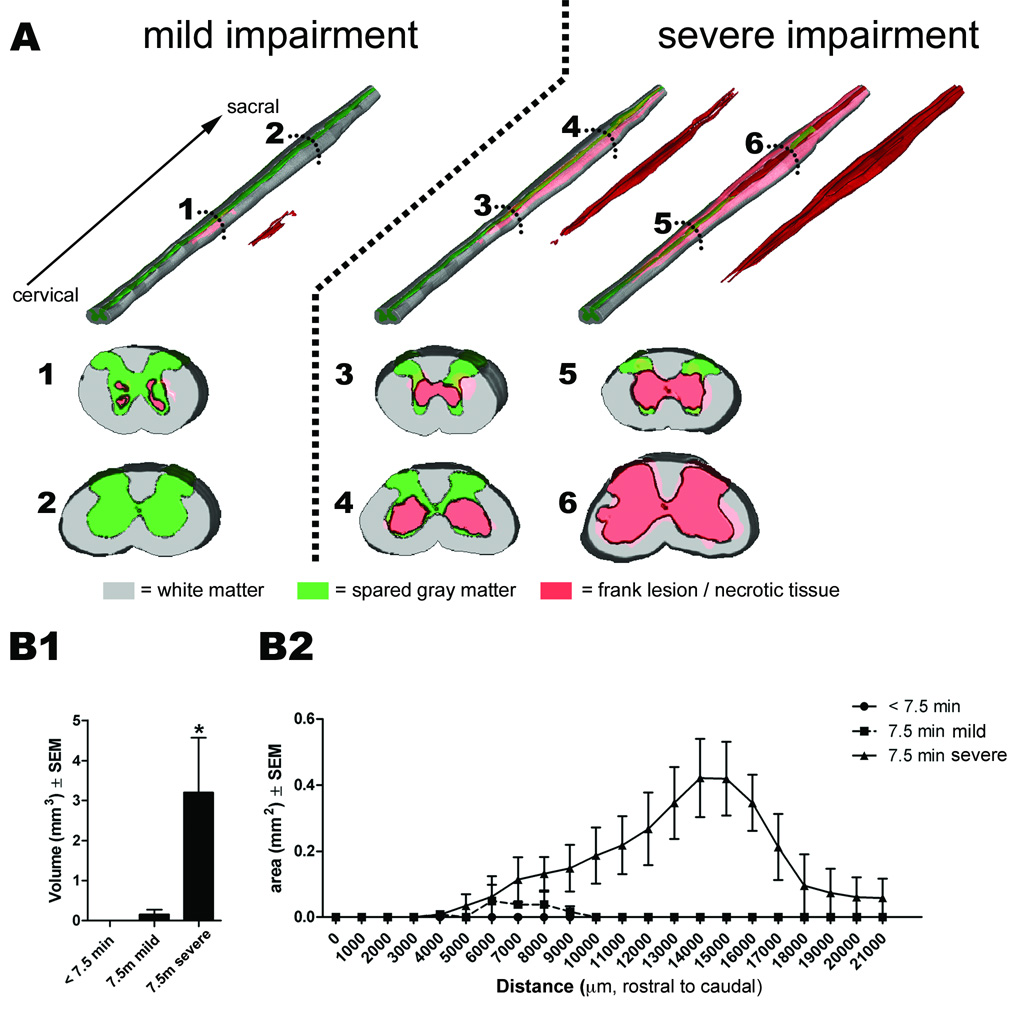
Aortic cross clamp times of 3–7 min failed to produce neurological impairment in (n = 10/11) mice that were evaluated up to 7 days postoperative (fig. 3). In one mouse, aortic cross clamp for 4 min caused immediate hindlimb paralysis and severe seizures. This mouse was euthanized at 40 h postsurgery. By extending the cross-clamp time to 7.5 min at 33°C, we reached a critical threshold at which it was possible to reproducibly cause paralysis without killing the mice. In fact, mice invariably awoke from anesthesia without signs of morbidity or neurological impairment (fig. 3). After ~24 h, gait abnormalities manifested as toe-drags, occasional missed steps, loss of forelimb-hindlimb coordination, a wide base of hindlimb support, external hindpaw rotation during the lift-off phase of the step cycle and/or trunk instability (mean onset of deficits = 40.5 h; range: 20–49 h. Mice that survived for at least 7 days could be stratified into two groups based on severity of their hindlimb deficits (fig. 3). In most cases (n = 19/27 mice), mice showed a progressive decline in hindlimb function that occurred over a period of 6–12 h, with permanent hindlimb paralysis being evident by ~48 h (severe; fig. 3). Conversely, a smaller subset of mice (n = 8 of 27) was able to step with only mild deficits in forelimb-hindlimb coordination or paw rotation (Basso Mouse Scale scores = 6–8). Regardless of the severity of impairment, none of the mice recovered normal locomotor function during the period of analysis (up to 63 days postsurgery; fig. 3). There was no evidence of gross forelimb deficits in any mice.
Figure 3.
Spinal cord pathology varies as a function of intraoperative core temperature and aortic cross-clamp time
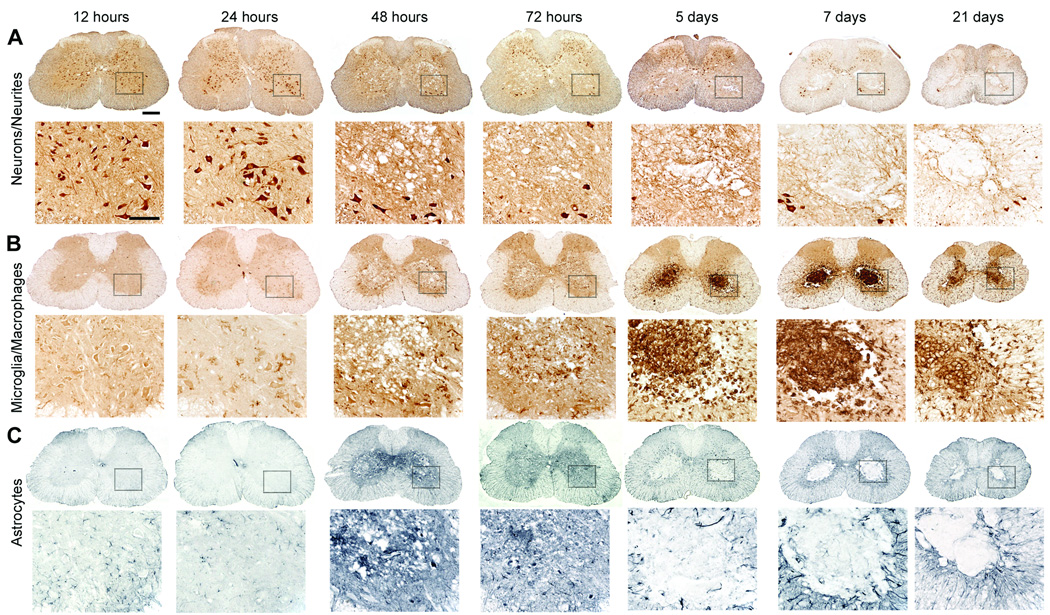
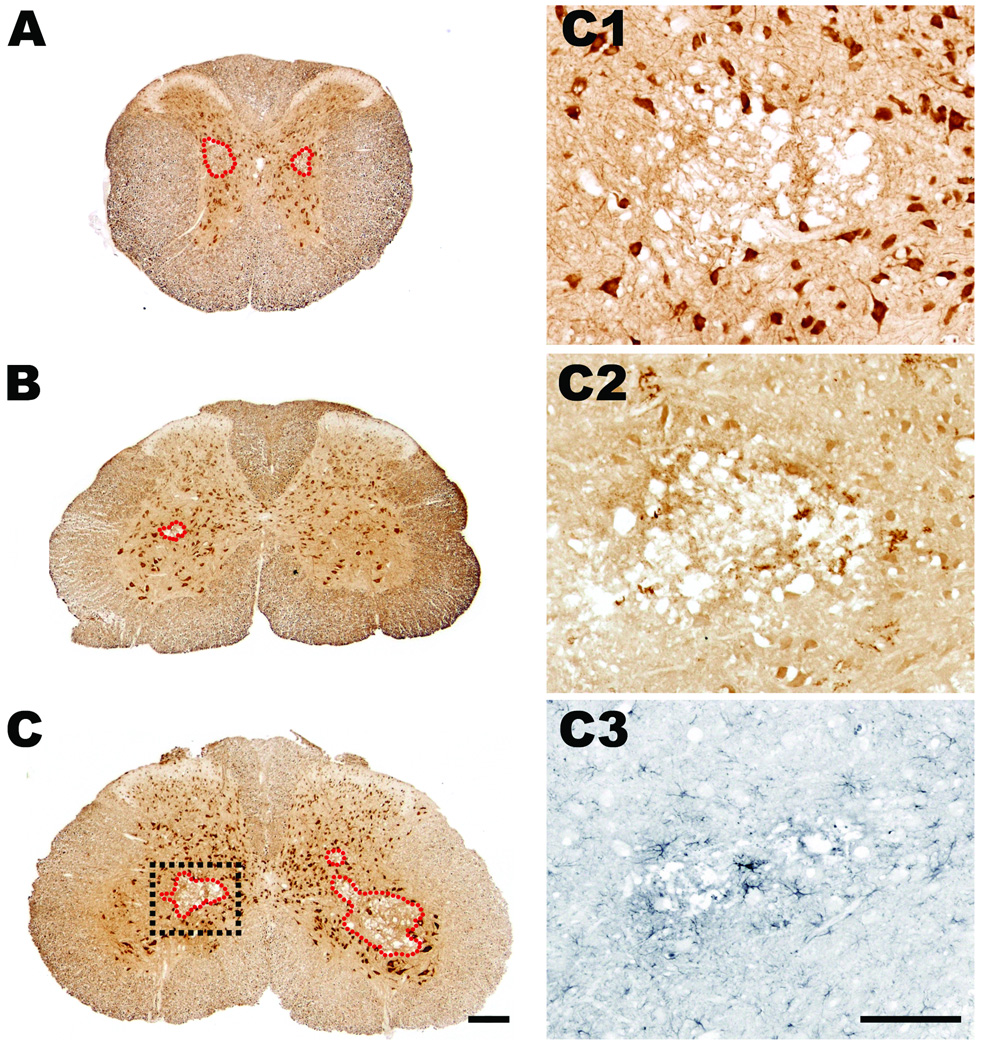
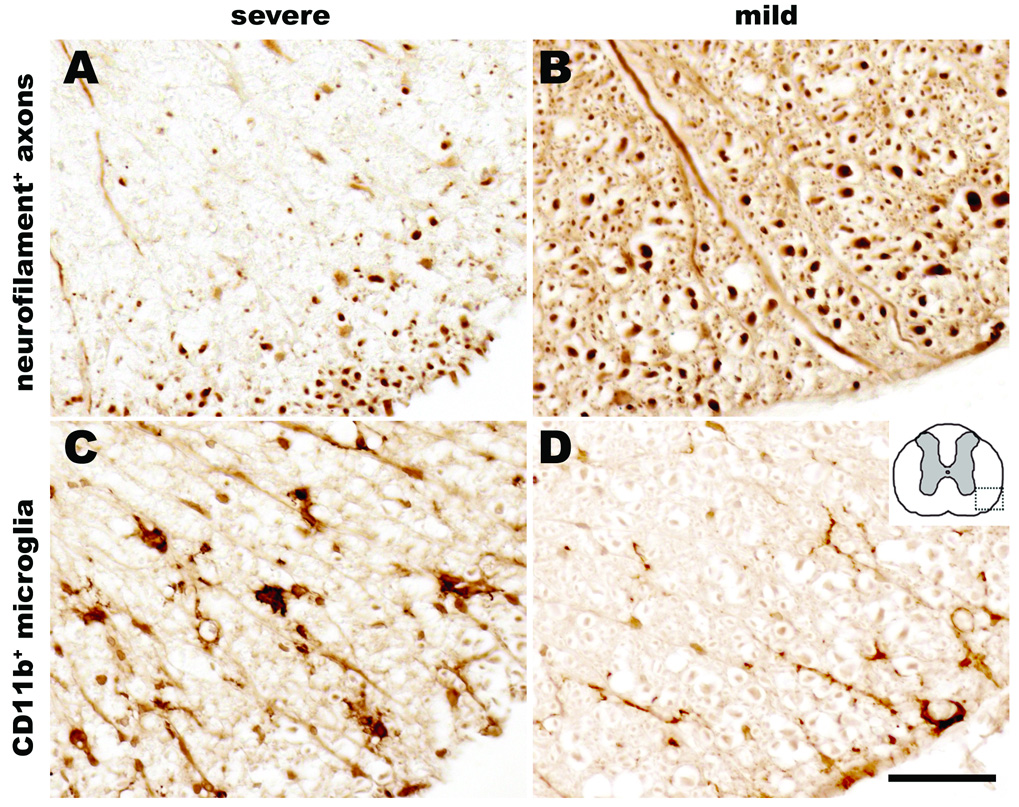
Using routine histology and immunohistochemistry, we qualitatively and quantitatively analyzed the temporal sequence of intraspinal pathology and neuroinflammation that occurs subsequent to cross clamp of the descending aorta for 7.5 min at 33°C. Spinal cords were analyzed at 12 h or 1, 2, 3, 5, 7, 21, 42, or 63 days postreperfusion (n = 4–6/group). In all cases, spinal cord pathology was limited to mid-thoracic (~ T6 spinal level) levels and below. Representative lumbar spinal cord pathology at 21 days postsurgery is shown in figures 4–6. Qualitatively similar pathology is seen at 42 and 63 days (not shown) and in the thoracic spinal cord (see Supplemental Digital Content 1, fig. 4, which shows the temporal progression of neuropathology at the level of the mid-thoracic spinal cord). Generally, the mild or severe functional deficits described in figure 3 predicted the onset, magnitude and rostrocaudal extent of intraspinal pathology, gliosis and neuroinflammation. Figures 4 and 5 illustrate the spatiotemporal progression of pathology in mice that developed severe neurological impairment while figures 6 and 7 illustrate quantitative differences between mild and severely affected mice.
Figure 4.
Figure 6.
Figure 5.
Figure 7.
In most mice, overt pathological changes were not evident until 24 h postsurgery (figs. 4 and 5), a time when hind limb deficits were beginning to appear (see fig. 3). Pathology was evident first as small isolated pockets of necrosis within gray matter, usually in Rexed’s lamina VII lateral to the central canal (fig. 5). Subtle changes in astrocyte and microglia morphology were seen around areas of necrosis and in intact gray matter throughout lamina V and VI and in the ventral horns surrounding neurons (figs. 4 and 5).
Necrosis and lesion expansion progressed between 2–7 days postsurgery, predominantly within the spinal cord gray matter. This pathology was characterized first by loss of ventral horn neurons and then by the formation of a contiguous necrotic lesion dominated by large phagocytic microglia/macrophages. By 7 days, these lesions extended over multiple segments of the mid-thoracic and lumbar spinal cord (fig. 6). Reactive CD11b+ microglia and glial-fibrillary acidic protein+ astrocytes were clearly visible within and surrounding regions of necrosis; activated glia also highlighted white matter tracts in which axons were undergoing anterograde and retrograde degeneration (see figs. 4 and 7). The progressive loss of gray and white matter likely caused the pronounced spinal cord atrophy that was visible by 21 days postsurgery (see fig. 4).
Discussion
During surgical repair of thoracic and thoracoabdominal aortic aneurysms, blood flow to the spinal cord is interrupted for minutes or hours. Throughout this time and reperfusion, irreversible damage to the spinal cord can occur. Although it is well recognized that neurological deficits, including paralysis, are devastating and all-to-common consequences of aortic aneurysm repair1,21, there is no adjunct therapy that effectively eliminates these adverse reactions. Also, there is no way to predict a priori whether paralysis will be immediate or delayed in onset or if specific mechanisms can explain these unique pathological presentations.
The need to minimize paresis and paralysis caused by ISCI subsequent to aortic cross clamp has stimulated the development of pig, canine, rabbit and rat models2,5–9. More recently, a mouse model was developed, making it possible to use transegenic and knock-out mice to reveal the effects of discrete genes and signaling pathways on recovery from ISCI10. In that model, an anterior thoracotomy was performed then aneurysm clips were used to cross clamp the aortic arch and left subclavian artery. The core temperature was maintained at 35–36°C. Using that approach, 60–80% of mice develop hindlimb paralysis but there was limited survival beyond 48 h of reperfusion. Despite our best efforts using that model, we were able to extend survival to 7 days in only a few mice; most mice died within 72 h of reperfusion. This is a significant shortcoming of the model and limits its use for testing therapeutic interventions or understanding mechanisms that regulate chronic changes in spinal cord structure or function caused by ischemia/reperfusion injury.
Here, we present a simplified mouse model of ISCI in which reproducible behavioral deficits and intraspinal pathology can be titrated by manipulating the duration of aortic cross clamp and the core temperature. Also, the effects of ischemia/reperfusion on hemodynamic and blood gas parameters are similar to those measured in humans undergoing aortic repair surgery21. When optimal surgical parameters are used, i.e., cross clamp for 7.5 min at 33°C, mice develop paralysis and > 90% of mice survive indefinitely. In our studies, we maintained mice for as long as 9 weeks postocclusion. The induction and maintenance of systemic hypothermia during the period of aortic cross-clamping and reperfusion appears to be key to ensure this survival. Our model maintains mice at 33°C, a temperature commonly used in the clinic as an adjunct neuroprotective measure during aortic repair and other cardiac surgeries22–25. Future studies will determine the optimal parameters for neuroprotective hypothermia, including the magnitude and duration of cooling and the rate of rewarming. Each of these variables are recognized as critical determinants for the successful translation of hypothermia as a treatment or adjunct to surgical intervention for spinal cord/brain ischemia or traumatic central nervous system injury26,27.
An interesting and unexpected consequence of our new model was the delay in onset of intraspinal pathology and hindlimb dysfunction or paralysis. Indeed, all mice exhibited normal locomotor function for at least 24 h postischemia/reperfusion, after which they all developed mild or severe hindlimb deficits. The onset and magnitude of intraspinal pathology correlated with degree of functional impairment. In nearly all other models of ischemic stroke and spinal cord injury, the onset of neurological impairment is immediate28,29. However, it is becoming increasingly more common in humans for the onset of paralysis subsequent to aortic repair surgery to be delayed4. The reasons for this are not clear, but our new model seems to be an ideal tool for defining the mechanims underlying discrete forms of mild or severe spinal cord pathology with associated changes in neurlogical function.
Our optimal surgical parameters (7.5 min of aortic cross clamp at 33°C) consistently produced two functionally distinct groups of mice; approximately 2/3 were severely-affected by ischemia/reperfusion and the remaining 1/3 were relatively resistent to ischemia/reperfusion injury. Lang-Lazdunski et al. made a similar observation in their model, with ~60% of mice developing complete paralysis and the remaining mice exhibiting milder deficits but with limited survival beyond 48 h10. Thus, it is unlikely that these distinct cohorts are a result of variability in the cross-clamp procedure. Indeed, Lang-Lazdunski et al. confirmed cessation of blood flow using laser doppler. We also used laser doppler (not shown) and real-time monitoring of blood pressure to ensure cessation of blood flow in the femoral artery. However, such detailed monitoring is not essential when the aorta is approached via a lateral thoracotomy (see fig. 1). Indeed, it is easy to visualize then lift the aorta by inserting a wire hook around the vessel. This ensures that an aneurysm clip can be placed across the full diameter of the aorta. We also found that when a second aneurysm clip was placed distal to the first clip (to further ensure complete blockage of perfusion below the clip), a subset of mice still developed only mild hindlimb deficits (not shown). Rather than incomplete occlusion, a more likely explanation for the functionally distinct cohorts of mice is inter-animal variation in vascular anatomy, specifically, collateral branching from the arch vessels and descending aorta above the location of the aneurysm clip30. Ongoing studies will use our new model to characterize the relationship between vascular anatomy and the magnitude of functional impairment caused by ISCI.
Because it is not currently possible to predict a priori whether a mouse will be severely or mildy affected by the aortic cross-clamp procedure, larger groups of animals will be required to detect statistically significant decreases in the ratio of severely- to mildly-affected mice. Such a change would indicate that a given drug has therapeutic effects. However, as our understanding of the mechanisms that regulate the natural stratification of neurological outcome improves, this new mouse model would become ideal for studying therapeutics that target only mild or severely affected mice. Indeed, the application of a “magic bullet” therapy to all forms of central nervous system ischemia/reperfusion injury (or traumatic injury) is impractical. Some drugs may only be effective in a subset of individuals who are less susceptible to the effects of ischemia/reperfusion or trauma. Our model lends itself to comparative analyses of this type, since long-term functional variability is limited to only two cohorts (mild vs. severe) and can be predicted within 48 h.
The lack of obvious pathology within 12 h of ischemia/reperfusion, with a further delay in the onset of functional impairments suggests that the metabolic derangements in neurons and glia caused by ischemia/reperfusion collaborate with other pathogenic cascades to cause cell injury and lesion expansion. Indeed, progressive necrosis, axonal degeneration and intraspinal inflammation occur over multiple segments of the spinal cord between 2 and 7 days of reperfusion. This delay means that it might be possible to minimize tissue damage and preserve neurological function by inhibiting cellular and molecular pathways activated downstream of ischemia and reperfusion. Several cascades including neuroinflammation, excitotoxicity, oxidative stress, channelopathy etc. have been implicated and can be explored with improved resolution with the mouse ISCI model1,9,31.
Recognizing subclinical pathology during or immediately after aortic repair surgery in humans is difficult since neither electromyography nor strength/sensory tests are performed routinely32 and most patients are discharged without reporting overt neurological complications. This, however, does not mean that the spinal cord is not damaged or that neurological impairment will not develop. In fact, during surgery it is not uncommon to see red blood cells and activated lymphocytes accumulate and turbidity increase in cerebrospinal fluid drained from the lumbosacral spinal cord of these individuals (personal observations, Hamdy Awad M.D., Novermber, 2007, Ohio State University, Columbus Ohio). It is not known if this or other signs of intraspinal damage can be used to predict subclinical pathology or neurological impairment; however, this should be possible using perioperative electromyography, rigorous postoperative neurological exams or biomarker analysis of cerebrospinal fluid samples obtained during routine cerebrospinal fluid drainage. Our new mouse model may be useful for establishing these correlations in both acute and chronic subjects.
Although our new model of ISCI recapitulates many aspects of the clinical condition in humans, a consequence of the ischemia/reperfusion insult that is unique to the mouse model is the acute but transient development of mild seizure-like events. These were seen in ~50% of the mice when cross-clamp was applied at 33°C. Presently, it is not clear why this happens but it seems logical that dramatic changes in cerebral blood pressure (as measured in the carotid arteries proximal to the site of occlusion; see Supplemental Digital Content 1, fig. 3) or systemic pathology caused by impaired acid-base balance could contribute (see table 2). In humans, complete or partial cardiopulmonary bypass during cross-clamp alleviates cerebral hypertension and facilitates maintenance of acid–base balance before blood is returned to the circulation. Regardless of mechanism, these events were transient and once they passed, did not adversely affect long-term survival. At this time, we also do not know if a truly postictal state exists in these mice. This is an important consideration since it could influence behavioral evaluation. While the seizure-like events are responsive to ketamine (known to inhibit spontaneous neuronal depolarization and cortical spreading depression)14–16, more sophisticated electrophysiological and behavioral analyses would be needed to define these post-ischemia/reperfusion events as conventional seizures. Both analyses would be difficult given the unpredictable nature of the seizure-like events. Even if the mice were truly postictal, preservation and recovery of spontaneous motor function in mice with seizure-like events was indistinguishable from that of mice that did not experience these events.
Our new model of mouse ISCI offers several advantages over existing models. First, mice are relatively inexpensive compared to pigs, dogs, rabbits or rats. Second, by using transgenic/knockout mice, it will be possible to manipulate specific genes and cell signaling pathways that are known to cause or contribute to secondary central nervous system injury or repair. Third, the surgical approach that we used and the changes in hemodynamics and blood gases that occur with this model of aortic cross clamp mimic what occurs in humans and . Finally, because mice survive indefinitely with our optimized protocol, the consequences of ischemia/reperfusion can be studied for weeks or months after injury. Together, these features of the model should simplify the preclinical development and testing of neuroprotective interventions for ISCI.
Supplementary Material
Acknowledgments
Funding: NS047175 (National Institute of Neurological Disorders and Stroke, Bethesda, Maryland), NS37846 (National Institute of Neurological Disorders and Stroke, Bethesda, Maryland), P30-NS045758 (National Institute of Neurological Disorders and Stroke, Bethesda, Maryland), NS059776 (National Institute of Neurological Disorders and Stroke, Bethesda, Maryland) and the Ohio State University Department of Anesthesiology (Columbus, Ohio).
Irina Shakhnovich, M.D., Surgical Resident, Department of Surgery, Ohio State University Medical Center, Columbus Ohio; Mohamed Abd-Eldayem, M.D., Cardiothoracic Fellow, Department of Anesthesiology, Ohio State University Medical Center, Columbus, Ohio; Ming Wang, M.D., Research Associate, Department of Neuroscience, Ohio State University Medical Center, Columbus Ohio.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Summary Statement: We report the development of a new murine model of ischemic spinal cord damage caused by transient aortic cross clamp. Using optimal parameters, this model produces > 95% survival and results in delayed paralysis and neuropathology.
References
- 1.Svensson LG. Paralysis after aortic surgery: In search of lost cord function. Surgeon. 2005;3:396–405. doi: 10.1016/s1479-666x(05)80050-2. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg RK, Lu Q, Roselli EE, Svensson LG, Moon MC, Hernandez AV, Dowdall J, Cury M, Francis C, Pfaff K, Clair DG, Ouriel K, Lytle BW. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: A comparison of endovascular and open techniques. Circulation. 2008;118:808–817. doi: 10.1161/CIRCULATIONAHA.108.769695. [DOI] [PubMed] [Google Scholar]
- 3.Cambria RP, Clouse WD, Davison JK, Dunn PF, Corey M, Dorer D. Thoracoabdominal aneurysm repair: Results with 337 operations performed over a 15-year interval. Ann Surg. 2002;236:471–479. doi: 10.1097/00000658-200210000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong DR, Coselli JS, Amerman K, Bozinovski J, Carter SA, Vaughn WK, LeMaire SA. Delayed spinal cord deficits after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2007;83:1345–1355. doi: 10.1016/j.athoracsur.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 5.Zivin JA, DeGirolami U. Spinal cord infarction: A highly reproducible stroke model. Stroke. 1980;11:200–202. doi: 10.1161/01.str.11.2.200. [DOI] [PubMed] [Google Scholar]
- 6.LeMay DR, Neal S, Zelenock GB, D'Alecy LG. Paraplegia in the rat induced by aortic cross-clamping: Model characterization and glucose exacerbation of neurologic deficit. J Vasc Surg. 1987;6:383–390. [PubMed] [Google Scholar]
- 7.Gulya K, Tekulics P, Kasa P. Effects of ischemia on opioid receptors in newborn pig lumbar spinal cord. Acta Biol Hung. 1989;40:229–235. [PubMed] [Google Scholar]
- 8.Lehr EJ, Coe JY, Ross DB. An intra-aortic shunt prevents paralysis during aortic surgery in sheep. J Surg Res. 2007;141:78–82. doi: 10.1016/j.jss.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 9.Awad H, Suntres Z, Heijmans J, Smeak D, Bergdall-Costell V, Christofi FL, Magro C, Oglesbee M. Intracellular and extracellular expression of the major inducible 70kDa heat shock protein in experimental ischemia-reperfusion injury of the spinal cord. Exp Neurol. 2008;212:275–284. doi: 10.1016/j.expneurol.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Lang-Lazdunski L, Matsushita K, Hirt L, Waeber C, Vonsattel JP, Moskowitz MA, Dietrich WD. Spinal cord ischemia. Development of a model in the mouse. Stroke. 2000;31:208–213. doi: 10.1161/01.str.31.1.208. [DOI] [PubMed] [Google Scholar]
- 11.Casey PJ, Black JH, Szabo C, Frosch M, Albadawi H, Chen M, Cambria RP, Watkins MT. Poly(adenosine diphosphate ribose) polymerase inhibition modulates spinal cord dysfunction after thoracoabdominal aortic ischemia-reperfusion. J Vasc Surg. 2005;41:99–107. doi: 10.1016/j.jvs.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 12.Black JH, Casey PJ, Albadawi H, Cambria RP, Watkins MT. Poly adenosine diphosphate-ribose polymerase inhibitor PJ34 abolishes systemic proinflammatory responses to thoracic aortic ischemia and reperfusion. J Am Coll Surg. 2006;203:44–53. doi: 10.1016/j.jamcollsurg.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Stone DH, Albadawi H, Conrad MF, Entabi F, Stoner MC, Casey PJ, Cambria RP, Watkins MT. PJ34, a poly-ADP-ribose polymerase inhibitor, modulates visceral mitochondrial activity and CD14 expression following thoracic aortic ischemia-reperfusion. Am J Surg. 2009;198:250–255. doi: 10.1016/j.amjsurg.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Guler G, Erdogan F, Golgeli A, Akin A, Boyaci A. Ketamine reduces lidocaine-induced seizures in mice. Int J Neurosci. 2005;115:1239–1244. doi: 10.1080/00207450590914617. [DOI] [PubMed] [Google Scholar]
- 15.Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49:248–255. doi: 10.1111/j.1528-1167.2007.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruss H, Holtkamp M. Ketamine successfully terminates malignant status epilepticus. Epilepsy Res. 2008;82:219–222. doi: 10.1016/j.eplepsyres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 18.Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119:2990–2999. doi: 10.1172/JCI39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- 20.Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol. 2004;190:17–31. doi: 10.1016/j.expneurol.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 21.Svensson LG, Hinder RA. Hemodynamics of aortic cross-clamping: Experimental observations and clinical applications. Surg Annu. 1987;19:41–65. [PubMed] [Google Scholar]
- 22.Bicknell CD, Riga CV, Wolfe JH. Prevention of paraplegia during thoracoabdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2009;37:654–660. doi: 10.1016/j.ejvs.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Kwon BK, Mann C, Sohn HM, Hilibrand AS, Phillips FM, Wang JC, Fehlings MG. Hypothermia for spinal cord injury. Spine J. 2008;8:859–874. doi: 10.1016/j.spinee.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Svensson LG, Khitin L, Nadolny EM, Kimmel WA. Systemic temperature and paralysis after thoracoabdominal and descending aortic operations. Arch Surg. 2003;138:175–179. doi: 10.1001/archsurg.138.2.175. [DOI] [PubMed] [Google Scholar]
- 25.Strauch JT, Lauten A, Spielvogel D, Rinke S, Zhang N, Weisz D, Bodian CA, Griepp RB. Mild hypothermia protects the spinal cord from ischemic injury in a chronic porcine model. Eur J Cardiothorac Surg. 2004;25:708–715. doi: 10.1016/j.ejcts.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma. 2009;26:301–312. doi: 10.1089/neu.2008.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Povlishock JT, Wei EP. Posthypothermic rewarming considerations following traumatic brain injury. J Neurotrauma. 2009;26:333–340. doi: 10.1089/neu.2008.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsala M, Vanicky I, Yaksh TL. Effect of graded hypothermia (27 degrees to 34 degrees C) on behavioral function, histopathology, and spinal blood flow after spinal ischemia in rat. Stroke. 1994;25:2038–2046. doi: 10.1161/01.str.25.10.2038. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto S, Matsumoto M, Yamashita A, Ohtake K, Ishida K, Morimoto Y, Sakabe T. The temporal profile of the reaction of microglia, astrocytes, and macrophages in the delayed onset paraplegia after transient spinal cord ischemia in rabbits. Anesth Analg. 2003;96:1777–1784. doi: 10.1213/01.ANE.0000064204.67561.73. [DOI] [PubMed] [Google Scholar]
- 30.Svensson LG, Hess KR, Coselli JS, Safi HJ. Influence of segmental arteries, extent, and atriofemoral bypass on postoperative paraplegia after thoracoabdominal aortic operations. J Vasc Surg. 1994;20:255–262. doi: 10.1016/0741-5214(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita K, Wu Y, Qiu J, Lang-Lazdunski L, Hirt L, Waeber C, Hyman BT, Yuan J, Moskowitz MA. Fas receptor and neuronal cell death after spinal cord ischemia. J Neurosci. 2000;20:6879–6887. doi: 10.1523/JNEUROSCI.20-18-06879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svensson LG, Patel V, Robinson MF, Ueda T, Roehm JO, Jr, Crawford ES. Influence of preservation or perfusion of intraoperatively identified spinal cord blood supply on spinal motor evoked potentials and paraplegia after aortic surgery. J Vasc Surg. 1991;13:355–365. doi: 10.1067/mva.1991.26137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.