Abstract
All decisions about initiating, continuing, or stopping therapy of the hepatitis B virus (HBV) during pregnancy must include an analysis of the risks and benefits for mother and fetus. The trimester of the pregnancy and the stage of the mother’s liver disease are important factors. Treatment in the third trimester may be initiated to aid in preventing perinatal transmission, which appears to be most pronounced in mothers with high viral loads. Consideration of initiating treatment in the third trimester should occur after a high viral load is documented in the latter part of the second trimester, to allow adequate time for initiation of antiviral therapy with significant viral suppression before delivery. This discussion should include the topic of breastfeeding, because it is generally not recommended while receiving antiviral therapy. Currently, lamivudine and tenofovir appear to be the therapeutic options with the most reasonable safety data in pregnancy.
Keywords: Hepatitis B, Pregnancy, Perinatal transmission, Treatment, Antiviral, Nucleoside, Nucleotide, Lamivudine, Tenofovir
Introduction
It is estimated that 350 million to 400 million individuals worldwide are chronically infected with the hepatitis B virus (HBV) [1]. In regions with high prevalence, infection is most commonly acquired through either perinatal or horizontal transmission [2, 3]. The risk of progression to chronic HBV infection is inversely proportional to the age at which the infection was acquired. Without immunoprophylaxis, up to 90% of infants born to hepatitis B e antigen (HBeAg)–positive mothers become infected. In contrast, only 20% to 30% of children exposed between ages 1 and 5 years, and fewer than 5% of adults, become infected [4–6]. Thus, women of childbearing age with chronic HBV infection remain an important source for continued spread of the virus.
This article addresses several issues pertinent to hepatitis B therapy in pregnancy. First, the woman of childbearing age who may require therapy for hepatitis B is discussed, with particular emphasis on the timing of therapy, choice of an agent, and the patient’s desire to have children in the future. Second, an approach is outlined for the woman who is newly diagnosed with hepatitis B early in pregnancy. In addition, whether therapy should be continued, switched, or stopped if an HBV-infected woman is on treatment and becomes pregnant is debated. Finally, the question of whether a pregnant woman should be treated in the third trimester to help prevent perinatal transmission is addressed in a proposed algorithm for the management of HBV in pregnancy.
Therapy for HBV in Women of Childbearing Age
Seven therapies are approved by the US Food and Drug Administration (FDA) for the treatment of hepatitis B, including interferon (both standard and pegylated), lamivudine, adefovir, entecavir, telbivudine, and tenofovir [3]. Factors that influence treatment choice in women of childbearing age include safety in pregnancy and breastfeeding, efficacy of the agent, its barrier to resistance, and proposed length of therapy. If pregnancy is contemplated in the near future, it may be prudent to delay therapy until after the child is born [7]. This approach requires a careful assessment of the degree of hepatic activity and fibrosis, with either liver biopsy or noninvasive methods. Although it is not used in the pregnant woman, interferon can be used in the woman of childbearing age, because therapy with this agent is for a defined period (48 weeks) and often results in clinical remission with HBeAg seroconversion [8]. This scenario is in contrast to the oral antiviral agents that generally require long-term therapy and result in much lower rates of HBeAg seroconversion [3, 7].
For those who require therapy, it is advisable to discuss the issue of pregnancy before starting treatment. A “planned pregnancy” is preferable and may influence the choice and timing of therapy, or potentially the timing of pregnancy. In addition, given the relative paucity of evidence for most recommendations, decisions about treatment during pregnancy can be made with the luxury of time for consideration of all relevant issues. Treatment with tenofovir is an ideal choice, given its efficacy, high barrier to resistance, and safety profile in pregnancy [9, 10]. Lamivudine, another agent that is considered safe in pregnancy, has a high chance of emergence of resistant virus with prolonged therapy and therefore is no longer a first-line agent in the nonpregnant patient [3, 7, 10].
Newly Diagnosed Chronic HBV in Pregnancy
The pregnant woman who is newly diagnosed with HBV early in pregnancy should undergo an assessment of her infection. Decisions about initiating therapy in this setting must include consideration of the risks and benefits for the mother and the fetus. The risk-benefit equation also depends upon the trimester of the pregnancy. The major determinant of the need for HBV therapy for the mother is the stage of her liver disease (both hepatic activity and fibrosis) [7]. Treatment is generally recommended if the mother is at risk of serious liver disease. Most women of childbearing age are likely to have mild disease; therefore, treatment can usually be postponed until after delivery. Because many of these women are in the immune-tolerant phase of infection (high HBV DNA levels with normal alanine transaminase [ALT] and inactive liver biopsy), therapy is generally not needed and no indication exists to start therapy during the early stages of pregnancy [3, 7].
Therapy for Hepatitis B in Early Pregnancy
No antiviral agent has been approved by the FDA for use in pregnancy. Thus, when a woman on HBV antiviral therapy becomes pregnant, a decision needs to be made whether she should continue therapy for the duration of the pregnancy or if therapy should be withdrawn immediately. As with all decisions during pregnancy, the health of the mother and the fetus must be considered independently. From the perspective of the fetus, the major concern is the risk of exposure to medication during early embryogenesis. From the perspective of the mother, the major issue is whether stopping or changing medication will adversely affect both short- and long-term liver disease outcomes. In general, if the mother is known to have significant fibrosis, therapy should be continued because the risk of flare with withdrawal of therapy could result in decompensation of her liver disease. This effect on the mother’s health could also impact the health of the fetus.
All HBV antivirals are inhibitors of either nucleoside or nucleotide polymerases. Although these drugs preferentially target the RNA-dependent DNA polymerase of HBV, they also interfere with replication of mitochondrial DNA, and this can result in mitochondrial toxicity leading to the lactic acidosis syndrome [11]. Although lactic acidosis syndrome is very uncommon in adults, less is known about the potential ramifications of mitochondrial toxicity in the developing fetus. These effects may be more diverse, because toxicity may affect organogenesis.
Safety data on HBV antivirals during pregnancy come from two major sources: the Antiretroviral Pregnancy Registry (APR) [10] and the Development of Antiretroviral Therapy Study (DART) [12]. The APR is an international, voluntary, prospective exposure registration cohort study of women exposed to antiretroviral therapies, most of whom are HIV-1 monoinfected. As of January 2010, data from 11,867 pregnancies were available. However, these data included only 112 women with HBV monoinfection, in whom birth defects were recorded in 2.7% of live births. This statistic compares favorably to the 2.72% rate reported by the Centers for Disease Control and Prevention (CDC) birth defect surveillance system. Table 1 shows the number of cases of early versus late exposure and the rate of birth defects for each of the FDA-approved HBV antivirals. No significant difference was reported in the rate of adverse outcomes if the initial exposure of any HBV drug was in the first trimester (2.7%) compared to the second or third trimester (2.5%) of pregnancy. Although these data are reassuring, it is important to look at the HBV agents specifically. Lamivudine and tenofovir are the two agents with the most in vivo experience in the first trimester and appear to be safe. For telbivudine and entecavir, only 5 and 12 pregnancies with exposure in the first trimester are recorded in the registry, with no adverse outcomes reported. Although the APR is extremely useful, it has limitations, including short follow-up and recording only defects identified at birth. Developmental anomalies (eg, cardiac or neurologic defects) identified at a later date may therefore be omitted.
Table 1.
Antiretroviral pregnancy registry data
| Agent | Earliest trimester of exposure | |
|---|---|---|
| 1st trimester birth defects/live births | 2nd/3rd trimester birth defects/live births | |
| Lamivudine | 99/3481 (2.8%) | 130/5194 (2.5%) |
| Adefovir | 0/37 | 0/0 |
| Telbivudine | 0/5 | 0/3 |
| Tenofovir | 19/879 (2.2%) | 11/501 (2.2%) |
| Entecavir | 0/12 | 0/2 |
| Any HBV nucleoside/nucleotide | 118/4414 (2.7%) | 141/5700 (2.5%) |
HBV—hepatitis B virus
The DART study is a 6-year, multicenter, randomized trial of antiretroviral therapy among adults with symptomatic HIV-1 infection or advanced disease/AIDS in Africa. The 3% rate of congenital anomalies reported in this study also compares favorably to the 2.72% reported by the CDC birth defect surveillance system [12].
Continuing, Switching, or Discontinuing Therapy?
If the decision is made to continue HBV therapy during pregnancy, the question then becomes whether the drug should be replaced with an agent that has more in vivo experience during pregnancy (ie, is thought to be “safer”). For example, because safety data are lacking for entecavir, and because it is an FDA pregnancy class C drug (Table 2), switching to an alternate therapy should be strongly considered [13]. If desired, the original treatment could eventually be resumed after delivery. The two most commonly used agents in pregnancy are lamivudine and tenofovir. Lamivudine is also categorized as a class C agent by the FDA because of reports of toxicity in rabbits with first trimester exposure. However, because it was the first oral agent approved for the treatment of HBV, extensive clinical experience exists. The APR data also suggest that lamivudine is safe despite its pregnancy C classification. Although there is less clinical experience with tenofovir, it is categorized as a class B agent by the FDA and has the added benefit of a very high genetic barrier to resistance, with no reported resistance identified to date [13, 14]. However, telbivudine, another class B agent, is seldom used, for two reasons [13]. First, there is minimal in vivo experience in pregnancy, and second, telbivudine has a low barrier to resistance [15].
Table 2.
Food and drug administration pregnancy categories
| Pregnancy category A | Adequate and well-controlled human studies have failed to demonstrate a risk to the fetus in the first trimester of pregnancy (and there is no evidence of risk in later trimesters) |
| Pregnancy category B | Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women, or animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester |
| Pregnancy category C | Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks |
| Pregnancy category D | There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks |
| Pregnancy category X | Studies in animals or humans have demonstrated fetal abnormalities and/or there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in use of the drug in pregnant women clearly outweigh potential benefits |
Rather than switching agents, withdrawal of treatment for the duration of pregnancy may be preferable, especially to the mother who wants to avoid any potential future risk to the fetus. What would be the consequence to the mother of stopping treatment completely? The natural history of chronic HBV in pregnancy has not been well described. Limited data exist to suggest that, rarely, severe complications of HBV occur late in pregnancy, with reports of liver failure requiring liver transplantation in previously asymptomatic individuals [16]. Data specifically addressing the risk of stopping therapy during pregnancy are anecdotal. Our knowledge of those risks relating to cessation of therapy is derived from early clinical trials in nonpregnant patients, with less advanced fibrosis. In these early studies, therapy was stopped after completion of the trial, even for patients who remained HBeAg-positive. Follow-up confirmed that HBV DNA levels rebounded, but rarely did this rebound result in clinically significant flares of hepatitis [17, 18]. In contrast, in those patients with severe fibrosis or cirrhosis at baseline, flares upon treatment withdrawal can result in decompensation [19].
Overall, it appears the risk of an adverse outcome with continuing antiviral therapy during pregnancy is likely very low. However, therapy could be discontinued with close observation of the mother to avoid continued fetal exposure during the first trimester, especially in the patient who does not have advanced fibrosis.
Rationale for Treatment in the Third Trimester
The majority of perinatal transmission is thought to occur at delivery, because a combination of passive immunization with hepatitis B immunoglobulin (HBIG) given within 12 h of birth and active immunization with three doses of the hepatitis B vaccine in the first 6 months of life resulted in preventing most infections in this setting. Early seminal studies by Beasley et al. [20, 21] showed that HBIG administration could reduce the rate of HBV transmission from more than 90% from HBeAg-positive mothers down to about 26%. When combined with the vaccine, the rates of transmission fell to 3% to 7% [22, 23]. When one looks at the vaccine and HBIG failures, almost all occur in HBeAg-positive women with very high viral loads, generally above 108 copies/mL [24]. A recent report suggested an overall 3% perinatal transmission rate in viremic mothers despite the use of immunoprophylaxis [25•]. The rate was as high as 7% in viremic HBeAg-positive mothers and 9% in those mothers who had viral loads greater than 108 copies/mL. This finding raises the question of whether antiviral therapy before delivery would lower the viral load adequately to prevent transmission.
Evidence for Treatment in the Third Trimester
Should pregnant women who are HBsAg-positive and highly viremic receive antiviral therapy in the third trimester to prevent perinatal transmission? No consensus on this issue has yet been reached [7]. The principal of treatment late in pregnancy to prevent or reduce the rate of perinatal transmission has been established with other viruses. In HIV-infected mothers, the effect of antiretroviral agents (including lamivudine) on the reduction of mother-to-child transmission of HIV was shown to be efficacious [26]. Antivirals have also been advocated late in pregnancy to prevent acquisition of neonatal herpes [27].
In a pilot study, eight women with HBV DNA levels greater than 109 copies/mL were given lamivudine at 34 weeks of gestation. Babies were vaccinated and received HBIG at birth, and only one became infected, compared to 7 of 25 (28%) cases of transmission in a matched historical control population [28]. This finding led to a randomized, double-blind, placebo-controlled trial of lamivudine to prevent transmission in highly viremic HBeAg-positive women [29•]. At 1 year of age, 18% of babies of lamivudine-treated mothers were HBsAg-positive compared to 39% in the placebo-treated arm. Both groups received vaccination and HBIG. Based on these results, the authors recommended treatment in the third trimester for women with high viral loads. Unfortunately, because of major problems with loss to follow-up, the data are extremely hard to interpret. At 1 year, 13% of babies in the lamivudine arm and 31% in the placebo arm were lost to follow-up. Evaluating only those with complete data, there was a trend but no significant difference in the rate of HBsAg positivity at 1 year (6% lamivudine vs 12% placebo, P = 0.37). The study was also underpowered (power = 53%), because of slow recruitment. No consequences were reported to mother or baby with lamivudine treatment in the study. More recently, a meta-analysis was reported to evaluate the efficacy of lamivudine in reducing in utero transmission of HBV [30•]. A total of 10 randomized controlled trials (RCTs) examining 951 HBV-carrier mothers were included [29•, 31–39]. The RCTs evaluated included newborns who received immunoprophylaxis at birth and women who were treated with lamivudine from 24 to 32 weeks of gestation, until delivery to 1 month post-delivery. Newborns in the lamivudine group had a 13% to 24% significantly lower incidence of intrauterine exposure, indicated by newborn HBsAg (P = 0.04) and HBV DNA (P < 0.001) positivity. In addition, newborns in the lamivudine treated group had a 1.4% to 2% lower perinatal infection rate at 9 to 12 months, as indicated by HBsAg (P < 0.01) and HBV DNA (P < 0.001) seropositivity. This report was limited by the quality of the studies included (most studies were only rated 3 of 5 on the Jadad scale [40]) and the heterogeneity of the definition used for high viral load that prompted therapy.
In a recent study of 31 pregnant women in China, treatment with telbivudine was commenced between 28 and 32 weeks and was continued to 30 days postpartum [41]. Compared with 30 pregnant patients who did not receive therapy and had similar viral loads at baseline and at parturition, the viral loads in the patients treated with telbivudine were reduced significantly, from 7.38 log10 at baseline to 4.08 log10 prior to parturition (P < 0.01). All babies received active and passive immunoprophylaxis. The infection rate was 0% in those treated with telbivudine and 13.3% in the untreated controls.
No comparable data exist on efficacy of tenofovir in this area. However, given its potency, tenofovir would be expected to be at least equally as effective as lamivudine in reducing perinatal transmission. This characteristic, together with its high barrier to resistance, makes it an attractive agent to consider in the third trimester.
Although treatment in the third trimester would be anticipated to result in a significant lowering of the viral load (which would help prevent perinatal transmission), this result has not been clearly shown and remains controversial. In addition, although a high viral load is clearly important, it is not the only factor predisposing to failure of immunoprophylaxis. This is highlighted by a case in which a child developed chronic HBV infection, despite suppression of HBV DNA to undetectable levels in the mother with lamivudine therapy throughout gestation and appropriate immunoprophylaxis after birth [42]. Rarely, infection may occur in utero, particularly in the setting of threatened preterm labor with very high maternal viral loads [43]. Furthermore, long-term safety data are lacking, and potential risks to the mother include the development of antiviral resistance (relatively unlikely, given the short duration of therapy) and flare of hepatitis after treatment withdrawal. In one study, 42% of those who did not receive antiviral therapy during pregnancy experienced a flare in the postpartum period, compared to 62% among those who had been treated and then discontinued therapy at delivery [44].
Algorithm for Management of HBV in the Pregnant Patient
An algorithm for the management and treatment of HBV in pregnancy is proposed in Fig. 1. Routine antepartum care includes testing a woman for the presence or absence of hepatitis B in the first trimester. If she is negative, her child will be vaccinated at birth. The mother does not have to be vaccinated during pregnancy, although it is considered safe and should therefore be administered to those with high-risk behavior for acquisition.
Fig. 1.
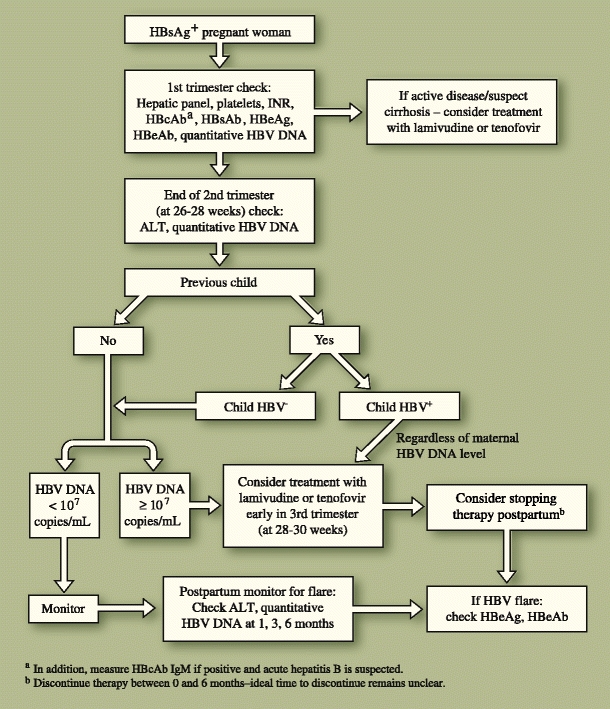
An algorithm for management of hepatitis B virus (HBV) in the pregnant patient. ALT—alanine transaminase; HBcAb—hepatitis B core antibody (total); HBeAb—hepatitis B e antibody; HBeAg—hepatitis B e antigen; HBsAb—hepatitis B surface antibody; HBsAg—hepatitis B surface antigen
For those who test positive early in pregnancy, an assessment of the mother’s HBV status should occur, including a hepatic panel, HBV serologies, and platelet count. If the patient has very active HBV (significantly elevated ALT with a high viral load), or if cirrhosis is suspected (low platelet count or suggestive imaging), then therapy should be initiated regardless of trimester. However, if therapy is not warranted (inactive disease with low ALT and low viral load), continued surveillance is suggested because pregnancy can result in a flare of hepatitis B, later in pregnancy and for several months postpartum [16, 44].
Subsequently, it is recommended that all women have their HBV DNA viral load quantified toward the end of the second trimester (at 26–28 weeks gestation) so that a final decision regarding therapy can occur shortly thereafter. This assessment will allow enough time in the third trimester to significantly drop the viral load after therapy is initiated to decrease the perinatal transmission rate. Women with high viral loads (>107 copies/mL) should consider initiating therapy early in the third trimester (28–30 weeks), after a thorough discussion of the risks and benefits, because data are too limited to mandate therapy. Once started, therapy is continued for the duration of the pregnancy and can be discontinued postpartum if desired. The decision to discontinue therapy is often influenced by the woman’s desire for subsequent pregnancies. The timing of therapy discontinuation postpartum remains unclear. In published studies, therapy is discontinued anywhere from birth to 1 month after delivery [30•]. In practice, therapy is often continued up to 6 months postpartum. Regardless of when therapy is discontinued, a small but real risk of flare exists, and the mother should be monitored closely after withdrawal for at least 6 months. Another factor that may influence the timing of treatment discontinuation postpartum is the mother’s desire to breastfeed. Few data are available regarding the safety of breastfeeding while on antiviral therapy; thus, breastfeeding while on treatment for HBV is not recommended [45, 46].
When deciding on therapy in the third trimester, the perinatal transmission outcome of prior pregnancies must be considered. If previous pregnancies did not result in perinatal transmission, then a viral load of greater than 107 copies/mL should be used to determine if therapy should be initiated (similar to the woman who has had no previous children). However, if perinatal transmission did occur with a prior pregnancy, then the risk of perinatal transmission in the current pregnancy is likely higher [7]. In such cases, strong consideration for initiating therapy in the third trimester is recommended, regardless of the mother’s viral load at the end of the second trimester.
Transmission of HBV Infection in Breastfed Babies
Although early studies claimed that HBV transmission could occur through breast milk, more recent studies have shown similar rates of acquisition, regardless of whether babies were fed with breast milk or formula. In 1975, before the availability of neonatal immunization, the rates of acquisition of HBV were found to be 53% in breast-fed and 60% in formula-fed babies born to HBsAg-positive mothers [47]. These data are limited because the high vertical transmission rates confounded the true rate of acquisition from breastfeeding. After the introduction of immunoprophylaxis, Hill et al. [48] found a similar rate of infection in breast-fed and formula-fed infants (0% and 3%). Thus, current guidelines state that breastfeeding is not contraindicated in HBV-infected mothers who are not on antiviral therapy whose infants receive immunoprophylaxis [3].
For mothers on antiviral therapy, breastfeeding is not recommended. According to prescribing information, it has not been recommended that women breastfeed their infants while taking lamivudine or tenofovir, to avoid risking postnatal transmission of HIV-1 infection [45, 46]. Although it is known that lamivudine and tenofovir are both excreted into human breast milk, little is known about the extent of exposure of antiviral agents during breastfeeding. Thus, little is known about the overall safety of breastfeeding in this setting.
Conclusions
Factors to consider when deciding whether HBV therapy is warranted for either the woman of childbearing age or the pregnant woman include extent of existing liver disease, efficacy, and safety of existing FDA-approved antiviral agents. Although none of these agents are approved for use in this setting, data are emerging regarding safety. With increasing data, fewer delays occur in treating pregnant women who have clinically active disease. However, it remains unclear if therapy is beneficial when initiated in the third trimester in highly viremic mothers. Additional studies are needed to address this question.
Acknowledgments
Disclosure
No potential conflict of interest relevant to this article was reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.World Health Organization: Hepatitis B: World Health Organization fact sheet 204 (revised August 2008). Available at http://www.who.int/mediacentre/factsheets/fs204/en. Accessed August 2010.
- 2.Yao GB. Importance of perinatal versus horizontal transmission of hepatitis B virus infection in China. Gut. 1996;38:S39–S42. doi: 10.1136/gut.38.Suppl_2.S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:1–36. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 4.McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 5.Tassopoulos NC, Papavangelou GJ, Sjogren MH, et al. Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology. 1987;92:1844–1850. doi: 10.1016/0016-5085(87)90614-7. [DOI] [PubMed] [Google Scholar]
- 6.Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol. 2000;15(Suppl):E16–E19. doi: 10.1046/j.1440-1746.2000.02096.x. [DOI] [PubMed] [Google Scholar]
- 7.Keeffe EB, Dieterich DT, Han SB, et al. A treatment algorithm for the management of chronic hepatitis B in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315–1341. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 9.Marcellin P, Heathcote EJ, Buti, M, et al.: Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. 2008, 359:2442–2455. [DOI] [PubMed]
- 10.Antiretroviral Pregnancy Registry: Available at http://www.apregistry.com. Accessed August 2010.
- 11.Fontana RJ. Side effects of long-term oral antiviral therapy for hepatitis B. Hepatology. 2009;49:S185–S189. doi: 10.1002/hep.22885. [DOI] [PubMed] [Google Scholar]
- 12.Munderi P, Wilkes H, Tumukunde D, et al.: Pregnancy and outcomes among women on triple-drug antiretroviral therapy (ART) in the DART trial [poster WEPEB261]. Presented at the Fifth IAS Conference on HIV Pathogenesis, Treatment and Prevention. Cape Town, South Africa; July 19–22, 2009.
- 13.FDA Pregnancy class definitions: Available at http://en.wikipedia.org/wiki/Pregnancy_category. Accessed August 2010.
- 14.Berg T, Marcellin P, Zoulim F, et al.: Tenofovir is effective a monotherapy or in combination with emtricitabine in adefovir treated patients with chronic hepatitis B infection. Gastroenterology 2010 (Epub ahead of print). [DOI] [PubMed]
- 15.Lai CL, Gane E, Liaw YF, et al. Telbivudine verus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen G, Garcia RT, Nguyen N, et al. Clinical course of hepatitis B virus infection during pregnancy. Aliment Pharmacol Ther. 2009;29:755–764. doi: 10.1111/j.1365-2036.2009.03932.x. [DOI] [PubMed] [Google Scholar]
- 17.de Man RA, Wolters LM, Nevens F, et al. Safety and efficacy of oral entecavir given for 28 days in patients with chronic hepatitis B virus infection. Hepatology. 2001;34:578–582. doi: 10.1053/jhep.2001.26815. [DOI] [PubMed] [Google Scholar]
- 18.Santantonio T, Mazzola M, Iacovazzi T, et al. Long-term follow-up of patients with anti-HBe/HBV DNA-positive chronic hepatitis B treated for 12 months with lamivudine. J Hepatol. 2000;32:300–306. doi: 10.1016/S0168-8278(00)80076-8. [DOI] [PubMed] [Google Scholar]
- 19.Lim SG, Wai CT, Rajnakova A, et al. Fatal hepatitis B reactivation following discontinuation of nucleoside analogues for chronic hepatitis B. Gut. 2002;51:597–599. doi: 10.1136/gut.51.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beasley RP, Hwang LY, Lin CC, et al. Hepatitis B immune globulin (HBIG) efficacy in the interruption of perinatal transmission of hepatitis B virus carrier state. Initial report of a randomised double-blind placebo-controlled trial. Lancet. 1981;2:388–393. doi: 10.1016/s0140-6736(81)90832-1. [DOI] [PubMed] [Google Scholar]
- 21.Beasley RP, Hwang LY, Stevens CE. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983;3:135–141. doi: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- 22.Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099–1102. doi: 10.1016/S0140-6736(83)90624-4. [DOI] [PubMed] [Google Scholar]
- 23.Lee C, Gong Y, Brok J, et al. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332:328–336. doi: 10.1136/bmj.38719.435833.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Canho R, Grosheide PM, Mazel JA, et al. Ten-year neonatal hepatitis B vaccination program, The Netherlands, 1982–1992: protective efficacy and long-term immunogenicity. Vaccine. 1997;15:1624–1630. doi: 10.1016/S0264-410X(97)00080-7. [DOI] [PubMed] [Google Scholar]
- 25.Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190:489–492. doi: 10.5694/j.1326-5377.2009.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 26.Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, et al. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA. 2001;285:2083–2093. doi: 10.1001/jama.285.16.2083. [DOI] [PubMed] [Google Scholar]
- 27.Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 28.van Zonneveld M, van Nunen AB, Niesters HG, et al. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294–297. doi: 10.1046/j.1365-2893.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 29.Xu WM, Cui YT, Wang L, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94–103. doi: 10.1111/j.1365-2893.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 30.Shi Z, Yang Y, Ma L, et al. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147–159. doi: 10.1097/AOG.0b013e3181e45951. [DOI] [PubMed] [Google Scholar]
- 31.Li XM, Yang YB, Hou HY, et al. Interruption of HBV intrauterine transmission: a clinical study. World J Gastroenterol. 2003;9:1501–1503. doi: 10.3748/wjg.v9.i7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi M, Li X, He J, et al. Lamivudine in interruption of HBV intrauterine infection. Clin Med Chin. 2005;21:77–78. [Google Scholar]
- 33.Han ZH, Chen YH, Li LW, et al. Effect and safety of preventing HBV vertical transmission by lamivudine treatment. Clin J Intern Med. 2005;44:378. [Google Scholar]
- 34.Li WF, Jiang R, Wie Z, Li Y. Clinical effect and safety of lamivudine in interruption of chronic HBV maternal to infant transmission. Chin Hepatol. 2006;11:106–107. [Google Scholar]
- 35.Feng HF, Zhang SF. Effect on interruption of hepatitis B virus vertical transmission by lamivudine. J Appl Clin Pediatr. 2007;22:1019–1020. [Google Scholar]
- 36.Xiang GJ, Sun JW, Jiang SQ, et al. Evaluation of therapeutic effect in HBV vertical transmission by lamivudine treatment combined with active-passive immunization for pregnant women. Clin Prac Med. 2007;2:14–16. [Google Scholar]
- 37.Yang JH. The clinical observation of effect of lamivudine on blocking mother to infant transmission of chronic HBV. Int Med Health Guid News. 2008;14:76–78. [Google Scholar]
- 38.Yang S, Liu M, Wang L. Effect of high viral hepatitis B virus DANN loads on vertical transmission of hepatitis B virus in late-pregnant women. Zhonghua Fu Chan Za Zhi. 2008;43:329–331. [PubMed] [Google Scholar]
- 39.Shi Z, Li X, Yang Y, Ma L: Clinical research on the interruption of mother to child transmission of HBV—a randomized, double-blind, placebo-controlled study. Presented at Unite for Sight 6th Annual Global Health Conference. New Haven, CT: Yale University; April 18–19, 2009.
- 40.Olivo SA, Macedo LG, Gadotti IC, et al. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88:156–175. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 41.Zhang LJ, Wang L. Blocking intrauterine infection by telbivudine in pregnant chronic hepatitis B patients [in Chinese] Zhonghua Gan Zang Bing Za Zhi. 2009;17:561–563. [PubMed] [Google Scholar]
- 42.Kazim SN, Wakil SM, Khan LA, et al. Vertical transmission of hepatitis B virus despite maternal lamivudine therapy. Lancet. 2002;359:1488–1489. doi: 10.1016/S0140-6736(02)08425-8. [DOI] [PubMed] [Google Scholar]
- 43.Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20–26. doi: 10.1002/jmv.2187. [DOI] [PubMed] [Google Scholar]
- 44.ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat. 2008;15:37–41. doi: 10.1111/j.1365-2893.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 45.Lamivudine [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009. Available at http://us.gsk.com/products/assets/us_epivir.pdf. Accessed August 2010.
- 46.Tenofovir DF [package insert]. Foster City, CA: Gilead Sciences; 2010. Available at http://www.gilead.com/pdf/viread_pi.pdf. Accessed August 2010.
- 47.Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breast-feeding as a mechanism for vertical transmission of hepatitis B. Lancet. 1975;2:740–741. doi: 10.1016/S0140-6736(75)90724-2. [DOI] [PubMed] [Google Scholar]
- 48.Hill JB, Sheffield JS, Kim MJ, et al. Risk of hepatitis b transmission in breast-fed infants of chronic hepatitis B carriers. Obstet Gynecol. 2002;99:1049–1052. doi: 10.1016/S0029-7844(02)02000-8. [DOI] [PubMed] [Google Scholar]


