Abstract
B7-H1 (PD-L1) is a B7-related protein that inhibits T-cell responses. B7-H1 participates in the immunoescape of cancer cells and is also involved in the long-term persistence of leukemic cells in a mouse model of leukemia. B7-H1 can be constitutively expressed by cancer cells, but is also induced by various stimuli. Therefore, we examined the constitutive and inducible expression of B7-H1 and the consequences of this expression in human acute myeloid leukemia (AML). We analyzed B7-H1 expression in a cohort of 79 patients with AML. In addition, we studied blast cells after incubation with interferon-gamma or toll-like receptors (TLR) ligands. Finally, we evaluated functionality of cytotoxic T-cell activity against blast cells. Expression of B7-H1 upon diagnosis was high in 18% of patients. Expression of TLR2, 4 and 9 was detected in one-third of AML samples. Expression of TLR2 and TLR4 ligands or IFN-γ induced by B7-H1 was found to protect AML cells from CTL-mediated lysis. Spontaneous B7-H1 expression was also found to be enhanced upon relapse in some patients. MEK inhibitors, including UO126 and AZD6244, reduced B7-H1 expression and restored CTL-mediated lysis of blast cells. In AML, B7-H1 expression by blasts represents a possible immune escape mechanism. The inducibility of B7-H1 expression by IFN-γ or TLR ligands suggests that various stimuli, either produced during the immune response against leukemia cells or released by infectious microorganisms, could protect leukemic cells from T cells. The efficacy of MEK inhibitors against B7-H1-mediated inhibition of CTLs suggests a possible cancer immunotherapy strategy using targeted drugs.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0909-y) contains supplementary material, which is available to authorized users.
Keywords: B7-H1, MEK, TLR, IFN-γ, AML
Introduction
Blast cells found in acute myeloid leukemia (AML) can grow in patients despite an autologous immune response. Numerous leukemia-associated antigens have been described, and cytotoxic T cells against these antigens have been detected [1]. However, leukemia cells may escape autologous or allogenic immune responses through various mechanisms, such as indoleamine 2, 3 dioxygenase activity in blast cells, production of inhibitory cytokines, or active suppression of NK cells and dendritic cells [2, 3].
In AML, B7-H1 is a candidate molecule for inhibition of T cells. In a mouse model of AML, expression of B7-H1 allowed dormant tumor cells to escape from CTLs [4–6] [7, 8]. B7-H1 (also known as PD-L1 or CD274) is a B7 family member, and it is a ligand for PD-1 (programmed death-1, a member of the CD28 family) and B7.1 [9–12]. B7-H1 is broadly distributed in various tissues and cell types and is often expressed following exposure to inflammatory cytokines, especially IFN-γ. B7-H1 can inhibit T-cell activation and CTL-mediated lysis. Various human carcinomas express B7-H1. We recently showed that malignant plasma cells from most multiple myeloma (MM) patients also express B7-H1, and expression is enhanced by IFN-γ- and toll-like receptor (TLR) stimulation via a MEK/ERK- and MyD88/TRAF6-dependent pathway [13]. This induced expression can inhibit T-cell responses, indicating B7-H1 expression as a possible immune evasion mechanism in MM. Thus, the immune response against tumor cells via IFN-γ release or stimulation of TLR by pathogen-associated molecular patterns (PAMP) released by microorganisms may actually promote tumor growth by inducing B7-H1 expression, which then inhibits CTLs.
If blast cells in human AML are also able to spontaneously express B7-H1 spontaneously or upon stimulation, they would escape from the autologous immune response and from the graft-versus-leukemia effect after allogenic stem cell transplantation. To investigate whether B7-H1 may play such a role in human leukemia, we analyzed blast cells from a cohort of patients with AML. We studied spontaneous B7-H1 expression and expression after stimulation by TLR ligands or IFN-γ. Here, we show that B7-H1 expression in AML cells is mostly inducible via TLR stimulation and IFN-γ may appear upon relapse, thus protecting blast cells from CTLs. MEK inhibitors are able to inhibit B7-H1 expression and restore sensitivity of blast cells to CTL, thereby offering new opportunities to develop immunotherapy strategies via small, targeted molecules.
Materials and methods
Patients and cell lines
Bone marrow mononuclear cells from 79 patients with AML were isolated by Ficoll–Hypaque centrifugation after the donors had given informed consent in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of Tumorotheque/CHU Lille. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics
| Total number of patients | 79 |
|---|---|
| Sex ratio | 0.95 |
| Median age (range) | 59 (23–89) |
| FAB | |
| M0 | 8 |
| M1 | 9 |
| M2 | 24 |
| M3 | 1 |
| M4 | 14 |
| M5 | 11 |
| M6 | 2 |
| AML evolved from MDS | 10 |
| Karyotype | |
| Good | 4 |
| Intermediate | 39 |
| Poor risk | 18 |
| Failed | 18 |
| Gene mutations | |
| NPM1 | 14 |
| CEBPA | 3 |
| FLT3-ITD | 12 |
| FLT3 m | 3 |
| WT1 | 3 |
| MLL | 2 |
K562, U937, HL60, THP-1, KG1a, Jurkat, Raji, WEHI-3b (all purchased from ATCC) and DA1-3b cell lines were cultured in RPMI 1640 supplemented with 10% fetal calf serum, l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in 5% CO2. Establishment of the DA1-3b BCR/ABL cell line has been described previously [8, 14].
Flow cytometry and Western blot analyses
Expression of B7 family molecules and TLR was evaluated by flow cytometry in blast cells gated with anti-CD45-PC5 (clone J33; Beckman Coulter, Miami, FL) monoclonal antibody (mAb). The following mAbs were used: anti-CD80-FITC (clone L307.4), anti-CD86-FITC (clone 2331), anti-B7-H1-PE (clone MIH1), anti-B7-DC-PE (clone MIH18), anti-B7-H4-PE (clone 7H3.1), anti-TLR2-FITC (clone TL2.1), anti-TLR 4-PE (HTA 125), anti-TLR9-PE (eBiosciences, San Diego, CA) and appropriate isotype controls with prior incubation with Fc-block reagent (Miltenyi Biotec, Bergisch Gladbach, Germany). In some experiments, B7-H1 expression was also evaluated by Western blot using the anti-human B7-H1 antibody (clone 130002) or anti-mouse B7-H1 mAb (clone 179711) (R&D Systems, Minneapolis, MN).
Quantitative real-time PCR
Total RNA was extracted from cell lines and blast cells using conventional techniques. Levels of B7-H1 mRNA expression were measured using the forward primer (5′-AGG AGA TTA GAT CCT GAG GAA AAC C-3′), reverse primer (5′-GGA CTC ACT TGG TAA TTC TGG GA) and TaqMan probe (5′FAM-CTG GCA CAT CCT C–3′MGB), with the TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA). Real-time PCR was performed with an ABI PRISM 7700HT sequence detection system. A twofold serial dilution of cDNA from a lung carcinoma cell line (A549) was used to generate a standard curve. A negative control containing no RNA template was used in each run. 18S ribosomal RNA (PDAR 18S; Applied Biosystems) was amplified as an internal control. An equal quantity of cDNA prepared from 10 ng of RNA was loaded for PCR reaction. Results were measured via standard curves and expressed as ratios.
Cytokines, TLR ligands and signal transduction experiments
To explore signals that induced B7-H1 expression, the following cytokines and reagents were used: AG490 JAK2 inhibitor (25 μM), PD98059 MEK1 inhibitor (25 μM), SB203580 p38MAPK inhibitor (3 μM), LY294002 PI-3K inhibitor (25 μM), rapamycin mTOR inhibitor (10 nM), 1L6hydroxymethyl-chiro-inositol-2-R-2-O-methyl-3-O-octadecyl-sn-glycerocarbonate AKT inhibitor (10 μM) (all from Calbiochem, San Diego, CA); SP600125 JNK inhibitor (25 μM; Biosource, Camarillo, CA), U0126 MEK1/2 inhibitor (20 μM; Cell Signaling Technology, Danvers, MA), AZD6244 MEK1/2 inhibitor (1 μM; kindly provided by Astra Zeneca), recombinant human IFN-γ (500 IU/ml; PeproTech, Rocky Hill, NJ), peptidoglycan TLR2 ligand (PGN, 2.5 μg/ml, from S. aureus), CpG oligonucleotide TLR9 ligand (type C, ODN M362, 5 μM) and its control CpG DNA, and TLR4 ligand Ultrapure lipopolysaccharide (500 ng/ml LPS, from E. coli strain O111:B4) (from InvivoGen/Cayla, Toulouse, France).
Generation of cytotoxic T cells
T cells from the peripheral blood of a healthy donor were isolated using a Pan T Cell Isolation Kit (Miltenyi Biotec) and cultured in RPMI 1640 (Life Technologies) supplemented with 10% fetal calf serum, 100 IU/ml penicillin, 100 mg/ml streptomycin, 2 mM l-glutamine, 50 μM β-mercaptoethanol and 20 IU/ml interleukin 2 (PeproTech, Rocky Hill, USA). The culture medium was changed every 2 days, and irradiated AML cells (1/1 ratio) were added once a week [15]. After 15 days, dead cells were removed and CD8a+ cells were purified using a CD8a+ T-cell Isolation Kit (Miltenyi Biotec). CTL activity was assessed with the Cytotox Non-Radioactive 96 kit (Promega, Madison, WI) using freshly thawed AML blasts as targets. To block B7-H1, target cells were pre-incubated with B7-H1 blocking antibodies (clone MIH1; eBiosciences) at 2 μg/ml 2 h before the CTL assay. The specificity of CTL-mediated lysis of AML cells was verified with HEK-293 cells as targets. MHC class I-restricted lysis was verified with an anti-HLA-ABC (clone W6/32; eBiosciences) or isotype control. The absence of NK cell-mediated cytotoxicity was verified with K562 cells as targets.
Statistical analyses
Statistical analyses were performed with the Sigma Stat 3.11 software (SPSS Sciences, Chicago, IL).
Results
Expression of B7-H1 in leukemic cell lines
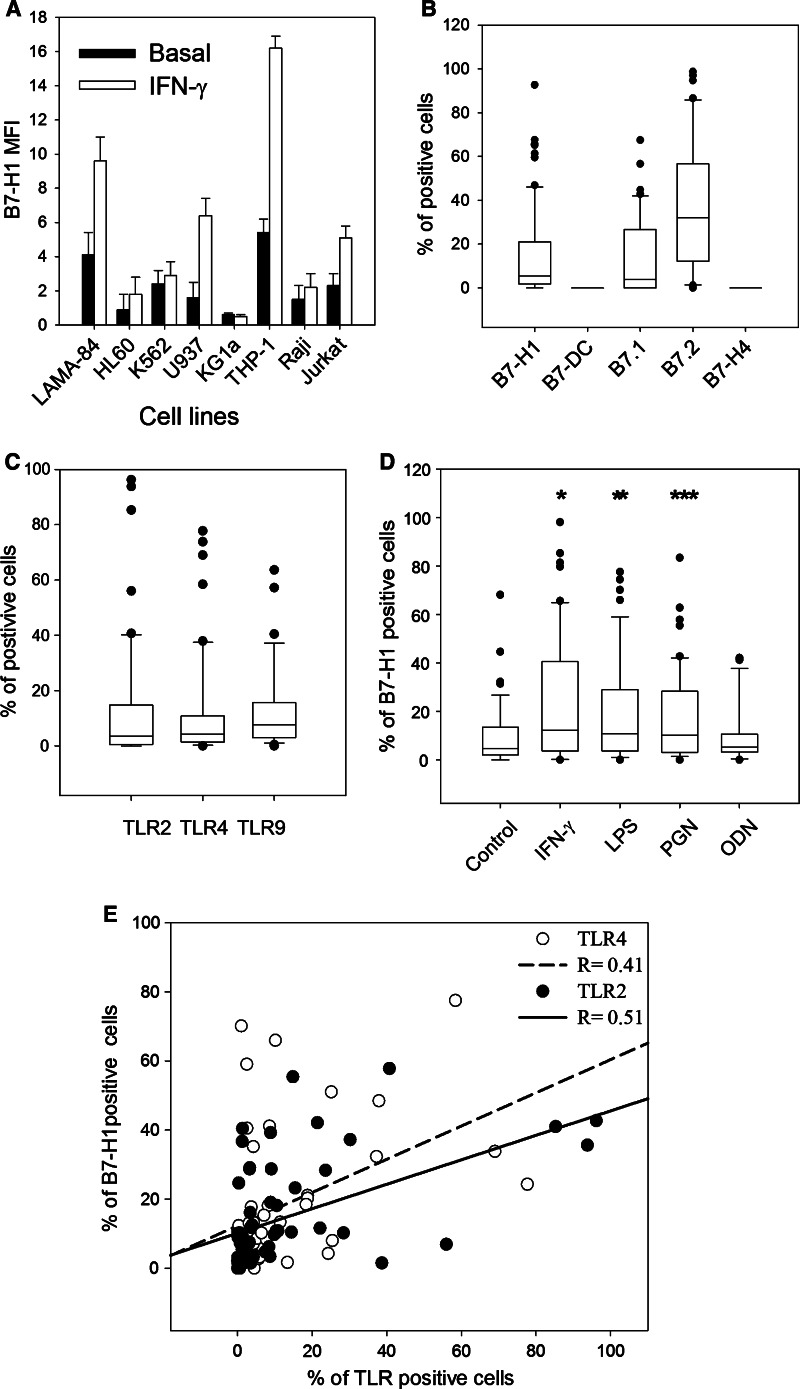
B7-H1 expression is reported to be high in several human cancers; however, its expression in human cancer cell lines has appeared to be modest. We looked at B7-H1 expression in several myeloid lines (U937, K562, KG1a, HL60, THP-1) and lymphoid lines (Raji, Jurkat). Under basal conditions, only THP-1 and LAMA-84 showed substantial expression of B7-H1 (Fig. 1a). Its expression increased after a 24-h incubation of THP-1 and LAMA-84 cells in 500 IU/ml IFN-γ. Expression also increased in U937 and Jurkat cells on 24-h incubation in 500 IU/ml IFN-γ, suggesting that leukemic cells could express B7-H1 under appropriate conditions.
Fig. 1.
B7-H1 and TLR expression in leukemic cell lines and blast cells from AML. a Flow cytometry analysis of B7-H1 expression in myeloid leukemic cell lines (LAMA-84, HL60, K562, U937, KG1a, THP-1) and lymphoid lines (Raji, Jurkat) with or without incubation with 500 IU/ml IFN-γ for 24 h. Data represent the mean and SD of three experiments. b B7-H1, B7-DC, B7.1, B7.2 and B7-H4 expression measured by flow cytometry in 79 blast samples from AML patients collected at the time of diagnosis. c Same as in (b), but this is for TLR2, TLR4 and TLR9 expression. d B7-H1 expression in 60 blast samples collected upon diagnosis with or without incubation with either 500 IU/mL IFN-γ or TLR ligands (500 ng/ml LPS, 2.5 μg/ml PGN or 5 μM ODN) for 24 h. *P < 1 × 10−6, **P < 1 × 10−5, ***P < 6 × 10−5, paired t test. e Correlation between TLR2 or TLR4 expression and percent of B7-H1-positive cells (Pearson’s correlation)
Expression of B7 family molecules in blast cells from AML
These results prompted us to study B7 family molecules in blasts from a large cohort of AML patients under basal conditions or after stimulation. On diagnosis, spontaneous expression of B7-H1 was detected in >30% of blast cells in 18% of patients. No correlations with age, FAB type, karyotype, leukocyte count or NPM, FLT3, MLL, CEBPA or WT1 mutations were found upon diagnosis. B7-DC, another PD-1 ligand (PD-1 is the B7-H1 receptor), was not detected. B7-H4, currently the only other known immunosuppressive B7 molecule [10, 12], was also absent (Fig. 1b). According to previous reports [16–18], B7.2 expression is high in most patients, and B7.1 expression resembles that of B7-H1.
As B7-H1 is usually inducible in normal cells, we investigated whether several stimuli known to play a role in the immune response could induce expression. TLR2, TLR4 and TLR9, which induce B7-H1 in MM [13], were expressed in 29, 27 and 36% of AML samples, respectively (Fig. 1c). No correlation between TLR expression and AML characteristics was observed. IFN-γ significantly enhanced B7-H1 expression (Fig. 1d). PGN and LPS, the TLR2 and TLR4 ligands, respectively, also induced B7-H1. However, ODN, the TLR9 ligand, had no effect on B7-H1 expression. Significant positive correlations were found between LPS-induced B7-H1 expression and TLR4 (r = 0.41, p = 0.01, Pearson’s correlation) and between PGN-induced expression and TLR2 (r = 0.51, p = 0.01), but not between ODN and TLR9 (r = 0.1, p = 0.5) (Fig. 1e). A significant correlation between PGN- and LPS-induced B7-H1 expression (r = 0.9, p = 0.001, Supplementary Figure S1a) and between IFNγ- and PGN- or LPS-induced B7-H1 expression (r = 0.8, p = 0.01, Supplementary Figure S1c,d) was also observed, indicating that in most samples B7-H1 expression was inducible through different stimuli. Correlation between TRL2 and TLR4 expression was significant, but less pronounced (r = 0.75, p = 0.01, Supplementary Figure S1b). These data suggest that blast cells that are refractory to stimulation with one B7-H1-inducing ligand are usually refractory to others. However, we cannot rule out that TLR ligands may induce B7-H1 in some patients via receptors other than TLRs.
Enhanced B7-H1 expression upon relapse
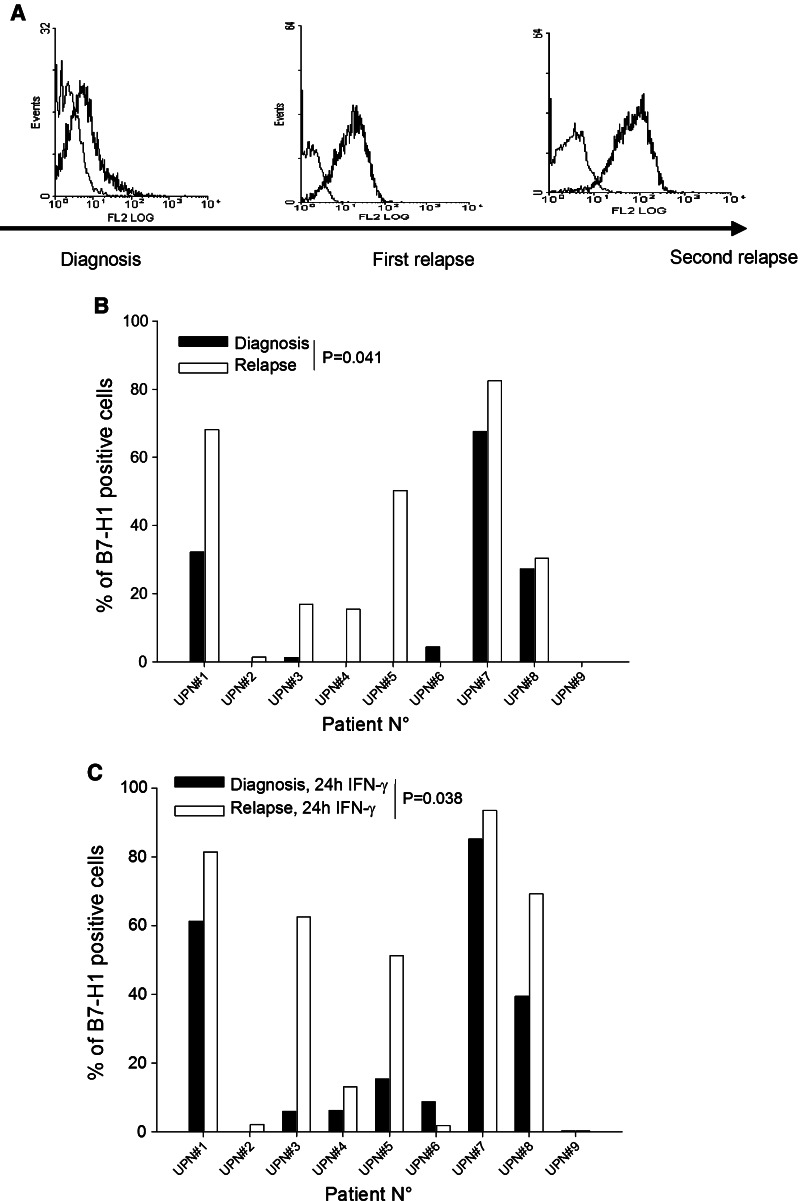
In a mouse model of tumor dormancy, enhanced B7-H1 expression in dormant tumor cells enables long-term persistence of these cells through inhibition of CTL-mediated killing [4–6]. In humans in complete remission, dormant leukemia cells cannot be obtained for study, so we can only speculate about the mechanisms that permit these cells to persist. In five out of nine patients with AML, spontaneous B7-H1 expression (p = 0.041, paired t test) and expression after IFN-γ exposure (p = 0.038) increased at relapse (Fig. 2a–c). Thus, some patients may show increased B7-H1 expression upon relapse. However, we could not determine whether this increase was acquired during the period of complete remission or upon relapse, and the low number of patients analyzed in this study requires cautious interpretation of these findings.
Fig. 2.
B7-H1 at relapse. a Histograms of B7-H1 flow cytometric analysis of blast cells collected from a patient with AML at the time of diagnosis and relapse. Thick line anti-B7-H1 mAb. Thin line control isotype. b B7-H1 expression upon diagnosis and relapse in nine AML samples. c Same patients as in (b), but cells were incubated for 24 h with 500 IU/mL IFN-γ
B7-H1-mediated resistance to CTLs is induced by TLR stimulation
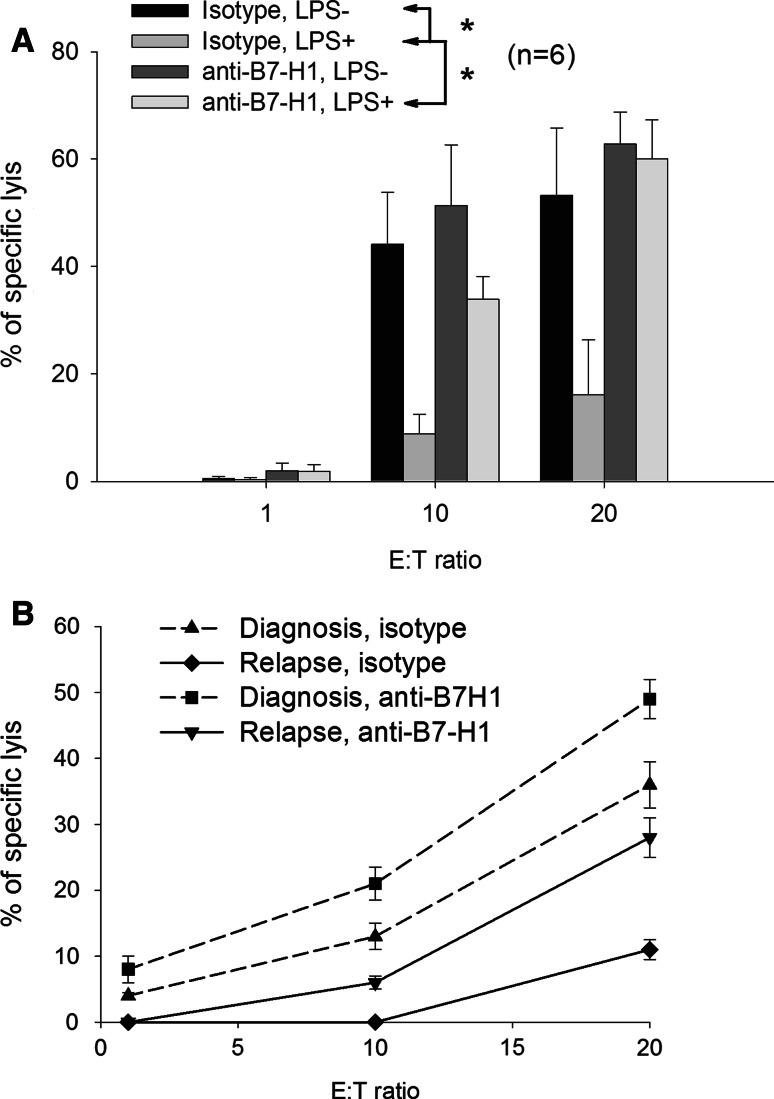
B7-H1 is a known inhibitor of T-cell functions, including CTL-mediated lysis. To evaluate the significance of B7-H1 expression in blast cells, we tested six blast samples with allogenic CTLs (Fig. 3a). Under basal conditions, blocking B7-H1 with a specific antibody had little effect on CTL lysis. However, when blast cells were pre-incubated for 24 h with LPS, they demonstrated a higher resistance to CTL-mediated killing. However, this protection was partially abolished when anti-B7-H1 was added to the medium. Thus, in AML, stimulation of TLRs in blast cells protects them from CTL killing via B7-H1 overexpression.
Fig. 3.
T-cell inhibition by blast cells. a CTL activity of CD8-sorted T cells expanded for 2 weeks against blast cells from six patients with AML. Before the CTL assay, blast cells were pre-incubated for 1 h with or without 500 ng/ml Ultrapure LPS, anti-B7-H1 mAb or isotype control. Data represent the mean and SD of experiments performed in quadruplicate. *indicates significant differences with LPS (Student’s t test). E:T effector:target ratio. b CTL activity of CD8-sorted T cells expanded for 2 weeks against blast cells from one patient with AML collected upon diagnosis and upon first relapse. Target cells were pre-incubated for 1 h with anti-B7-H1 or isotype control before the CTL assay. Data represent the mean and SD of experiments performed in quadruplicate
The differential expression of B7-H1 between the time of diagnosis and relapse seen in some patients may also have a functional consequence. Blasts from a patient during AML relapse were more resistant to CTL-mediated killing than blasts isolated upon diagnosis, and this difference was reversible by incubation with an anti-B7-H1 antibody (Fig. 3b).
Blocking MEK but not PI3K/mTOR decreases B7-H1 expression
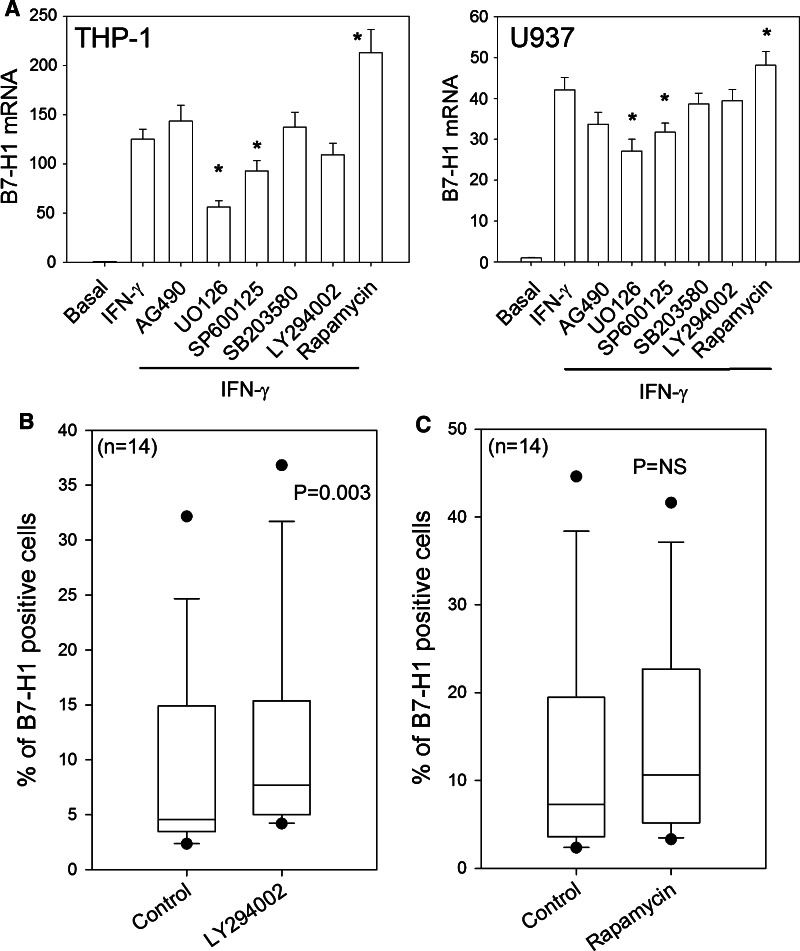
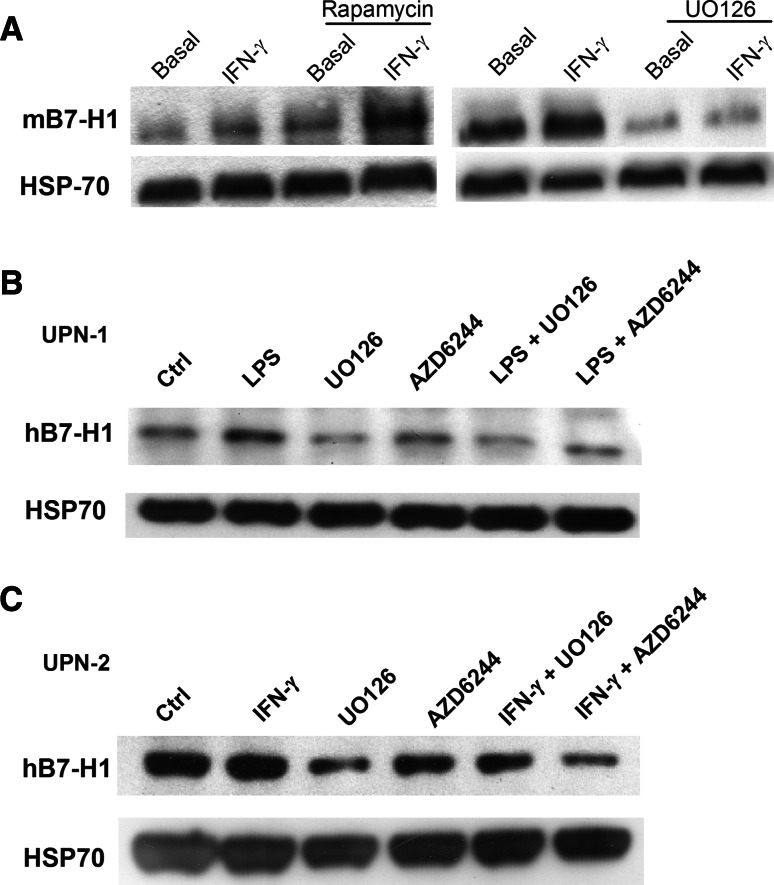
In MM, B7-H1 expression is activated by IFN-γ and TLR ligands via a common signaling pathway involving MyD88/MEK/ERK/STAT1, which activates transcription [13]. We confirmed that in the AML cell lines THP-1 and U937, blocking MEK inhibits B7-H1 transcription (Fig. 4a). B7-H1 protein expression was measured by Western blot because the autofluorescence of leukemic cells treated with UO126 made comparison between different MEK inhibitors difficult by flow cytometry. Blast cells from patients with AML and a mouse leukemia cell line showed reduced B7-H1 protein expression after exposure to MEK inhibitors, confirming that MEK is an important regulator of B7-H1 expression in leukemic cells (Fig. 5). Interestingly, inhibition was found with UO126 and with AZD6244, which is an MEK inhibitor that is currently under clinical development for the treatment of various types of tumors. Previous reports proposed that PI3K/AKT may also enhance expression of B7-H1 [19]. Surprisingly, blocking PI3K/mTOR with LY294002 or blocking mTORC1 with rapamycin slightly enhanced B7-H1 expression in human and mouse leukemic cell lines that were either untreated or exposed to IFN-γ (Figs. 4a, 5). This result was also found, but to a lesser degree, in human AML blasts treated with LY294002. However, rapamycin (Fig. 4b, c) and the AKT inhibitor (data not shown) had no significant effect on B7-H1 expression. Thus, in AML, clinical targeting of the PI3K/AKT/mTOR pathway may not actually inhibit B7-H1 expression.
Fig. 4.
B7-H1 expression and signal transduction inhibitors. a B7-H1 mRNA levels (relative to rRNA) in THP-1 (left panel) and U937 (right panel) cells measured by RQ-PCR following 24 h incubation with IFN-γ with or without 1-h pre-treatment with signal transduction inhibitors. Inhibitors included the following: 20 μM UO126 (MEK1/2), 25 μM PD98059 (MEK1), 25 μM SP600125 (JNK), 25 μM AG490 (JAK2), 25 μM LY294002 (PI3K), 3 μM SB203850 (p38MAPK) and 10 nM rapamycin (mTOR). *indicates significant differences from cells treated with IFN-γ alone (Student’s t test). b B7-H1 expression in 60 blast samples with or without incubation with 25 μM of the PI3K inhibitor LY294002. c Same as in (b), but with incubation with the mTOR inhibitor rapamycin
Fig. 5.
B7-H1 protein expression and MEK inhibitors. a Western blot analysis of B7-H1 levels with anti-mouse B7-H1 mAb (clone 179711) in DA1-3b mouse leukemic cells exposed to 500 IU/ml IFN-γ with or without 1-h pre-incubation with 20 μM UO126 (right panel) or 25 μM rapamycin (left panel). b Western blot analysis of B7H1 levels with anti-human B7-H1 mAb (clone 130002) in blast cells from one patient with AML. Cells were exposed to 500 ng/ml Ultrapure LPS for 24 h with or without pre-incubation with 20 μM UO126 or 1 μM AZD6244. c Same as in (b), but in another AML patient and with exposure to 500 IU/ml IFN-γ instead of LPS
MEK inhibitors enhance CTL-mediated lysis of blast cells
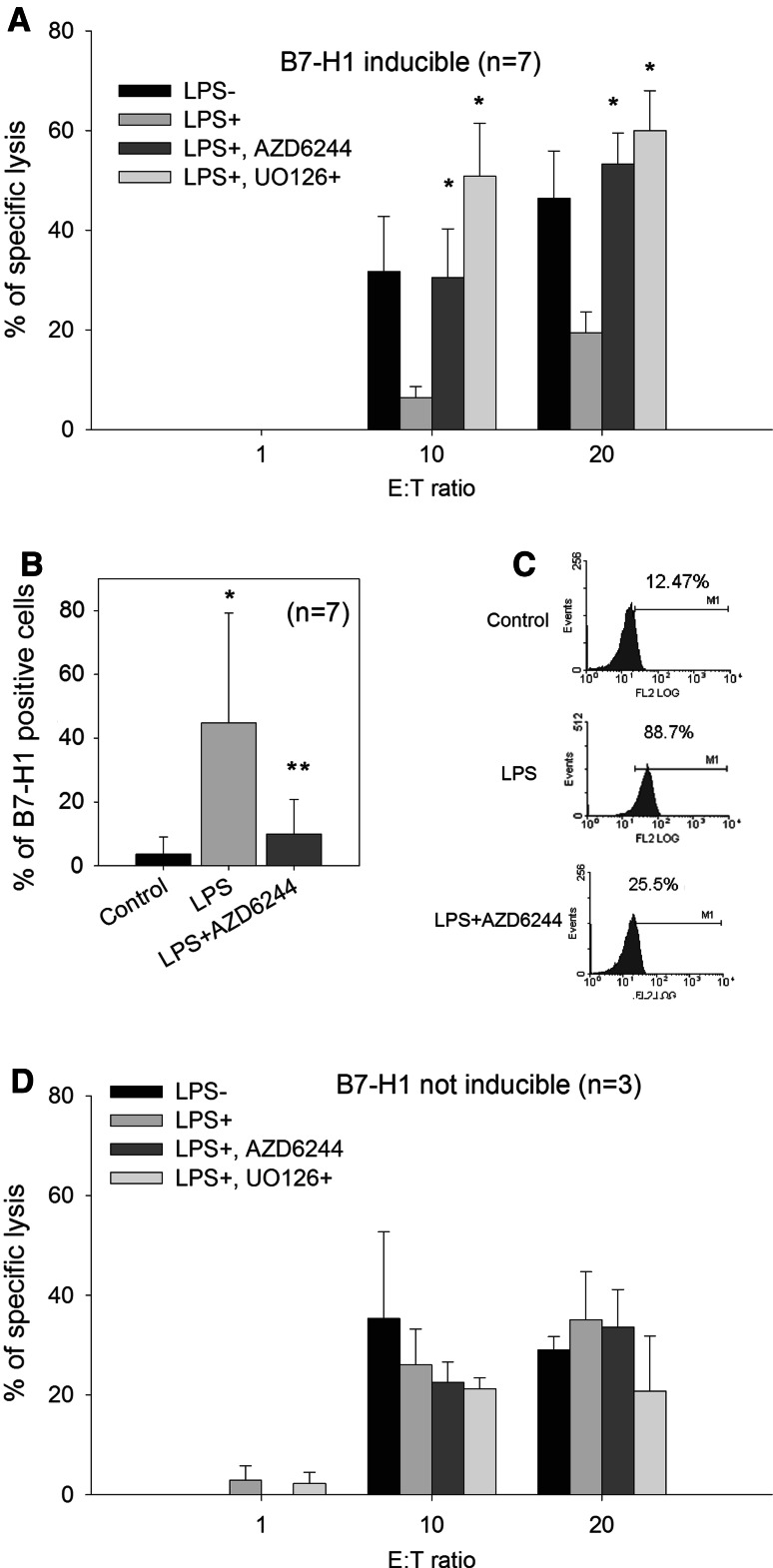
As MEK inhibitors are able to inhibit B7-H1 expression, we tested the effect of the MEK inhibitors, UO126 and AZD6244, on the sensitivity of blast cells from ten patients with AML to allogenic CTL-mediated lysis (Fig. 6a, d). B7-H1 expression was inducible with LPS in seven of the ten tested samples and was significantly reduced by the MEK inhibitor, AZD6244 (Fig. 6b, c). Pre-incubation of these B7-H1-inducible leukemic cells with UO126 resulted in increased cytotoxicity. Furthermore, pre-incubation with AZD6244 also resulted in increased cytotoxicity, but to a lesser degree at the doses selected (Fig. 6a). The three patients who did not express B7-H1 after LPS treatment showed no improvement in cytotoxicity upon treatment with AZD6244 or UO126 (Fig. 6d).
Fig. 6.
CTL-mediated killing of blast cells and MEK inhibitors. a CTL activity of CD8-sorted T cells expanded for 2 weeks against blast cells from seven patients with AML; the cells were chosen for their expression of B7-H1 after pre-incubation for 1 h with 500 ng/ml Ultrapure LPS. Blast cells were exposed to 20 μM UO126 or 1 μM AZD6244. Data represent the mean and SD of seven patients performed in quadruplicate. *indicates significant differences with LPS (Student’s t test). b B7-H1 expression in blast cells from (a) after exposure to LPS and 1 μM AZD6244. *indicates significant differences between control and LPS; **indicates significant differences between LPS and LPS plus AZD6244 (Student’s t test). c B7-H1 expression in one representative sample from (b). d Same as in (a), but with three patients who did not express B7-H1 after exposure to LPS
Discussion
AML blasts are known to be immunosuppressive, but the precise nature of the signals produced by these cells to inhibit T-cell function remains elusive [20–22]. B7 family molecules may be responsible for this immunosuppressive effect. B7.1 and B7.2, which both activate T cells via binding to CD28 and suppress T cells via CTLA-4, are expressed by leukemic cells [17, 18]. Furthermore, both ligands activate or inhibit T cells in AML, depending on their level of expression, which can be modified in vitro and in vivo by chemotherapy [18]. Other more recently described members of the B7 family have not been investigated as thoroughly. B7-H2, which interacts with ICOS on activated T cells, is expressed on AML cells and induces CD4+ proliferation as well as IL4 and IL10 production [16]. B7-H1 may suppress T-cell function through its receptor, PD-1, and possibly through direct interaction with B7.1 [23].
Marked expression of B7-H1 has been reported in several human and mouse tumors [13, 24, 25]. However, reports on AML are conflicting. Tamura et al. detected B7-H1 expression only in a minority of 69 de novo AML cases [16]. Another study reported significant expression in 17 of 30 cases of leukemia of various types [26], although inhibition of T-cell proliferation mediated by B7-H1 was not observed. However, both reports demonstrated B7-H1 expression in several leukemic cell lines. Our data support the conclusion that B7-H1 expression upon AML at diagnosis is restricted; upon diagnosis, only 18% of patients expressed B7-H1 in most cells.
B7-H1 is an inducible molecule; IFN-γ, TNF-α and GM-CSF can enhance B7-H1 expression under physiological conditions [27–29]. Here, we confirmed previous reports showing that IFN-γ induces B7-H1 in leukemic cell lines [16, 26]. We previously demonstrated in the DA1-3b mouse leukemic model that B7-H1 dose dependently inhibits CTL [4]. Here, we observed that IFN-γ induces and enhances B7-H1 expression in most AML samples. Thus, in addition to basal B7-H1 expression observed in some patients, we hypothesize that IFN-γ produced by T cells in the AML microenvironment may induce T-cell inhibition by inducing B7-H1 expression.
We also observed that TLR ligands induced B7-H1 expression in blast cells and increased resistance to CTLs. B7-H1 expression induced by PGN, a TLR2 ligand, correlated positively with B7-H1 expression induced by LPS, a TLR4 ligand, and by IFNγ. AML blast cells that are refractory to stimulation with one ligand are usually refractory to others. Activation of TLR2, TLR4 and TLR9 induces B7-H1 in MM, leading to resistance to CTLs [13]. Activation of TLR4 on mouse tumor cells can also induce B7-H1 expression, leading to immunoescape. TLR4 activation in Langerhans cells has recently been shown to induce B7-H1 and favor tolerogenic properties in the oral cavity [30, 31]. Until now, TLR expression in hematological malignancies has been mostly reported in B-cell tumors [32–38]. Data regarding TLR expression and function in AML remain scarce. Numerous publications have reported the possibility that AML blast cells differentiate into leukemic dendritic cells (DC) using various combinations of agents, including LPS [1, 15]. These reports imply that AML blast cells may recognize LPS in vitro when combined with cytokines, such as GM-CSF and IL4. However, LPS can stimulate target cells through other receptors besides the TLRs, and LPS alone is not sufficient to mature blast cells into dendritic cells. In the THP-1 leukemic cell line, induction of apoptosis by LPS plus IFN-α has been reported, but primary AML samples seemed less sensitive to this combination [39]. Altogether, these experiments provide little information about the role of TLRs in AML.
Recently, using gene expression profiling in a large cohort of cytogenetically normal AMLs, Marcucci et al. reported that TLR2, TLR4 and TLR8 expression was inversely correlated with the levels of microRNA-181 family, and this correlation was associated with a worse prognosis [40]. Here, we observed that TLR2, TLR4 and TLR9 proteins were commonly expressed on AML blast cells. Stimulation of TLR2 and TLR4 induced B7-H1 expression and increased resistance of blast cells to CTLs. These data indicate that receptors of innate immunity could play a role in the development of AML.
A recent report studying gene expression profiles in mouse hematopoietic stem cells during aging showed that genes involved in inflammation and stress responses were up-regulated in aged mice [41, 42], and Tlr4 was among these genes. Thus, aging stem cells may be more sensitive to inflammatory response. An attractive hypothesis is that aging patients might be more prone to AML, in part because their stem cells are more sensitive to stimulation by TLR ligands, leading to immunoevasion of emerging leukemic clones; but this hypothesis remains untested.
In some patients, blasts studied upon relapse showed increased levels of B7-H1. This increase may have allowed blasts to resist CTL-mediated killing, perhaps during the complete remission period. However, given the low number of paired samples analyzed during diagnosis and relapse, further investigations using larger cohorts and on blast cells isolated from minimal residual disease are necessary to confirm this hypothesis.
We also confirmed that in leukemic cells, B7-H1 expression requires MEK, as previously shown in MM and dermal fibroblasts, and as recently confirmed in bladder carcinoma [13, 43, 44]. In addition to MAPK pathways, PI3K/AKT plays a critical role in B7-H1 expression in malignant gliomas, prostate and breast carcinoma, and dermal fibroblasts [19, 45]. We tested different inhibitors of PI3K and mTOR and observed the effect on B7-H1 expression. We found no inhibition in leukemic cell lines or in AML blast cells; rather, we found a slight enhancement. This finding suggests that regulation of B7-H1 expression varies widely between cell types; thus, drugs that target signal transduction pathways might have different immunological effects in different tumors. MEK inhibition not only inhibited B7-H1 expression, but also sensitized blast cells expressing B7-H1 after stimulation with LPS to CTL-mediated lysis. Current immunotherapy strategies primarily use monoclonal antibodies or cell therapy. Another approach would consist of using small, targeted molecules to block immunoescape mechanisms developed by cancer cells. MEK inhibitors could represent a means to this end. Several of these molecules are currently under clinical development, including AZD6244. These drugs have been developed to block tumor cell proliferation or induce cell death by blocking growth and survival signals, but our data show that they could also be used to kill tumor cells indirectly via suppression of B7-H1 expression and CTL-mediated killing. We can also imagine targeting B7-H1 via MEK after allogenic stem cell transplantation to facilitate a graft-versus-leukemia effect, but this strategy must be explored by further experiments.
In conclusion, we have identified B7-H1 as an immunoescape molecule in blast cells from patients with AML. Expression of B7-H1 increases when these cells are exposed to immune response, pathogens and sometimes upon relapse, and increased expression may be targeted via MEK inhibitors to facilitate CTL-mediated killing. These findings suggest that B7-H1 may be a possible target for immunotherapy via small molecules.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the Ligue Nationale Contre le Cancer (Comité du septentrion, and Equipe Labellisées program) and Institut National du Cancer «IMMUDORM» project. JL is the recipient of a grant from the Fondation pour la Recherche Médicale and from the Institut de Recherche sur le Cancer de Lille. VD received a grant from the Fondation de France. We thank AstraZeneca for providing AZD6244.
Conflict of interest
BQ received AZD6244 from AstraZeneca.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
C. Berthon and V. Driss contributed equally to this work.
References
- 1.el-Shami K, Smith BD. Immunotherapy for myeloid leukemias: current status and future directions. Leukemia. 2008;22:1658–1664. doi: 10.1038/leu.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corm S, Berthon C, Imbenotte M, Biggio V, Lhermitte M, Dupont C, Briche I, Quesnel B. Indoleamine 2, 3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-gamma. Leuk Res. 2009;33:490–494. doi: 10.1016/j.leukres.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, Horenstein AL, Fiore F, Massaia M, Colombo MP, Baccarani M, Lemoli RM. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 4.Saudemont A, Hamrouni A, Marchetti P, Liu J, Jouy N, Hetuin D, Colucci F, Quesnel B. Dormant tumor cells develop cross-resistance to apoptosis induced by CTLs or imatinib mesylate via methylation of suppressor of cytokine signaling 1. Cancer Res. 2007;67:4491–4498. doi: 10.1158/0008-5472.CAN-06-1627. [DOI] [PubMed] [Google Scholar]
- 5.Saudemont A, Jouy N, Hetuin D, Quesnel B. NK cells that are activated by CXCL10 can kill dormant tumor cells that resist CTL-mediated lysis and can express B7-H1 that stimulates T cells. Blood. 2005;105:2428–2435. doi: 10.1182/blood-2004-09-3458. [DOI] [PubMed] [Google Scholar]
- 6.Saudemont A, Quesnel B. In a model of tumor dormancy, long-term persistent leukemic cells have increased B7-H1 and B7.1 expression and resist CTL-mediated lysis. Blood. 2004;104:2124–2133. doi: 10.1182/blood-2004-01-0064. [DOI] [PubMed] [Google Scholar]
- 7.Quesnel B. Dormant tumor cells as a therapeutic target? Cancer Lett. 2008;267:10–17. doi: 10.1016/j.canlet.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Joha S, Idziorek T, Corm S, Hetuin D, Philippe N, Preudhomme C, Quesnel B. BCR-ABL mutants spread resistance to non-mutated cells through a paracrine mechanism. Leukemia. 2008;22:791–799. doi: 10.1038/leu.2008.3. [DOI] [PubMed] [Google Scholar]
- 9.Wei S, Shreiner AB, Takeshita N, Chen L, Zou W, Chang AE. Tumor-induced immune suppression of in vivo effector T-cell priming is mediated by the B7-H1/PD-1 axis and transforming growth factor beta. Cancer Res. 2008;68:5432–5438. doi: 10.1158/0008-5472.CAN-07-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butte MJ, Pena-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol. 2008;45:3567–3572. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nature Rev. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 14.Vereecque R, Buffenoir G, Gonzalez R, Preudhomme C, Fenaux P, Quesnel B. A new murine aggressive leukemic model. Leuk Res. 1999;23:415–416. doi: 10.1016/S0145-2126(98)00180-5. [DOI] [PubMed] [Google Scholar]
- 15.Saudemont A, Corm S, Wickham T, Hetuin D, Quesnel B. Induction of leukemia-specific CD8+ cytotoxic T cells with autologous myeloid leukemic cells maturated with a fiber-modified adenovirus encoding TNF-alpha. Mol Ther. 2005;11:950–959. doi: 10.1016/j.ymthe.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Tamura H, Dan K, Tamada K, Nakamura K, Shioi Y, Hyodo H, Wang SD, Dong H, Chen L, Ogata K. Expression of functional B7-H2 and B7.2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res. 2005;11:5708–5717. doi: 10.1158/1078-0432.CCR-04-2672. [DOI] [PubMed] [Google Scholar]
- 17.Vereecque R, Buffenoir G, Gonzalez R, Cambier N, Hetuin D, Bauters F, Fenaux P, Quesnel B. Gamma-ray irradiation induces B7.1 expression in myeloid leukaemic cells. Br J Haematol. 2000;108:825–831. doi: 10.1046/j.1365-2141.2000.01967.x. [DOI] [PubMed] [Google Scholar]
- 18.Vereecque R, Saudemont A, Quesnel B. Cytosine arabinoside induces costimulatory molecule expression in acute myeloid leukemia cells. Leukemia. 2004;18:1223–1230. doi: 10.1038/sj.leu.2403391. [DOI] [PubMed] [Google Scholar]
- 19.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 20.Stripecke R, Levine AM, Pullarkat V, Cardoso AA. Immunotherapy with acute leukemia cells modified into antigen-presenting cells: ex vivo culture and gene transfer methods. Leukemia. 2002;16:1974–1983. doi: 10.1038/sj.leu.2402701. [DOI] [PubMed] [Google Scholar]
- 21.Buggins AG, Lea N, Gaken J, Darling D, Farzaneh F, Mufti GJ, Hirst WJ. Effect of costimulation and the microenvironment on antigen presentation by leukemic cells. Blood. 1999;94:3479–3490. [PubMed] [Google Scholar]
- 22.Barrett AJ, Savani BN. Does chemotherapy modify the immune surveillance of hematological malignancies? Leukemia. 2009;23:53–58. doi: 10.1038/leu.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xerri L, Chetaille B, Seriari N, Attias C, Guillaume Y, Arnoulet C, Olive D. Programmed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Hum Pathol. 2008;39:1050–1058. doi: 10.1016/j.humpath.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Kozako T, Yoshimitsu M, Fujiwara H, Masamoto I, Horai S, White Y, Akimoto M, Suzuki S, Matsushita K, Uozumi K, Tei C, Arima N. PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia. 2009;23:375–382. doi: 10.1038/leu.2008.272. [DOI] [PubMed] [Google Scholar]
- 26.Salih HR, Wintterle S, Krusch M, Kroner A, Huang YH, Chen L, Wiendl H. The role of leukemia-derived B7-H1 (PD-L1) in tumor-T-cell interactions in humans. Exp Hematol. 2006;34:888–894. doi: 10.1016/j.exphem.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 28.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 29.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 30.Allam JP, Peng WM, Appel T, Wenghoefer M, Niederhagen B, Bieber T, Berge S, Novak N. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allerg Clin Immunol. 2008;121:368–374. doi: 10.1016/j.jaci.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 32.Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, Bataille R, Jego G. Toll-like receptors: lessons to learn from normal and malignant human B cells. Blood. 2008;112:2205–2213. doi: 10.1182/blood-2008-02-140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb RN, Cruse JM, Lewis RE. Differential cytokine and Toll-like receptor expression in leukemia. Exp Mol Pathol. 2007;83:464–470. doi: 10.1016/j.yexmp.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Spaner DE, Shi Y, White D, Mena J, Hammond C, Tomic J, He L, Tomai MA, Miller RL, Booth J, Radvanyi L. Immunomodulatory effects of Toll-like receptor-7 activation on chronic lymphocytic leukemia cells. Leukemia. 2006;20:286–295. doi: 10.1038/sj.leu.2404061. [DOI] [PubMed] [Google Scholar]
- 35.Abbas S, Abu-Amer Y. Dominant-negative IkappaB facilitates apoptosis of osteoclasts by tumor necrosis factor-alpha. J Biol Chem. 2003;278:20077–20082. doi: 10.1074/jbc.M208619200. [DOI] [PubMed] [Google Scholar]
- 36.Spaner DE, Foley R, Galipeau J, Bramson J. Obstacles to effective Toll-like receptor agonist therapy for hematologic malignancies. Oncogene. 2008;27:208–217. doi: 10.1038/sj.onc.1210905. [DOI] [PubMed] [Google Scholar]
- 37.Spaner DE, Masellis A. Toll-like receptor agonists in the treatment of chronic lymphocytic leukemia. Leukemia. 2007;21:53–60. doi: 10.1038/sj.leu.2404456. [DOI] [PubMed] [Google Scholar]
- 38.Corthals SL, Wynne K, She K, Shimizu H, Curman D, Garbutt K, Reid GS. Differential immune effects mediated by Toll-like receptors stimulation in precursor B-cell acute lymphoblastic leukaemia. Br J Haematol. 2006;132:452–458. doi: 10.1111/j.1365-2141.2005.05893.x. [DOI] [PubMed] [Google Scholar]
- 39.Lehner M, Bailo M, Stachel D, Roesler W, Parolini O, Holter W. Caspase-8 dependent apoptosis induction in malignant myeloid cells by TLR stimulation in the presence of IFN-alpha. Leuk Res. 2007;31:1729–1735. doi: 10.1016/j.leukres.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Marcucci G, Radmacher MD, Maharry K, Mrozek K, Ruppert AS, Paschka P, Vukosavljevic T, Whitman SP, Baldus CD, Langer C, Liu CG, Carroll AJ, Powell BL, Garzon R, Croce CM, Kolitz JE, Caligiuri MA, Larson RA, Bloomfield CD. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- 41.Chambers SM, Goodell MA. Hematopoietic stem cell aging: wrinkles in stem cell potential. Stem Cell Rev. 2007;3:201–211. doi: 10.1007/s12015-007-0027-1. [DOI] [PubMed] [Google Scholar]
- 42.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian Y, Deng J, Geng L, Xie H, Jiang G, Zhou L, Wang Y, Yin S, Feng X, Liu J, Ye Z, Zheng S. TLR4 signaling induces B7-H1 expression through MAPK pathways in bladder cancer cells. Cancer Invest. 2008;26:816–821. doi: 10.1080/07357900801941852. [DOI] [PubMed] [Google Scholar]
- 44.Lee SK, Seo SH, Kim BS, Kim CD, Lee JH, Kang JS, Maeng PJ, Lim JS. IFN-gamma regulates the expression of B7-H1 in dermal fibroblast cells. J Dermatol Sci. 2005;40:95–103. doi: 10.1016/j.jdermsci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, Parsa AT. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2008;28:306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.