Abstract
The pancreatic manifestations seen in patients with von Hippel-Lindau (VHL) disease are subdivided into 2 categories: pancreatic neuroendocrine tumors (NET), and cystic lesions, including simple cyst and serous cystadenoma. The VHL-associated cystic lesions are generally asymptomatic and do not require any treatment, unless they are indistinguishable from other cystic tumor types with malignant potential. Because pancreatic NET in VHL disease are non-functioning and have malignant potential, it is of clinical importance to find and diagnose these as early as possible. It will be recommended that comprehensive surveillance using dynamic computed tomography for abdominal manifestations, including pancreatic NET, should start from the age of 15 years in VHL patients. Unlike sporadic non-functioning NET without VHL disease, in which surgical resection is generally recommended, VHL patients at lower metastatic risk of pancreatic NET should be spared the risks of operative resection.
Keywords: Von Hippel-Lindau disease; Pancreas; Neuroendocrine tumor, Diagnosis; Clinical protocols
INTRODUCTION
Von Hippel-Lindau (VHL) disease is an autosomal dominant disorder that develops a variety of tumors and cysts in the central nervous system (CNS) and visceral organs[1]. The prevalence of patients with VHL disease was reported to be 1 in 100 000 of the population and 1 family in 1 million of the population[2]. Tumor types seen in VHL disease include hemangioblastomas in the CNS and retina, renal cell carcinoma, pheochromocytomas and pancreatic neuroendocrine tumors (NET)[1]. During their growth, these tumors impair the function of the primary organs and sometimes metastasize to distant organs, and thus are thought to have malignant potential. A number of studies in the United States and Europe have reported the clinical characteristics of these tumors, including pancreatic NET[3-9].
PANCREATIC MANIFESTATIONS IN VHL DISEASE
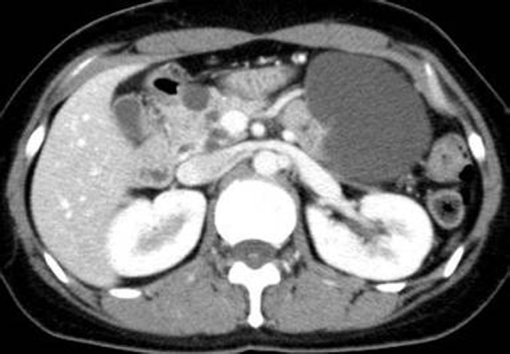
The pancreatic manifestations seen in patients with VHL disease are subdivided into 2 categories: NET as solid tumors, and cystic lesions, including a simple cyst and serous cystadenoma[1,5,10]. Fortunately, cystic lesions complicated with VHL disease are generally asymptomatic and do not require any treatment (Figure 1)[1]. It is necessary to differentially diagnose them from other cystic tumor types, such as intraductal papillary mucin-producing tumors or mucinous cystic tumors, because these mucinous cystic tumors have malignant potential. When cystic lesions seen in patients with VHL disease are indistinguishable from these tumor types or are causative of compression symptom onto adjacent organs, operative resection of the cystic lesion in the pancreas would be considered.
Figure 1.
Abdominal computed tomography shows several cystic lesions in the pancreas (32-year-old female).
Unlike cystic lesions seen in the pancreas of patients with VHL disease, NET can be locally invasive and can metastasize, resulting in much higher clinical significance[1,6]. NET occur in 8%-17% of patients with VHL disease[1]. The malignant potential of sporadic pancreatic NET, which is not associated with VHL disease, varies depending on the functional properties of the tumors. None of the patients with pancreatic NET associated with VHL disease has been reported to present with hormonal syndrome[3,8]. Sporadic non-functioning NET behave in a malignant fashion with a metastatic spread in 60% to 90%, in marked contrast to the findings in cases with pancreatic NET associated with VHL disease, as previously described (metastatic disease in 11%-20%)[11]. The reason is thought to be as follows. In the case of sporadic non-functioning pancreatic NET, there are no hormonal symptoms, hence the tumors are first identified when they grow larger than 5 cm. In contrast, in the case of pancreatic NET in patients with VHL disease, the tumors can be diagnosed at a relatively early stage by screening examination for abdominal manifestations of the disease[11].
In general, pancreatic NET with or without VHL disease show a slow growth phenotype and thus the patients have a good prognosis. Blansfield et al[12] reported that the death rate as a result of metastatic pancreatic NET was 0.3% in patients (n = 633) with VHL disease. Pancreatic NET tend to have a high frequency in patients with pheochromocytoma (VHL type 2) as previously described[3,11]. However, Hammel et al[5] reported that patients with pancreatic lesions had significantly fewer pheochromocytomas than those without pancreatic lesions (14/122 vs 16/36, P < 0.0001). Taken together, there is no consensus to date regarding coexistence of pancreatic NET and pheochromocytoma.
DIAGNOSIS OF PANCREATIC NET IN VHL DISEASE
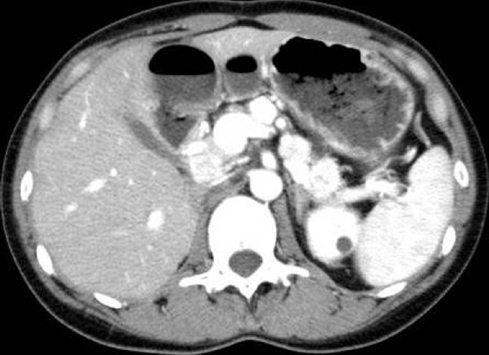
Ultrasonography, computed tomography (CT) or magnetic resonance imaging (MRI) can be used to detect primary NET and their metastases. Octreotide scintigraphy has a sensitivity that exceeds the combination of the others. However, smaller tumors can be difficult to visualize with octreotide scintigraphy. Positron emission tomography with 5-hydroxytryptophan or L-dopa can be an option for detection of small tumors[13], although only a limited number of institutes have employed these methodologies. In almost all hospitals over the world, dynamic CT is the most sensitive method for detection at present, since pancreatic NET are strongly enhanced on dynamic CT (Figure 2)[14]. MRI is also an effective method for metastatic liver lesions [15].
Figure 2.
Contrast-enhanced abdominal computed tomography reveals several pancreatic mass lesions that are strongly enhanced (33-year-old female)[14].
MANAGEMENT OF PANCREATIC NET IN VHL DISEASE
In past reports, the youngest age at diagnosis of pancreatic NET in patients with VHL disease is 12 years old[16], and the second youngest age is 16 years old[12]. The surveillance of renal cell carcinoma (RCC) in VHL disease has been begun from the age of 15, therefore it will be recommended that comprehensive surveillance of abdominal organs including pancreas starts from the age of 15 by abdominal dynamic CT in view of the risk from radiation exposure and renal dysfunction caused by contrast media. In addition, patients with VHL disease require particular attention to distinguish pancreatic NET from metastatic RCC, because pancreatic metastasis from RCC is visualized as a hypervascular tumor as well as pancreatic NET. If pancreatic NET are not found by dynamic CT in the first abdominal surveillance (at the age of 15 years), the patient can be followed with comprehensive surveillance including that for RCC and pheochromocytoma every 2-3 years[12].
Sporadic non-functioning NET without VHL disease behave in a malignant fashion, therefore surgery is recommended to avoid later development of malignancy in all cases with tumor size greater than 2 cm[17,18]. In contrast, in the case of pancreatic NET with VHL disease, the indication for surgery should be carefully decided, because the patients commonly have multiple or recurrent tumors. The problem of surveillance is how to manage pancreatic NET without metastasis.
Blansfield et al[12] proposed 3 criteria to predict metastatic disease of pancreatic NET in patients with VHL disease: (1) tumor size greater than or equal to 3 cm; (2) presence of a mutation in exon 3; and (3) tumor doubling time less than 500 d (Table 1). If the patient has none of these criteria, they suggested that the likelihood of the patient’s lesion resulting in metastatic disease is very low and that the patient can be followed with a medical history and physical examination and radiologic surveillance on 2-3 years cycles. If the patient has 1 criterion, the patient should be followed more closely every 6 mo to 1 year to detect the emergence of a second criterion. If the patient has 2 or 3 criteria, the patient should be considered for surgical management because of the greater likelihood of future malignancy from pancreatic NET[12]. The treatment strategy in patients with the metastatic disease is still controversial, depending on histological tumor types.
Table 1.
Treatment recommendations for pancreatic neuroendocrine tumors with von Hippel-Lindau disease[12]
| Treatment recommendation | |
| Prognostic criteria | |
| Tumor size ≥ 3 cm | |
| Mutation in exon 3 | |
| Tumor doubling time ≤ 500 d | |
| None of the criteria | Followed by CT/MRI every 2-3 yr |
| 1 criterion | Followed by CT/MRI every 6-12 mo |
| 2 or 3 criteria | Consider surgical intervention |
CT: Computed tomography; MRI: Magnetic resonance imaging.
CONCLUSION
It is of clinical importance to find and diagnose pancreatic NET in patients with VHL as early as possible. It is recommended that comprehensive surveillance for abdominal manifestations in VHL patients including pancreatic NET should start from the age of 15. In general, pancreatic NET with or without VHL disease show a slow growth phenotype and patients have a good prognosis. VHL patients at lower metastatic risk from pancreatic NET should be spared the risks of surgical resection.
Footnotes
Supported by The Health and Labor Sciences Research Grant for a nationwide clinical survey and establishment of guidelines in the diagnosis and treatment for von Hippel-Lindau disease in Japan
Peer reviewer: Yasuhiro Fujino, MD, PhD, Director, Department of Surgery, Hyogo Cancer Center, 13-70 Kitaoji-cho, Akashi 673-8558, Japan
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM
References
- 1.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 2.Maher ER, Iselius L, Yates JR, Littler M, Benjamin C, Harris R, Sampson J, Williams A, Ferguson-Smith MA, Morton N. Von Hippel-Lindau disease: a genetic study. J Med Genet. 1991;28:443–447. doi: 10.1136/jmg.28.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binkovitz LA, Johnson CD, Stephens DH. Islet cell tumors in von Hippel-Lindau disease: increased prevalence and relationship to the multiple endocrine neoplasias. AJR Am J Roentgenol. 1990;155:501–505. doi: 10.2214/ajr.155.3.1974734. [DOI] [PubMed] [Google Scholar]
- 4.Eras M, Yenigun M, Acar C, Kumbasar B, Sar F, Bilge T. Pancreatic involvement in Von Hippel-Lindau disease. Indian J Cancer. 2004;41:159–161. [PubMed] [Google Scholar]
- 5.Hammel PR, Vilgrain V, Terris B, Penfornis A, Sauvanet A, Correas JM, Chauveau D, Balian A, Beigelman C, O'Toole D, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087–1095. doi: 10.1053/gast.2000.18143. [DOI] [PubMed] [Google Scholar]
- 6.Hough DM, Stephens DH, Johnson CD, Binkovitz LA. Pancreatic lesions in von Hippel-Lindau disease: prevalence, clinical significance, and CT findings. AJR Am J Roentgenol. 1994;162:1091–1094. doi: 10.2214/ajr.162.5.8165988. [DOI] [PubMed] [Google Scholar]
- 7.Libutti SK, Choyke PL, Bartlett DL, Vargas H, Walther M, Lubensky I, Glenn G, Linehan WM, Alexander HR. Pancreatic neuroendocrine tumors associated with von Hippel Lindau disease: diagnostic and management recommendations. Surgery. 1998;124:1153–1159. doi: 10.1067/msy.1998.91823. [DOI] [PubMed] [Google Scholar]
- 8.Libutti SK, Choyke PL, Alexander HR, Glenn G, Bartlett DL, Zbar B, Lubensky I, McKee SA, Maher ER, Linehan WM, et al. Clinical and genetic analysis of patients with pancreatic neuroendocrine tumors associated with von Hippel-Lindau disease. Surgery. 2000;128:1022–1027; discussion 1027-1028. doi: 10.1067/msy.2000.110239. [DOI] [PubMed] [Google Scholar]
- 9.Neumann HP, Dinkel E, Brambs H, Wimmer B, Friedburg H, Volk B, Sigmund G, Riegler P, Haag K, Schollmeyer P. Pancreatic lesions in the von Hippel-Lindau syndrome. Gastroenterology. 1991;101:465–471. doi: 10.1016/0016-5085(91)90026-h. [DOI] [PubMed] [Google Scholar]
- 10.Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995;194:629–642. doi: 10.1148/radiology.194.3.7862955. [DOI] [PubMed] [Google Scholar]
- 11.Yamasaki I, Nishimori I, Ashida S, Kohsaki T, Onishi S, Shuin T. Clinical characteristics of pancreatic neuroendocrine tumors in Japanese patients with von Hippel-Lindau disease. Pancreas. 2006;33:382–385. doi: 10.1097/01.mpa.0000240604.26312.e4. [DOI] [PubMed] [Google Scholar]
- 12.Blansfield JA, Choyke L, Morita SY, Choyke PL, Pingpank JF, Alexander HR, Seidel G, Shutack Y, Yuldasheva N, Eugeni M, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs) Surgery. 2007;142:814–818; discussion 818.e1-818.e2. doi: 10.1016/j.surg.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plöckinger U, Rindi G, Arnold R, Eriksson B, Krenning EP, de Herder WW, Goede A, Caplin M, Oberg K, Reubi JC, et al. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS) Neuroendocrinology. 2004;80:394–424. doi: 10.1159/000085237. [DOI] [PubMed] [Google Scholar]
- 14.Maeda H, Nishimori I, Okabayashi T, Kohsaki T, Shuin T, Kobayashi M, Onishi S, Hanazaki K. Total pancreatectomy for multiple neuroendocrine tumors of the pancreas in a patient with von Hippel-Lindau disease. Clin J Gastroenterol. 2009;2:222–225. doi: 10.1007/s12328-009-0071-2. [DOI] [PubMed] [Google Scholar]
- 15.Reznek RH. CT/MRI of neuroendocrine tumours. Cancer Imaging. 2006;6:S163–S177. doi: 10.1102/1470-7330.2006.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langrehr JM, Bahra M, Kristiansen G, Neumann HP, Neumann LM, Plöckinger U, Lopez-Hänninen E. Neuroendocrine tumor of the pancreas and bilateral adrenal pheochromocytomas. A rare manifestation of von Hippel-Lindau disease in childhood. J Pediatr Surg. 2007;42:1291–1294. doi: 10.1016/j.jpedsurg.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Triponez F, Goudet P, Dosseh D, Cougard P, Bauters C, Murat A, Cadiot G, Niccoli-Sire P, Calender A, Proye CA. Is surgery beneficial for MEN1 patients with small (< or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg. 2006;30:654–662; discussion 663-664. doi: 10.1007/s00268-005-0354-9. [DOI] [PubMed] [Google Scholar]
- 18.Lairmore TC, Chen VY, DeBenedetti MK, Gillanders WE, Norton JA, Doherty GM. Duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg. 2000;231:909–918. doi: 10.1097/00000658-200006000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]