Abstract
AIM: To investigate the effects of RNA interference targeting hepatocyte progenitor kinase-like kinase (HGK) in the invasion and adhesion of hepatocellular carcinoma (HCC) cell line HepG2.
METHODS: Three paired insert DNA fragments specific to HGK gene and one negative control DNA fragment were synthesized and inserted into RNAi-Ready pSIREN-RetroQ-ZsGreen vector. Western blotting assay and real-time reverse transcriptase polymerase chain reaction (RT-PCR) were used to screen the vector with a highest inhibitory rate. The vector was used to generate recombinant retrovirus specific to HGK. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2h-tetrazolium bromide (MTT) assay was used to examine cell growth; wound closure assay and cell adhesion assay were employed to investigate cell migration and adhesion respectively; and transwell assay and three-dimensional culture invasion assay were used to detect cell invasion. The expressions of matrix metalloproteinase (MMP)-2, MMP-9 and nuclear factor (NF)-κB were detected by Western blotting assay.
RESULTS: The real time RT-PCR and Western blotting assay showed that cells transfected with retrovirus mediating RNAi targeting of HGK (RV-shHGK)-1 vector had the strongest inhibition of HGK protein, with an inhibition rate of 76%, and this vector was used to generate recombinant retrovirus RV-shHGK-1. Cell adhesion assay and MTT assay found that cell adhesion and growth of the cells infected with RV-shHGK-1 were significantly lower than those of the control cells (P < 0.05). Wound closure assay, transwell assay and three-dimensional culture invasion assay showed that the cell invasiveness was significantly less in HGK knockdown cells than in the control cells (P < 0.05). The expressions of MMP-2, MMP-9 and NF-κB were inhibited in HepG2 cells infected with RV-shHGK-1.
CONCLUSION: Down-regulation of HGK can obviously inhibit the migration and invasion of HepG2 cells in vitro. HGK may be a new therapeutic target for treatment of HCC.
Keywords: Hepatocellular carcinoma, Hepatocyte progenitor kinase-like kinase, RNA interference, Invasion, Metastasis
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most fatal cancers due to delayed diagnosis and lack of effective treatment options[1]. However, little is known of its pathogenesis by the currently available methods[2]. Hepatocyte progenitor kinase-like kinase (HGK) is a member of the mammalian STE20/MAPK family that is widely overexpressed in certain types of human tumors, including pancreatic carcinoma, prostatic carcinoma, lung cancer and HCC[3-6]. Recent studies suggest that HGK plays an important role in cell migration and invasiveness of prostate and breast cancer cells[3,5,6]. However, the role of HGK overexpression in HCC has been scarcely studied. In this study, we constructed a retrovirus vector mediating RNAi targeting HGK (RV-HGK shRNA). The efficacy of RV-HGK shRNA vectors in interference with HGK was confirmed by real-time reverse transcriptase polymerase chain reaction (RT-PCR) and Western blotting assay. We observed that retrovirus mediating RNAi targeting of HGK (RV-shHGK)-1 suppressed invasion by markedly decreasing the expression of HGK, matrix metalloproteinase (MMP)-2, MMP-9 and nuclear factor (NF)-κB in HepG2 cells, but RV-shHGK-C (control) showed no effect in HepG2 cells. Based on these results, HGK may be a new therapeutic target for treatment of HCC[4,6].
MATERIALS AND METHODS
Cells and reagents
HCC cell line HepG2 was purchased from American Type Culture Collection; RNAi-Ready pSIREN-RetroQ-ZsGreen vector, PT-67 retrovirus packaging cells and Retro-XTM qRT-PCR titer kit from Clontech (Clontech Laboratories, Palo Alto, CA); DMEM high glucose medium and fetal calf serum from Hyclone (Hyclone Laboratories, Logan, Utah); cell lysis solution trizol reagent, liposome lipofectamine™ 2000 and Opti-MEM culture medium from Invitrogen Corporation (Carlsbad, CA, USA); RIPA lysis buffer and real-time RT-PCR kit from Takara Company (Takara Suzo, Kyoto, Japan); HGK and MMP-2 polyclonal antibodies from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA); NF-κB and MMP-9 polyclonal antibodies from Cell Signaling Technology (Beverly, MA); HRP-labeled goat anti-rabbit IgG from Wuhan Boster Company; matrigel from the BD (BD Biosciences, San Jose, CA); fibronectin (FN), laminin (LN) and collagen IV from Sigma (St. Louis, MO); transwell chambers from Corning (Coming Glass Works, Corning, NY, USA); and Western luminescent detection kit [electrochemiluminescence (ECL)] from Pierce Company (Rockford, Illinois, USA). The DNA sequences used in this study were all synthesized in Jiangsu Nantong Bio-Technology Co. Ltd. Other chemicals were obtained in their commercially available highest purity grade.
Generation of recombinant retrovirus
To target the HGK protein for down-regulation by siRNA, we established the RNAi-Ready pSIREN-RetroQ-ZsGreen vector (Clontech) of the RNA interference retrovirus vector specific to HGK gene. The retrovirus vector contains a CMV-driven ZsGreen reporter, which makes it easy to detect the transfection efficiency, and a U6 promoter upstream of the cloning restriction sites (BamHI and EcolI). Three paired insert DNA fragments according to the coding regions of HGK (NM_145686.2), starting at 393, 985 and 2224, were designed using software offered by Qiagen (Valencia, CA). Three vectors were named RV-shHGK-1, RV-shHGK-2 and RV-shHGK-3, respectively (Table 1). RNAi-Ready pSIREN-RetroQ-ZsGreen control vector was constructed according to the manufacturer’s instructions (Clontech), named RV-shHGK-C. All the DNA sequences were synthesized in a pattern as BamHI-sense DNA-loop (TTCAAGACG)-antisense DNA-EcolI. The most effective vector was selected to produce recombinant retrovirus. Recombinant retrovirus vectors were transfected into PT-67 packaging cells (Clontech) by liposome lipofectamine™ 2000 (Invitrogen, USA). After 48 h, the supernatant containing the viral particles was collected, filtered through the 0.45 μm low protein binding syringe filter and the titer of viruses was determined in 293T cells by real time RT-PCR (Takara).
Table 1.
Three vectors specifically interfering with hepatocyte progenitor kinase-like kinase expression and a control vector
| Name | Paired DNA fragment |
| RV-shHGK-1 | 5'-GGATCCTTACAGACCTTGTGAAGAATTCAAGACGTTCTTCACAAGGTCTGTAATTTTTGAATTC-3' |
| 5'-GAATTCAAAAATTACAGACCTTGTGAAGAAGGTGTTGAATTCTTCACAAGGTCTGTAAGGATCC-3' | |
| RV-shHGK-2 | 5'-GGATCCGAAGAAGAGAGGCGAGAAAATTCAAGACGTTTCTCGCCTCTCTTCTTCTTTTTGAATTC-3' |
| 5'-GAATTCAAAAAGAAGAAGAGAGGCGAGAAACGTCTTGAATTTTCTCGCCTCTCTTCTTCGGATCC-3' | |
| RV-shHGK-3 | 5'-GGATCCGAGCAATGGTGAAACGGAATTCAAGACGTTCCGTTTCACCATTGCTCTTTTTTGAATTC-3' |
| 5'-GAATTCAAAAAGAGCAATGGTGAAACGGAACGTCTTGAATTCCGTTTCACCATTGCTCGGATCC-3' | |
| RV-shHGK-C | 5'-GGATCCGGCCAGAGGTTGAAAGTGATTCAAGACGTCACTTTCAACCTCTGGCCTTTTTGAATTC-3' |
| 5'-GAATTCAAAAAGGCCAGAGGTTGAAAGTGACGTCTTGAATCACTTTCAACCTCTGGCCGGATCC-3' |
RV-shHGK: Retrovirus mediating RNAi targeting of hepatocyte progenitor kinase-like kinase.
Recombinant retrovirus infection
HepG2 cells were cultured and plated into 6-well culture plates at 5 × 105 cells/well in DMEM high glucose culture medium with 100 mL/L fetal bovine serum (FBS) at 37°C in a saturated humidified atmosphere containing 50 mL/L CO2. After 24 h, the cells were infected with different viral supernatants, respectively, at a multiplicity of infection (MOI) of 4 transducing units/cell for 24 h[7].
Real-time RT-PCR
The total cellular RNA was isolated from the collected cells by Trizol. The HGK mRNA copies were quantified using the real-time RT-PCR Kit (Takara) on a Bio-Rad iQ5 Sequence Detection System (Bio-Rad, Hercules, CA, USA). The primers of HGK and β-actin were as follows: forward primer 5'-GAGCAGTGCTGAAGGCCAAAG-3', reverse primer 5'-ACTAAAGTCCTGTGGCGATGGAA-3' and forward primer 5'-CACCAACTGGGACGACAT-3', reverse primer 5'-ATCTGGGTCATCTTCTCGC-3'. The housekeeping gene β-actin was amplified to normalize the HGK mRNA expression. The copy numbers of β-actin and HGK were determined according to each standard curve. Relative HGK mRNA levels were determined by comparing the PCR cycle thresholds between the cDNA of HGK and that of β-actin.
Western blotting assay
The cellular total proteins were extracted by RIPA Lysis Buffer (Takara) and 50 μg was added to each well for 100 g/L sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After semi-dry electric transfer at 45 V for 1 h, polyvinylidine fluoride membrane was sealed with 50 g/L skim milk powder at room temperature (15-25°C) for 6 h, and incubated at 4°C overnight with rabbit anti-human HGK, NF-κB, MMP-2 and MMP-9 polyclonal antibodies (1:500) and mouse anti-human β-actin (1:1000) monoclonal antibody, respectively. After being washed, it was incubated at 37°C for 2 h with second-antibody (1:1000) and colored by ECL. It was scanned for the relative value of protein expression in gray scale by Image-Pro plus software 6.0.
Cell viability assays
Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2h-tetrazolium bromide (MTT) assay in 96-well micro-culture plates. In brief, HepG2 cells (1 × 104/well) were routinely cultured in DMEM with 100 mL/L FBS for 12 h. After infection for 1, 2, 3 and 4 d, 10 μL of 5 g/L MTT (Sigma) was added into each well and incubated for another 4 h. The supernatant was then discarded and 0.1 mL dimethyl sulfoxide was added into each well. After oscillated for 5 min, the absorbance of 96-well culture plate was read by a Bio-Rad 550 Microplate Reader (Hercules, CA) at a wavelength of 490 nm. There were two groups in this study: HepG2 cells infected with RV-shHGK-1 and RV-shHGK-C. Each group had 3 parallel wells to replicate the test 3 times. A L02 cell sample of gradiently diluted parental HepG2 cells was used to draw the standard curve of cell number.
Cell adhesion assay
HepG2 cells (5 × 104/well) infected with RV-shHGK-1, RV-shHGK-C and parental HepG2 cells were suspended and added into 96-well micro-culture plates coated with FN, LN, and collagen IV (3 μg/well), respectively. After cultured in the incubator at 37°C for 1 h, the cells were allowed to adhere, and then washed three times with PBS, fixed with 40 g/L formaldehyde, stained with 5 g/L crystal violet in 200 mL/L methanol/water and viewed under microscope. The amount of bound cells was estimated by reading the absorbance at a wavelength of 490 nm[8]. Triplicate determinations were done for each group.
Wound closure assay
The 6-well culture plates were coated with PBS containing 10 g/L collagen IV. HepG2 cells trypsinized with 2.5 g/L trypsin, were recultured (5 × 105 cells/well) in DMEM high glucose medium containing 100 mL/L FBS for 6 h to get adherent monolayer growth state. The cells in 6-well culture plates were scratched with the tip of 200 μL pipet, and recultured in the incubator at 37°C for 24 h. At the time points of 0, 12 and 24 h after scratched, four visions of each well were photographed under microscope and observed for the scratch healing.
Transwell assay
The transwell chamber (Corning) containing an 8-μm pore size polycarbonate membrane filter was coated with a matrigel (Sigma) and inserted in a 24-well culture plate. Cells collected after infection for 48 h were adjusted to a density of 1 × 109 cells/L with serum-free DMEM high glucose culture medium. The cell suspension of 200 μL was added into the upper transwell chamber and 500 μL DMEM high glucose medium containing 200 mL/L FBS was added into the lower transwell chamber. After recultured with 50 mL/L CO2 at 37°C for 24 h, the transwell chambers were inverted and stained with hematoxylin and eosin. Five fields were randomly selected and the number of trans-membrane cells was counted.
Three-dimensional culture invasion assay
Matrigel 100 μL (Sigma) was dropped onto 8 mm × 8 mm coverslips in a 24-well culture plate. The matrigel was allowed to polymerize for 30 min at room temperature. HepG2 cells were trypsinized and re-suspended in complete medium (DMEM + 100 mL/L FBS) at 2.5 × 107 cells/L, and 500 μL of the cell suspension was dropped onto the matrigel to analyze the ability of cell invasion. After 24 h incubation at 37°C, cells were observed under inverted microscope[5,9].
Statistical analysis
SPSS 13.0 software was used for statistical analysis, and t test was used in the comparison between two groups. One-way analysis of variance was used for multiple comparisons. There was statistical significance when P value was less than 0.05.
RESULTS
Retrovirus mediated knockdown of HGK by RNA interference
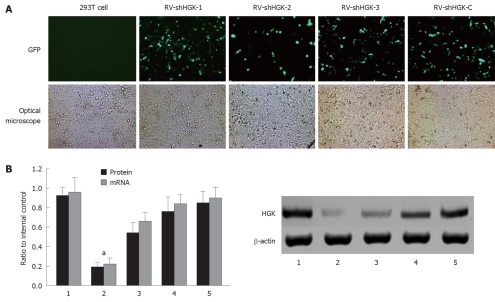
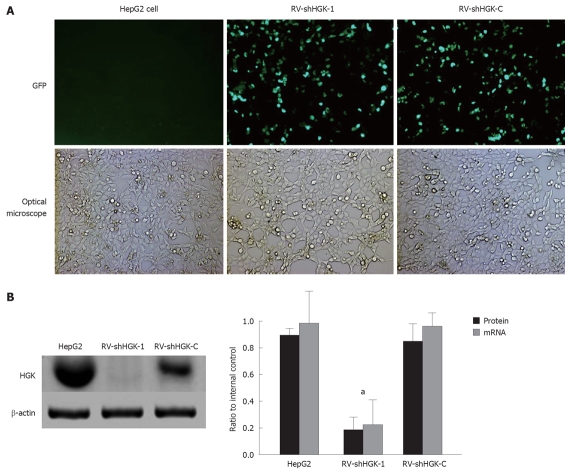
Three plasmids containing shHGK (1-3) and plasmid shHGK-C were transfected into HepG2 cells, respectively. The expression of reporter ZsGreen in HepG2 cells was observed under a fluorescent microscope 48 h after transfection with plasmids containing shHGK (1-3) and shHGK-C (Figure 1A). To evaluate the interfering effects of vectors in the expression of HGK, real-time quantitative RT-PCR and Western-blotting were performed. The relative ratios of HGK mRNA and protein of the HepG2 cells transfected with vectors were analyzed (Figure 1B). The results showed that vector shHGK-1 could significantly suppress the expression of HGK. It was the most effective RNA interference vector. In the following experiment, vectors shHGK-1 and shHGK-C were used to produce retrovirus RV-shHGK-1 and RV-shHGK-C, the virus titers were approximately 1 × 1011 v.p./L. To evaluate the effect of RV-shHGK-1 in the expression of HGK, real-time quantitative RT-PCR and Western-blotting were performed to determine the mRNA and protein level in HepG2 cells infected by RV-shHGK-1 at an indicated MOI (MOI = 4). The HGK expression was significantly suppressed in cells infected with RV-shHGK-1 compared with RV-shHGK-C (P < 0.05, Figure 2A and B), showing the markedly inhibitory effect of RV-shHGK-1 system on HGK expression in HepG2 cells.
Figure 1.
Selection of the most effective hepatocyte progenitor kinase-like kinase specific shRNA expression vector in 293T cells. A: Phase contrast and GFP expression under a fluorescent microscope after 48 h in 293T cells; B: Protein mRNA and hepatocyte progenitor kinase-like kinase (HGK) levels after HepG2 cells were treated with different vectors detected by real-time reverse transcriptase polymerase chain reaction and Western boltting assay. The vector retrovirus mediating RNAi targeting of HGK (RV-shHGK)-1 significantly inhibited HGK expression in HepG2 cells, aP < 0.05 vs HepG2 cells and HepG2 cells transfected with RV-shHGK-C vector. 1: 293T cells; 2: Transfection of RV-shHGK-1 vector in 293T cells; 3: Transfection of RV-shHGK-2 vector; 4: Transfection of RV-shHGK-3 vector; 5: Transfection of RV-shHGK-C vector (original magnification × 200).
Figure 2.
Hepatocyte progenitor kinase-like kinase expression suppressed by RV-shHGK-1 retrovirus in HepG2 cells. A: HepG2 cells infected with retrovirus mediating RNAi targeting of hepatocyte progenitor kinase-like kinase (RV-shHGK)-1 or RV-shHGK-C (multiplicity of infection = 4), GFP expression and the phase contrast images after 48 h (original magnification × 200); B: Protein and mRNA levels of hepatocyte progenitor kinase-like kinase (HGK) after HepG2 cells were treated with different retrovirus detected by real-time reverse transcriptase polymerase chain reaction and Western boltting assay. The retrovirus RV-shHGK-1 significantly inhibited HGK expression in HepG2 cells, aP < 0.05 vs HepG2 cells and HepG2 cells infected with RV-shHGK-C retrovirus.
Down-regulation of HGK inhibits HepG2 cell growth
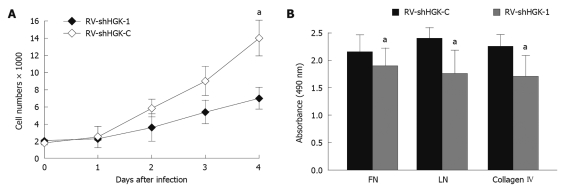
To validate the HGK functions in cell growth regulation, cell proliferation was monitored for 4 d after HepG2 cells were infected with RV-shHGK-1 and RV-shHGK-C. At day 4, the growth of HepG2 cells was reduced to 45% (Figure 3A), indicating that the suppression of HGK expression apparently reduces the growth of HepG2 cells.
Figure 3.
Down-regulation of hepatocyte progenitor kinase-like kinase inhibits HepG2 cell growth and adhesion (methyl thiazolyl tetrazolium assay). A: HepG2 cell growth was significantly suppressed by retrovirus mediating RNAi targeting of hepatocyte progenitor kinase-like kinase (RV-shHGK)-1 vs RV-shHGK-C group; B: RV-shHGK-1 inhibited HepG2 adhesion to fibronectin (FN), laminin (LN) and collagen IV. aP < 0.05 vs control.
Down-regulation of HGK inhibits HepG2 cell adhesion to extracellular matrix proteins
To verify the effects of HGK expression in adhesion to extracellular matrix (ECM) proteins in HCC, HepG2 cells infected with RV-shHGK-1, RV-shHGK-C and parental HepG2 cells were examined by cell adhesion assay. As shown in Figure 3B, down-regulation of HGK could inhibit HepG2 cell adhesion to FN, LN and collagen IV. The results indicated that HGK participated in cell adhesion.
Down-regulation of HGK inhibits HepG2 cell invasion
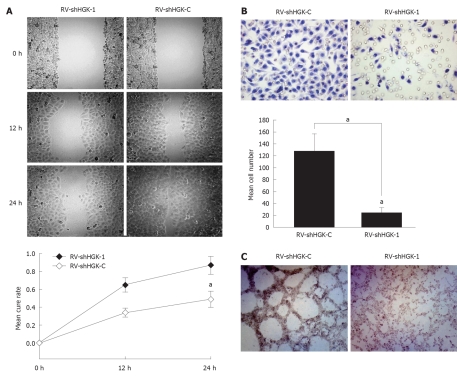
In order to confirm whether HGK is involved in the process of HepG2 cell motility and invasiveness, the wound closure assay and transwell assay were used to determine the impact of RV-shHGK-1 on HepG2 cell invasion. Cells infected with RV-shHGK-1 migrated more tardily and filled in the wound more slowly than RV-shHGK-C infected cells (P < 0.05, Figure 4A). Consistent with the data of wound closure assay, the results of transwell assay showed that the invasiveness through matrigel was significantly decreased in RV-shHGK-1 infected HepG2 cells, compared with that in RV-shHGK-C infected cells (P < 0.05, Figure 4B).
Figure 4.
Down-regulation of hepatocyte progenitor kinase-like kinase inhibits HepG2 cell invasion. A: HepG2 cell invasiveness was significantly suppressed by retrovirus mediating RNAi targeting of hepatocyte progenitor kinase-like kinase (RV-shHGK)-1 vs RV-shHGK-C group (original magnification × 200, aP < 0.05); B: The blue-stained cells are those invading the ECMatrix and migrating through the polycarbonate membrane to the lower surface of the membrane (original magnification × 200, aP < 0.05 vs HepG2 cells infected with RV-shHGK-C); C: HepG2 cells infected with RV-shHGK-1 do not form the structure patterned network of interconnected loops (original magnification × 200).
The effects of HGK on tumor metastasis and invasion in HCC were further examined in HepG2 cells under three-dimensional cell culture. As shown in Figure 4C, the structure patterned network of interconnected loops was not found in RV-shHGK-1 infected HepG2 cells under three-dimensional cell culture, but present in RV-shHGK-C infected cells. These results indicated that RV-shHGK-1 system could dramatically suppress the aggressive ability of HepG2 cells.
Down-regulation of HGK inhibits MMP-2, MMP-9 and NF-κB expression in HepG2 cells
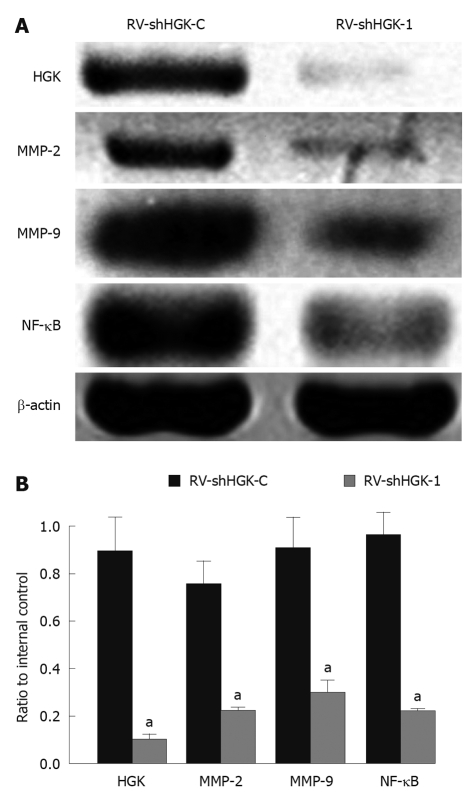
Because MMPs and NF-κB are known to play important roles in HCC cell invasion and metastasis, and down-regulation of HGK can inhibit HepG2 cell invasion ability, Western blotting assay was performed to investigate whether the down-regulation of HGK in HepG2 cells affects MMPs and NF-κB expression levels. The results showed that the expressions of MMP-2, MMP-9 and NF-κB were inhibited in HepG2 cells infected with RV-shHGK-1 compared with that in control cells (Figure 5A and B).
Figure 5.
Hepatocyte progenitor kinase-like kinase, matrix metalloproteinase-2, matrix metalloproteinase-9 and nuclear factor-κB expressions in HepG2 cells (Western blotting). A: Retrovirus mediating RNAi targeting of hepatocyte progenitor kinase-like kinase (RV-shHGK)-1 significantly inhibited matrix metalloproteinase (MMP)-2, MMP-9 and nuclear factor (NF)-κB in HepG2 cells. MMP-2 protein expression in each group (Western blotting); B: Densitometric analysis hepatocyte progenitor kinase-like kinase (HGK), MMP-2, MMP-9 and NF-κB protein expression in HepG2 cells infected with RV-shHGK-1 or RV-shHGK-C, respectively. aP < 0.05 vs HepG2 cells infected with RV-shHGK-C.
DISCUSSION
Mitogen-activated protein (MAP) kinases are cellular regulators that play significant roles in various diverse processes as apoptosis, differentiation, and proliferation[10-13]. Their activation can be mediated by many upstream kinases that regulate the downstream MAP kinases[14]. Sterile 20 kinases were identified in the past few years. One of the protein kinase families can act upstream of MAP kinases[15]. These kinases can be divided into two structural classes, the p21-activated protein kinases and the germinal center protein kinases[16,17]. HGK is a member of the germinal center protein kinases. More recently, increasing data have shown that HGK can control cellular events ranging from cell motility, cell adhesion and invasion in some cancer cells, including breast cancer, lung cancer, pancreatic cancer and colon carcinoma[5,6,18].Thus, the potentiality of regulating cancer cell invasion and metastasis is to control HGK expression. However, the effect of HGK in HCC is unclear.
To evaluate the functional role of HGK in HCC, knockdown experiments were performed in HepG2 cells. Recently, RNAi has been widely used to silence the expression of many targets. In this study, three RNA interference retrovirus vectors targeting HGK were designed and synthesized, and the most effective vector was selected to generate the recombinant retrovirus named RV-shHGK-1. Meanwhile, the control retrovirus was produced and named RV-shHGK-C. Because the inhibitory effect of RNAi is related to the specificity to its target sequence, real-time RT-PCR and Western blotting assay were used to confirm the effect of RV-shHGK-1 in HepG2 cells. The results showed that the expressions of HGK mRNA and protein were visibly blocked, indicating that RV-shHGK-1 can down-regulate the expression of HGK effectively.
Metastasis is known to be the biggest problem to oncologists and the main cause of death in cancer patients[19]. The metastatic process involves a series of interdependent events, including cancer cell growth, invasion and adhesion[20]. In this study, the effects of RV-shHGK-1 on HepG2 cell growth and adhesion were investigated by MTT assay and cell adhesion assay. The results indicated that the growth and the adhesion of HepG2 cells were inhibited by RV-shHGK-1. Notably, the down-regulation of cell adhesion and the disassembly of basement membrane (BM) are the key elements of cell metastasis[21,22]. FN, LN and collagen IV are known to be the major components of BM[23]. In cell adhesion assay, the adhering ability to BM of HepG2 cells infected with RV-shHGK-1 was inhibited compared with HepG2 cells infected with RV-shHGK-C. The results revealed that HGK may correlate with cell adhesion. In addition, the ability of cell invasion was examined by transwell assay and three-dimensional invasion assay. The results showed that down-regulation of HGK could inhibit the invasiveness of HepG2 cells. To further investigate the involvement of HGK in cell invasion, whether the down-regulation of HGK in HepG2 cells affects MMPs and NF-κB expression level was also observed by Western blotting assay. MMPs and NF-κB are considered to be important in cancer cell invasion by degrading components of the BMs and ECM[24,25]. The effect of HGK in regulation of cell adhesion and invasion may be related to regulation of the HGK expression.
Overall, these observations are in agreement with recent reports that examined the effects of HGK in other cancer cells[5,26]. These findings have laid a foundation for further investigation into the manipulation of HGK in the treatment of HCC.
COMMENTS
Background
Hepatocyte progenitor kinase-like kinase (HGK) is a member of the mammalian STE20/MAPK family that is overexpressed in human hepatocellular carcinoma (HCC) compared with normal liver tissues.
Research frontiers
Recent studies suggest that HGK plays an important role in cell migration and invasiveness of prostate and breast cancer cells. However, the role of HGK overexpression in HCC has been scarcely studied. To explore the role of HGK in HCC, the authors investigated the effects of HGK through RNA interference mediated by retrovirus.
Innovations and breakthroughs
The effects of HGK in regulation of cell adhesion and invasion may be related to regulation of the HGK expression. HGK may be a new therapeutic target for treatment of HCC.
Applications
The down-regulation of the HGK expression appears to inhibit the invasiveness and HCC cell migration. HGK may be an important factor promoting HCC progression. Therefore, it can be used as a new therapeutic target for treatment of HCC.
Terminology
HGK is a member of the germinal center protein kinases, which has been found to control cellular events ranging from cell motility, cell adhesion and invasion in some cancer cells, including breast cancer, lung cancer, pancreatic cancer and colon carcinoma.
Peer review
The authors have knocked-down the expression of HGK in HepG2 using RNAi and investigated the effect on cell proliferation, migration and invasion in vitro. The results have shown that decreasing the expression of HGK inhibited cellular proliferation, invasiveness and migratory capability using different assay systems. Expression of matrix metalloproteinase (MMP)-2, MMP-9 and nuclear factor-κB were decreased with HGK knocked-down. All the experiments were well conducted and controlled. The conclusions made are valid.
Footnotes
Supported by The Natural Science Foundation of China, No. 81071692
Peer reviewer: Dr. Eric WC Tse, MB, PhD, Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong, China
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma: novel molecular approaches for diagnosis, prognosis, and therapy. Annu Rev Med. 2010;61:317–328. doi: 10.1146/annurev.med.080608.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins CS, Hong J, Sapinoso L, Zhou Y, Liu Z, Micklash K, Schultz PG, Hampton GM. A small interfering RNA screen for modulators of tumor cell motility identifies MAP4K4 as a promigratory kinase. Proc Natl Acad Sci USA. 2006;103:3775–3780. doi: 10.1073/pnas.0600040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urzúa U, Roby KF, Gangi LM, Cherry JM, Powell JI, Munroe DJ. Transcriptomic analysis of an in vitro murine model of ovarian carcinoma: functional similarity to the human disease and identification of prospective tumoral markers and targets. J Cell Physiol. 2006;206:594–602. doi: 10.1002/jcp.20522. [DOI] [PubMed] [Google Scholar]
- 5.Wright JH, Wang X, Manning G, LaMere BJ, Le P, Zhu S, Khatry D, Flanagan PM, Buckley SD, Whyte DB, et al. The STE20 kinase HGK is broadly expressed in human tumor cells and can modulate cellular transformation, invasion, and adhesion. Mol Cell Biol. 2003;23:2068–2082. doi: 10.1128/MCB.23.6.2068-2082.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang JJ, Wang H, Rashid A, Tan TH, Hwang RF, Hamilton SR, Abbruzzese JL, Evans DB, Wang H. Expression of MAP4K4 is associated with worse prognosis in patients with stage II pancreatic ductal adenocarcinoma. Clin Cancer Res. 2008;14:7043–7049. doi: 10.1158/1078-0432.CCR-08-0381. [DOI] [PubMed] [Google Scholar]
- 7.Morgan JR, LeDoux JM, Snow RG, Tompkins RG, Yarmush ML. Retrovirus infection: effect of time and target cell number. J Virol. 1995;69:6994–7000. doi: 10.1128/jvi.69.11.6994-7000.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian AR, Zhang W, Cao JP, Yang PF, Gao X, Wang Z, Xu HY, Weng YY, Shang P. Downregulation of CD147 expression alters cytoskeleton architecture and inhibits gelatinase production and SAPK pathway in human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2008;27:50. doi: 10.1186/1756-9966-27-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cong R, Sun Q, Yang L, Gu H, Zeng Y, Wang B. Effect of Genistein on vasculogenic mimicry formation by human uveal melanoma cells. J Exp Clin Cancer Res. 2009;28:124. doi: 10.1186/1756-9966-28-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence MC, Jivan A, Shao C, Duan L, Goad D, Zaganjor E, Osborne J, McGlynn K, Stippec S, Earnest S, et al. The roles of MAPKs in disease. Cell Res. 2008;18:436–442. doi: 10.1038/cr.2008.37. [DOI] [PubMed] [Google Scholar]
- 12.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury P, Udupa KB. Nicotine as a mitogenic stimulus for pancreatic acinar cell proliferation. World J Gastroenterol. 2006;12:7428–7432. doi: 10.3748/wjg.v12.i46.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Yao Z, Zhou G, Wang XS, Brown A, Diener K, Gan H, Tan TH. A novel human STE20-related protein kinase, HGK, that specifically activates the c-Jun N-terminal kinase signaling pathway. J Biol Chem. 1999;274:2118–2125. doi: 10.1074/jbc.274.4.2118. [DOI] [PubMed] [Google Scholar]
- 16.Delpire E. The mammalian family of sterile 20p-like protein kinases. Pflugers Arch. 2009;458:953–967. doi: 10.1007/s00424-009-0674-y. [DOI] [PubMed] [Google Scholar]
- 17.Fanger GR, Gerwins P, Widmann C, Jarpe MB, Johnson GL. MEKKs, GCKs, MLKs, PAKs, TAKs, and tpls: upstream regulators of the c-Jun amino-terminal kinases? Curr Opin Genet Dev. 1997;7:67–74. doi: 10.1016/s0959-437x(97)80111-6. [DOI] [PubMed] [Google Scholar]
- 18.Yustein JT, Xia L, Kahlenburg JM, Robinson D, Templeton D, Kung HJ. Comparative studies of a new subfamily of human Ste20-like kinases: homodimerization, subcellular localization, and selective activation of MKK3 and p38. Oncogene. 2003;22:6129–6141. doi: 10.1038/sj.onc.1206605. [DOI] [PubMed] [Google Scholar]
- 19.Rudmik LR, Magliocco AM. Molecular mechanisms of hepatic metastasis in colorectal cancer. J Surg Oncol. 2005;92:347–359. doi: 10.1002/jso.20393. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama T, Miyanishi K, Hayashi T, Sato Y, Niitsu Y. Colorectal cancer: genetics of development and metastasis. J Gastroenterol. 2006;41:185–192. doi: 10.1007/s00535-006-1801-6. [DOI] [PubMed] [Google Scholar]
- 22.Wendt MK, Schiemann WP. Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis. Breast Cancer Res. 2009;11:R68. doi: 10.1186/bcr2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian B, Li Y, Ji XN, Chen J, Xue Q, Ye SL, Liu YK, Tang ZY. Basement membrane proteins play an active role in the invasive process of human hepatocellular carcinoma cells with high metastasis potential. J Cancer Res Clin Oncol. 2005;131:80–86. doi: 10.1007/s00432-004-0614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das R, Philip S, Mahabeleshwar GH, Bulbule A, Kundu GC. Osteopontin: it's role in regulation of cell motility and nuclear factor kappa B-mediated urokinase type plasminogen activator expression. IUBMB Life. 2005;57:441–447. doi: 10.1080/15216540500159424. [DOI] [PubMed] [Google Scholar]
- 25.Lu KW, Tsai ML, Chen JC, Hsu SC, Hsia TC, Lin MW, Huang AC, Chang YH, Ip SW, Lu HF, et al. Gypenosides inhibited invasion and migration of human tongue cancer SCC4 cells through down-regulation of NFkappaB and matrix metalloproteinase-9. Anticancer Res. 2008;28:1093–1099. [PubMed] [Google Scholar]
- 26.Rangaswami H, Bulbule A, Kundu GC. JNK1 differentially regulates osteopontin-induced nuclear factor-inducing kinase/MEKK1-dependent activating protein-1-mediated promatrix metalloproteinase-9 activation. J Biol Chem. 2005;280:19381–19392. doi: 10.1074/jbc.M414204200. [DOI] [PubMed] [Google Scholar]