Abstract
AIM: To assess long-term efficacy of initially successful endo-sponge assisted therapy.
METHODS: Between 2006 and 2009, consecutive patients who had undergone primary successful endo-sponge treatment of anastomotic leakage following rectal cancer surgery were enrolled in the study. Patients were recruited from 6 surgical departments in Vienna. Clinical and oncologic outcomes were assessed through routine endoscopic and radiologic follow-up examination.
RESULTS: Twenty patients (7 female, 13 male) were included. The indications for endo-sponge treatment were anastomotic leakage (n = 17) and insufficiency of a rectal stump after Hartmann’s procedure (n = 3). All patients were primarily operated for rectal cancer. The overall mortality rate was 25%. The median follow-up duration was 17 mo (range 1.5-29.8 mo). Five patients (25%) developed a recurrent abscess. Median time between last day of endo-sponge therapy and occurrence of recurrent abscess was 255 d (range 21-733 d). One of these patients was treated by computed tomography-guided drainage and in 3 patients Hartmann’s procedure had to be performed. Two patients (10%) developed a local tumor recurrence and subsequently died.
CONCLUSION: Despite successful primary outcome, patients who receive endo-sponge therapy should be closely monitored in the first 2 years, since recurrence might occur.
Keywords: Rectal surgery, Anastomotic leakage, Endo-sponge, Endo-vacuum treatment
INTRODUCTION
Anastomotic leakage following rectal cancer surgery is regarded as one of the most feared postoperative complications[1]. Together with a significant increase in both morbidity and mortality rate, several studies found anastomotic leaks after curative colorectal resections for malignancy associated with poor survival and a higher tumor recurrence rate[2,3].
Once leakage occurs, treatment strategies are limited and depend on the septic condition of affected patients. The anastomosis can be renewed or taken down; simultaneously a protective stoma is usually created. In cases of mild sepsis and localised abscesses, computed tomography (CT)-guided drainage can be attempted.
The use of an endoluminal vacuum therapy represents a novel method to treat patients with anastomotic leakage after rectal resection[4]. An open-pored sponge is inserted into the abscess cavity and connected via draining tube to a vacuum drainage system. Through continuous negative pressure a shrinking and cleaning of the wound can be achieved.
To date, only few studies are available focusing on endo-sponge treatment[5-7]. Taking into account the limited number of included patients, promising short-term results have been reported. Weidenhagen et al[4] described a success rate of nearly 96% in their series. Another study observed complete healing in only 75% of patients[8].
Long-term results after primary successful endo-sponge therapy have not been published to date. Therefore, it is still under debate as to whether vacuum therapy-derived granulation tissue is stable and not prone to develop recurrent abscesses. This problem might occur especially in those patients who require further chemotherapy after endo-sponge therapy has been completed. Another question arises as to whether endo-sponge therapy negatively influences oncologic results. It is tempting to speculate that through stimulation of granulation tissue angiogenesis, growth of residual local tumor cells becomes reactivated.
The present multicenter study was designed to evaluate long-term efficacy of initially successful endo-sponge assisted therapy after anastomotic leakage following rectal resection for malignancy. Additionally, oncologic outcome was assessed.
MATERIALS AND METHODS
Between 2006 and 2009, consecutive patients who had undergone initially successful endo-sponge assisted treatment of anastomotic leakage following rectal cancer surgery were included in the study. Patients were recruited from 6 surgical departments in Vienna. The study was approved by the institutional Ethics Committee.
All patients were initially operated for rectal cancer. Rectal tumor was located in the upper third of the rectum in 2 patients, in the middle third in 8 patients and in the lower third in 10 patients. In 15 patients a low anterior resection, in 3 patients a rectal resection with a coloanal anastomosis, and in 2 patients a Hartmann’s procedure with resection of the tumor were performed. The type of anastomosis was end-end in 14 patients, side-end in 1 patient and a J-Pouch in 3 patients. A protective stoma was created in 14 patients [ileostomy: n = 10 patients, colostomy: n = 4 patients, none (including Hartmann’s procedure): n = 6 patients]. One patient had neoadjuvant short-term radiotherapy and 5 patients required long- term radio/chemotherapy before primary rectal resection. The remaining 14 patients did not receive any additional treatment prior to surgery. According to the International Union Against Cancer (UICC) classification, the rectal cancers were grouped as stage I (n = 9), stage II (n = 5), stage III (n = 4) and stage IV (n = 2).
The indication for endo-sponge assisted therapy was an anastomotic leakage in 17 patients and insufficiency of a rectal stump after Hartmann’s procedure in 3 patients. The Hartmann’s procedures were conducted after anastomotic leakage following anterior rectal resection, after rectal perforation following palliative endoscopic stent implantation, and as primary treatment of bleeding rectal cancer in a patient who was in poor condition.
Time from primary operation to anastomotic leakage showed a median duration of 12.5 d (range 3-668 d).
In 2 patients (10%), a surgical approach (abscess drainage and stoma formation) and in 3 patients (15%) CT-guided drainage were attempted prior to endo-sponge treatment.
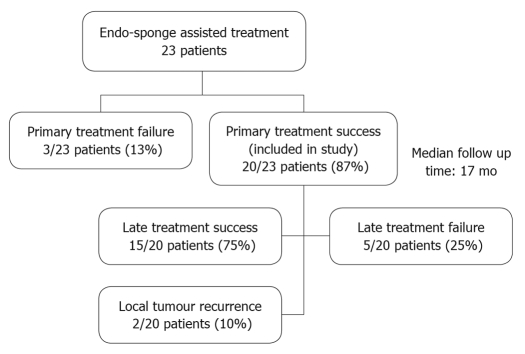
Those patients with an unsuccessful primary endo-sponge treatment were excluded. The study design is outlined in Figure 1.
Figure 1.
Study outline.
Patients were investigated during annual routine follow-up examination. A colonoscopy was performed to assess the anastomotic site with regard to local recurrence and reappearance of any leakage. Whenever possible a CT or magnetic resonance tomography scan was performed to obtain additional information.
Patient data were obtained from the institutional database of each participating hospital and individual chart reviews retrospectively. Additional information was gathered through a face to face questioning.
The technique of inserting an endo-sponge (B. Braun, Austria, GmbH) and installing an endoluminal vacuum system has been described previously[9].
Statistical analysis
Continuous data are shown with median and minimum-maximum values. Associations between late treatment failure and variables were analyzed by nonparametric Wilcoxon rank sum test. A P-value ≤ 0.05 was considered significant. All data were collected and statistically analyzed using SPSS (14.0.1. Chicago, USA).
RESULTS
Twenty patients (7 female, 13 male) who had primary successful endo-sponge therapy were included in the study. The median body mass index (BMI) was 26.5 kg/m2 (range 17.3-31.3 kg/m2) and the median age was 66.3 years (range 54.8-91.2 years). The median follow-up duration was 17.1 mo (range 1.5-29.8 mo). Five patients (25%) died during the follow-up period but were included in the analysis: four patients died due to tumor progression (2 patients had liver metastases at the time of endo-sponge therapy) and one patient died because of acute liver failure due to known liver cirrhosis.
One patient developed a benign anal stenosis which was improved by anal dilatation.
Interestingly, we identified five patients (25%) who developed a recurrent symptomatic abscess. The following interventions were performed for recurrent abscess: in 3 patients anastomosis was taken down by Hartmann’s procedure, which classified abscesses as stage C (definition by the International Study Group of Rectal Cancer)[10]; one abscess was classified as stage B, requiring CT-guided drainage; one patient had only minimal clinical signs, therefore abscess was classified as stage A and further therapy (e.g. Hartmann’s procedure) is still under discussion.
Additionally, we analyzed whether we could identify predictive factors for recurrent abscess formation (Table 1). None of the demographic factors, such as age or BMI were significantly different between successful and refractory groups. The same factors related to standard tumor care were similar in both groups. Specifically, neoadjuvant radiotherapy or radiochemotherapy and adjuvant chemotherapy administration was evenly distributed in both groups. With respect to anti-angiogenic treatment with Bevacizumab (Avastin®, Roche), there was 1 patient in the successful group and 1 patient in the treatment failure group.
Table 1.
Characteristics of patients with and without successful long-term outcome after endo-sponge treatment1
| Successful outcome | No successful outcome | |
| No. of patients | 15 | 5 |
| Age (yr) | 67 (55-91.2) | 63.4 (54.8-69.8) |
| BMI | 24.9 (17.3-31.2) | 24.5 (19.7-28.9) |
| Male:female ratio | 10:5 | 3:2 |
| Neoadjuvant treatment | ||
| None | 10 | 4 |
| Radiotherapy | 1 | 0 |
| Chemo/radiotherapy | 4 | 1 |
| Indication | ||
| Anastomotic leakage | 12 | 5 |
| Rectal stump insufficiency | 3 | 0 |
| Interval between primary operation and anastomotic leakage (d) | 12.5 (3-668) | 42 (21-668) |
| Duration of endo-sponge therapy (d) | 21 (7-56) | 21 (19-106) |
| Chemotherapy after endo-sponge therapy | 3 | 1 |
| Follow up time (mo) | 16.1 (1.5-29.8) | 17.1(8.38-26.2) |
| Interval between final endo-sponge therapy and recurrent abscess (d) | 240 (21-730) | |
| Local tumor recurrence | 1 | 1 |
Values are given as absolute numbers and frequencies (%) or as medians (min-max). BMI: Body mass index.
One patient received Bevacizumab before endo-sponge therapy was started. This patient also developed a recurrent abscess.
Similarly, there was no difference in the indication for endo-sponge therapy (anastomotic leakage vs rectal stump insufficiency). At the time of endo-sponge treatment 9 patients (45%) had a diverting ileostomy and 8 patients (40%) a colostomy. Three patients (15%) were treated without protective stoma. There was no difference regarding recurrence between stoma groups during therapy. In 13 patients (76.5%) the stoma was closed after successful endo-sponge treatment.
We then analyzed endo-sponge therapy-related factors. The procedure for endo-sponge was the same in both groups with a sponge change at 2-3 d intervals. One patient had a fibrin glue injection after successful treatment to improve local healing. In one patient a stent was placed through the anal canal for 7 d until sufficient healing was achieved. Both patients had no recurrence during the follow-up period. The median duration of endo-sponge therapy was 21 d in both groups. Median interval between primary operation and onset of anastomotic leakage was longer in the non-successful group. This difference was statistically significant (P < 0.05).
With regard to the oncological outcome, two patients (10%) developed a local tumor recurrence during the follow-up period and subsequently died. The UICC stage was II and III, respectively. Neither patient received any additional chemo- or radiotherapy prior to or after primary rectal resection. Notably, this was declined by one of them. Two patients (10%) developed distant liver metastasis.
DISCUSSION
This multicenter study was conducted to assess long-term outcome after primary successful endo-sponge treatment in patients with rectal cancer. The major finding of this analysis is the fact that 25% of patients developed recurrent abscess formation.
The endo-sponge system has been shown to be a suitable instrument for the treatment of anastomotic leaks following rectal cancer surgery. Glitsch et al[11] reported primary successful healing in 16 of 17 included patients. Each patient had a follow-up colonoscopy 2 mo after complete closure of the abscess cavity. Other studies with slightly longer follow-up periods found initial success rates ranging from 75% to nearly 100%[4-6].
Thus, this is the first study that indicates that late treatment failure might also occur. The consequence of our finding is a recommendation of ongoing surveillance (at least 2 years) for recurrent abscess, since this might be easier to treat if diagnosed early.
In the present study, for 2 of 5 patients who developed a new abscess, recurrence occurred 30 d after endo-sponge therapy had been finished. The reason for reappearance, which obviously occurred soon after the therapy was considered a success, remains unclear. It is tempting to speculate that the wound healing was superficial, whereas in deeper areas of the cavity a new abscess formation was established. Notably, both patients had a late anastomotic leak (668 and 427 d after initial surgery), which could have further contributed to the worse outcome.
In one patient endo-sponge treatment was continued for 106 d. This extended duration was due to the firm desire of the patient not to receive a stoma formation. Although after that time period the abscess cavity was healed, such prolonged treatment might be predictive for a less successful outcome. This observation was comparable to previous published results[9].
We have also attempted to identify predictive parameters for reoccurrence of abscess. Taking into account the limited number of patients available for statistical analysis, we found that late onset of anastomotic leakage is associated with a tendency for a high probability of recurrent abscess formation. This correlated with the fact that late onset of endo-sponge therapy correlates with a worse outcome of primary therapy[8].
Interestingly, concomitant chemo- or radiotherapy was not associated with an increased risk of recurrent abscess. This is in contrast to the finding of von Bernstorff et al[12], who did observe a negative influence of radio-chemotherapy on the primary success rate. Therefore, the present finding is important as it relieves fear associated with application of chemotherapy in this setting.
The humanized monoclonal antibody Bevacizumab represents another therapy associated with potential colorectal anastomotic complications[13]. Of the 20 patients analyzed herein, 10% received Bevacizumab after initial endo-sponge therapy. At present, we do not see antiangiogenic therapy as an obstacle for endo-sponge therapy.
In addition, we observed 2 local recurrences of tumor growth. We cannot differentiate whether this high number of patients is an effect of anastomotic leakage or correlates with endo-sponge treatment. There are controversies regarding the impact of anastomotic leakage on oncologic outcome. Several studies found anastomotic leaks significantly associated with a worse cancer specific mortality and increased local recurrence[3,14,15]. However, others could not detect any correlation[16,17].
Since endo-sponge therapy is believed to rely on induction of angiogenesis, induction of tumor growth is a potential theoretical concern[18]. The question remains whether this has a biological consequence since angiogenesis formation is also part of chronic inflammation. Furthermore, tumor recurrence might also be induced by chronic inflammation. There is growing evidence that systemic inflammatory response, characterized by raised levels of circulating C-reactive protein, predicts poor survival in patients undergoing curative resection for colorectal malignancies[19,20]. Thus, it is possible that an ongoing septic condition caused by anastomotic leakage might negatively influence oncologic outcome. On the other hand, a more rapid healing as induced by endo-sponge therapy might even be beneficial.
Before the era of endo-sponge treatment anastomotic leakage was treated by defunctioning stoma. This procedure could save anastomosis in around 60% to 75% of cases[21,22]. Thus, the question arises, whether despite obvious macroscopic early improvements, endo-sponge treatment is of great benefit. Based on late treatment failures requiring Hartmann’s procedures, we believe that a randomized trial with 2 years of follow-up will be necessary to solve this question. In the meantime, endo-sponge treatment can still be recommended as a therapeutic option for anastomotic leakage. The great advantages of this novel method are still rapid leak control and the avoidance of a defunctioning stoma in selected cases.
In conclusion, in the current multicenter study we observed that 25% of patients developed recurrent abscesses after primary successful endo-sponge assisted treatment for anastomotic leakage following rectal resection. Notably, 2 patients showed a local tumor recurrence during the follow-up period. This has to be investigated in larger series of patients.
COMMENTS
Background
Anastomotic leakage following rectal cancer surgery is regarded as one of the most feared postoperative complications with a considerable morbidity and mortality rate. The incidence varies from 2% to 19%. Strategies to treat anastomotic leaks are limited and depend on the septic condition of affected patients. The use of an endoluminal vacuum therapy (endo-sponge) represents a novel method to treat patients with anastomotic leakage after rectal resection.
Research frontiers
An open-pored endo-sponge is inserted into the abscess cavity and connected via draining tube to a vacuum drainage system. Through continuous negative pressure a shrinking and cleaning of the wound can be achieved. The research hotspot is to assess the long-term effect of endo-sponge therapy. It is still under debate, as to whether vacuum therapy-derived granulation tissue is stable and not prone to develop recurrent abscesses.
Innovations and breakthroughs
It has been recently published that endo-sponge assisted closure can be used as an alternative in the treatment of colorectal anastomotic leakage. The few available studies focusing on endo-sponge treatment found short-term healing rates of nearly 96%. Others concluded that extended leakages should be treated by different approaches having little probability of successful healing but these can lead to discomfort for the patient.
Applications
In the present study it was observed that 25% of patients developed recurrent abscesses after primary successful endo-sponge assisted treatment for anastomotic leakage following rectal resection. Consequently, patients need to be monitored closely as abscess recurrence might occur. Notably, 2 patients showed a local tumor recurrence during the follow-up period. This has to be investigated in larger series of patients.
Terminology
Endo-sponge treatment means the insertion of an open-pored sponge into the abscess cavity. The endo-sponge is connected via draining tube to a vacuum drainage system. Through continuous negative pressure a shrinking and cleaning of the wound can be achieved.
Peer review
This is a well-written hypothesis-generating paper. Although there might be many contributing factors, the authors present data that suggest endo-sponge may promote abscess formation a long time after treatment of a failed low anastomosis. This is worth knowing.
Footnotes
Peer reviewer: Julio Mayol, MD, PhD, Department of Digestive Surgery, Hospital Clinico San Carlos, MARTIN-LAGOS S/n, Madrid, 28040, Spain
S- Editor Wang JL L- Editor Logan S E- Editor Lin YP
References
- 1.Jech B, Felberbauer FX, Herbst F. Complications of elective surgery for rectal cancer. European Surgery. 2007;39:8–14. [Google Scholar]
- 2.Jung SH, Yu CS, Choi PW, Kim DD, Park IJ, Kim HC, Kim JC. Risk factors and oncologic impact of anastomotic leakage after rectal cancer surgery. Dis Colon Rectum. 2008;51:902–908. doi: 10.1007/s10350-008-9272-x. [DOI] [PubMed] [Google Scholar]
- 3.Petersen S, Freitag M, Hellmich G, Ludwig K. Anastomotic leakage: impact on local recurrence and survival in surgery of colorectal cancer. Int J Colorectal Dis. 1998;13:160–163. doi: 10.1007/s003840050158. [DOI] [PubMed] [Google Scholar]
- 4.Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008;22:1818–1825. doi: 10.1007/s00464-007-9706-x. [DOI] [PubMed] [Google Scholar]
- 5.Mees ST, Palmes D, Mennigen R, Senninger N, Haier J, Bruewer M. Endo-vacuum assisted closure treatment for rectal anastomotic insufficiency. Dis Colon Rectum. 2008;51:404–410. doi: 10.1007/s10350-007-9141-z. [DOI] [PubMed] [Google Scholar]
- 6.Nagell CF, Holte K. Treatment of anastomotic leakage after rectal resection with transrectal vacuum-assisted drainage (VAC). A method for rapid control of pelvic sepsis and healing. Int J Colorectal Dis. 2006;21:657–660. doi: 10.1007/s00384-005-0083-4. [DOI] [PubMed] [Google Scholar]
- 7.Van Koperen PJ, Van Berge Henegouwen MI, Slors JF, Bemelman WA. Endo-sponge treatment of anastomotic leakage after ileo-anal pouch anastomosis: report of two cases. Colorectal Dis. 2008;10:943–944. doi: 10.1111/j.1463-1318.2008.01485.x. [DOI] [PubMed] [Google Scholar]
- 8.van Koperen PJ, van Berge Henegouwen MI, Rosman C, Bakker CM, Heres P, Slors JF, Bemelman WA. The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc. 2009;23:1379–1383. doi: 10.1007/s00464-008-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riss S, Stift A, Meier M, Haiden E, Grünberger T, Bergmann M. Endo-sponge assisted treatment of anastomotic leakage following colorectal surgery. Colorectal Dis. 2010;12:e104–e108. doi: 10.1111/j.1463-1318.2009.01885.x. [DOI] [PubMed] [Google Scholar]
- 10.Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Glitsch A, von Bernstorff W, Seltrecht U, Partecke I, Paul H, Heidecke CD. Endoscopic transanal vacuum-assisted rectal drainage (ETVARD): an optimized therapy for major leaks from extraperitoneal rectal anastomoses. Endoscopy. 2008;40:192–199. doi: 10.1055/s-2007-995384. [DOI] [PubMed] [Google Scholar]
- 12.von Bernstorff W, Glitsch A, Schreiber A, Partecke LI, Heidecke CD. ETVARD (endoscopic transanal vacuum-assisted rectal drainage) leads to complete but delayed closure of extraperitoneal rectal anastomotic leakage cavities following neoadjuvant radiochemotherapy. Int J Colorectal Dis. 2009;24:819–825. doi: 10.1007/s00384-009-0673-7. [DOI] [PubMed] [Google Scholar]
- 13.August DA, Serrano D, Poplin E. "Spontaneous," delayed colon and rectal anastomotic complications associated with bevacizumab therapy. J Surg Oncol. 2008;97:180–185. doi: 10.1002/jso.20938. [DOI] [PubMed] [Google Scholar]
- 14.Bell SW, Walker KG, Rickard MJ, Sinclair G, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg. 2003;90:1261–1266. doi: 10.1002/bjs.4219. [DOI] [PubMed] [Google Scholar]
- 15.McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92:1150–1154. doi: 10.1002/bjs.5054. [DOI] [PubMed] [Google Scholar]
- 16.den Dulk M, Marijnen CA, Collette L, Putter H, Påhlman L, Folkesson J, Bosset JF, Rödel C, Bujko K, van de Velde CJ. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96:1066–1075. doi: 10.1002/bjs.6694. [DOI] [PubMed] [Google Scholar]
- 17.Jörgren F, Johansson R, Damber L, Lindmark G. Anastomotic leakage after surgery for rectal cancer: a risk factor of local recurrence, distant metastasis and reduced cancer-specific survival? Colorectal Dis. 2009:Epub ahead of print. doi: 10.1111/j.1463-1318.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- 18.Schintler MV, Prandl EC. Vacuum-assisted closure - what is evidence based? Eur Surg. 2008;40:11–18. [Google Scholar]
- 19.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen HJ, Christensen IJ, Sørensen S, Moesgaard F, Brünner N. Preoperative plasma plasminogen activator inhibitor type-1 and serum C-reactive protein levels in patients with colorectal cancer. The RANX05 Colorectal Cancer Study Group. Ann Surg Oncol. 2000;7:617–623. doi: 10.1007/BF02725342. [DOI] [PubMed] [Google Scholar]
- 21.Khan AA, Wheeler JM, Cunningham C, George B, Kettlewell M, Mortensen NJ. The management and outcome of anastomotic leaks in colorectal surgery. Colorectal Dis. 2008;10:587–592. doi: 10.1111/j.1463-1318.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 22.Nesbakken A, Nygaard K, Lunde OC. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br J Surg. 2001;88:400–404. doi: 10.1046/j.1365-2168.2001.01719.x. [DOI] [PubMed] [Google Scholar]