Abstract
AIM: To determine the role of epidermal growth factor-like repeats and discoidin I-like domains 3 (EDIL3) in pathogenesis of hepatocellular carcinoma (HCC) by investigating the EDIL3 expression in HCC and its prognostic value for HCC.
METHODS: EDIL3 expression was detected in 101 HCC surgical tissue samples with immunohistochemistry method, and its relation with clinicopathologic features and prognosis of HCC patients was analyzed.
RESULTS: EDIL3 was highly expressed in 48.5% of the HCC patients. Although the EDIL3 expression level did not correlate with any clinicopathological parameters, Kaplan-Meier survival analysis showed that high expression level of EDIL3 resulted in a significantly poor prognosis of HCC patients (log-rank test, P = 0.010). Multivariate Cox’s analysis showed that the EDIL3 expression level was a significant and independent prognostic parameter for the overall survival rate of HCC patients (hazard ratio = 1.978, 95% confidence interval = 1.139-3.435, P = 0.015).
CONCLUSION: High expression level of EDIL3 predicts poor prognosis of HCC patients. EDIL3 may be a potential target of antiangiogenic therapy for HCC.
Keywords: Epidermal growth factor-like repeats and discoidin I-like domains 3, Hepatocellular carcinoma, Prognosis, Angiogenesis
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide[1]. Due to its high recurrence rate after surgical resection and chemotherapy resistance, the total survival rate of HCC patients is only 3%-5%[2]. There is evidence that angiogenesis plays a critical role in the development and progression of HCC, a highly vascularized tumor. It has been shown that microvascular density is significantly correlated with HCC prognosis and postoperative recurrence[3-6]. Therefore, it is crucial to understand the molecular mechanism underlying angiogenesis of HCC.
Epidermal growth factor-like repeats and discoidin I-like domains 3 (EDIL3), also known as endothelial cell locus 1 (DEL1), is a glycoprotein secreted by endothelial cells, which was isolated and identified from the embryonic mouse lung in 1998[7]. EDIL3, containing a signal peptide, 3 epidermal growth factor-like repeats, and 2 discoidin I-like repeats, initiates angiogenesis by binding to integrin αvβ5 on resting endothelium and may play an important role in vessel wall remodeling and development during angiogenesis[8]. It has been confirmed that EDIL3 can induce mesentery and cerebral angiogenesis in mice[9,10]. Both animal experiments and clinical studies have demonstrated that EDIL3 gene therapy is effective for ischemic disease[11-13]. There is evidence that EDIL3 is involved in tumor angiogenesis and plays an important role in interaction between HCC cells and endothelial cells[14-16]. It has been reported that the EDIL3 gene is over-expressed in HCC[17]. As far as we know, no report is available on the actual expression level of EDIL3 and the correlation between clinicopathologic features and prognosis of HCC patients. Therefore, in this study, we investigated the EDIL3 protein expression profile in 101 primary HCC patients, and found that EDIL3 was highly expressed in 48.5% of the HCC patients, suggesting that the high expression level of EDIL3 is a reliable indicator for the poor prognosis of HCC patients.
MATERIALS AND METHODS
Patients and tissue samples
A total of 101 patients with primary HCC, who underwent routine surgery at Sun Yat-Sen University Cancer Center in 1998-2002, were enrolled in this study. Their diagnosis was made by a pathological examination, and all patients did not receive any preoperative treatment before admission. Histological cell types were assigned following the WHO classification criteria. Paraffin-embedded HCC tissue samples (n = 101) were obtained from surgical pathology files at Sun Yat-Sen University Cancer Center. All tissue blocks were cut into consecutive 4-μm thick sections. The patients were followed up for 3-81 mo with a median follow-up time of 30 mo.
Immunohistochemistry
The sections were deparaffinized in xylene and rehydrated with graded ethanol. Following rehydration, endogenous peroxidase was inactivated with 0.3% hydrogen peroxide. Antigen was retrieved by putting the sections in a boiling ethylenediaminetetraacetic acid buffer (1 mmol/L, pH 8.0) for 15 min. After rinsed with phosphate buffered saline (PBS), the sections were incubated with rabbit anti-EDIL3 antibody (Sigma-Aldrich, Inc. USA) diluted in a working solution (1:200) at 37°C for 1 h, and then with horseradish peroxidase (ChemMate™ EnVision™ detection kit) at 37°C for 30 min. Finally, the visualization signal was developed with 3,3’-diaminobenzidine tetrahydrochloride and all sections were then counterstained with hematoxylin. For negative controls, the sections were incubated in a solution without anti-EDIL3 antibody under the same experimental conditions. The percent of positive cells was scored as ‘0’ (< 5%, negative), ‘1’ (5%-25%, sporadic), ‘2’ (25%-50%, focal), and ‘3’ (> 50%, diffuse), respectively. The staining intensity was scored as ‘0’ (no staining), ‘1’ (weakly stained), ‘2’ (moderately stained) and ‘3’ (strongly stained), respectively. Both the percent of positive cells and cell staining intensity were decided in a double-blinded manner. The final EDIL3 immunostaining score was calculated using the percent of positive cell score × staining intensity score ranging 0-9. High EDIL3 expression level was defined as a total score ≥ 4, and low EDIL3 expression level as a total score < 4.
Statistical analysis
All statistical analyses were performed using the SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Correlation of EDIL3 expression with immunohistochemistry and clinicopathologic parameters was evaluated by chi-square test or Fisher’s exact probability test. Overall survival rate was calculated with the Kaplan-Meier method and the difference in survival curves was analyzed by the log-rank test. The follow-up time was calculated from the date of surgery to the date of death, or the last known follow-up. Independent prognostic factors were analyzed by the Cox proportional hazards regression model. P < 0.05 was considered statistically significant.
RESULTS
Expression of EDIL3 in HCC tissues
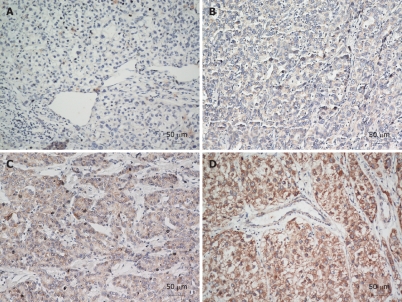
The expression level of EDIL3 protein in 101 HCC tissue samples was measured with immunohistochemical staining. The immunoreactivity of EDIL3 in cytoplasm was detected (Figure 1). Overall, EDIL3 was positively and negatively expressed in 95 (94.06%) and 6 (5.94%) of the 101 HCC patients, respectively (Figure 1B-D, total score ≥ 1; Figure 1A, total score = 0). EDIL3 was highly and lowly expressed in 49 (48.5%) and 52 (51.5%) of the 101 HCC patients, respectively (Figure 1C and D, total score ≥ 4; Figure 1A and B, total score < 4). No significant difference was found in EDIL3 expression level and clinicopathologic parameters including age, gender, tumor size, histological differentiation, liver cirrhosis, metastasis, recurrence, hepatitis B virus infection, and serum α-fetoprotein (Table 1).
Figure 1.
Immunohistochemical analysis of epidermal growth factor-like repeats and discoidin I-like domains 3 in hepatocellular carcinoma patients. A: Negative expression of epidermal growth factor-like repeats and discoidin I-like domains 3 protein; B: Low expression level of epidermal growth factor-like repeats and discoidin 1-like domains 3 protein; C, D: High expression level of epidermal growth factor-like repeats and discoidin 1-like domains 3 protein. A, B: Original magnification × 100; C, D: Original magnification × 200.
Table 1.
Relation between epidermal growth factor-like repeats and discoidin I-like domains 3 expression in hepatocellular carcinoma and clinicopathological features of hepatocellular carcinoma patients
| Clinicopathologic features (n) | Low EDIL3 (total score < 4) n = 52 | High EDIL3 (total score ≥ 4) n = 49 | P value |
| Age (yr) | 0.889 | ||
| < 60 (57) | 29 | 28 | |
| ≥ 60 (44) | 23 | 21 | |
| Gender | 0.648 | ||
| Male (87) | 44 | 43 | |
| Female (14) | 8 | 6 | |
| Tumor size (cm) | 0.491 | ||
| < 5 (26) | 16 | 10 | |
| 5-10 (58) | 28 | 30 | |
| > 10 (17) | 8 | 9 | |
| Histological differentiation | 0.379 | ||
| Well (17) | 9 | 8 | |
| Moderate (63) | 35 | 28 | |
| Poor (21) | 8 | 13 | |
| Liver cirrhosis | 0.617 | ||
| Yes (50) | 27 | 23 | |
| No (51) | 25 | 26 | |
| Metastasis | 0.202 | ||
| Yes (15) | 10 | 5 | |
| No (86) | 42 | 44 | |
| Recurrence | 0.817 | ||
| Yes (38) | 19 | 19 | |
| No (63) | 33 | 30 | |
| HBSAg status | 0.527 | ||
| Positive (90) | 45 | 45 | |
| Negative (11) | 7 | 4 | |
| Serum AFP | 0.707 | ||
| Positive (62) | 31 | 31 | |
| Negative (39) | 21 | 18 | |
EDIL3: Epidermal growth factor-like repeats and discoidin I-like domains 3; HBSAg: Hepatitis B surface antigen; AFP: α-fetoprotein.
Correlation between high EDIL3 protein expression level and low survival rate of HCC patients
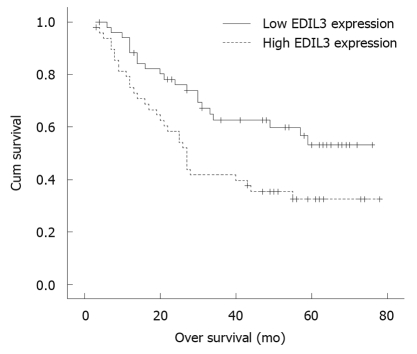
The prognostic effect of EDIL3 on the overall survival rate of HCC patients with a high or low EDIL3 protein expression level was compared using Kaplan-Meier survival curves and the log-rank test respectively, showing that high expression level of EDIL3 protein was a significant prognostic factor for poor overall survival rate of HCC patients. The 5-year survival rate of HCC patients with a high or a low EDIL3 protein expression level was 32.4% and 53.2%, respectively. A significant difference was observed on the Kaplan-Meier survival curves for HCC patients with a high or a low expression level of EDIL3 (P = 0.010, log-rank test, Figure 2). Univariate Cox regression analysis showed that EDIL3 expression level and tumor size were the significant prognostic factors for HCC patients (Table 2). The relative risk was 2.019 times higher for patients with a high EDIL3 expression level than for those with a low EDIL3 expression level. Multivariate Cox regression analysis showed that EDIL3 expression might play a role in prediction of the overall survival rate of HCC patients (P = 0.015, Table 2).
Figure 2.
Overall survival rate of hepatocellular carcinoma patients estimated according to the epidermal growth factor-like repeats and discoidin I-like domains 3 expression level in hepatocellular carcinoma tissue samples (Kaplan-Meier method) with immunohistochemical staining.
Table 2.
Univariate and multivariate analysis showing the overall survival rate for hepatocellular carcinoma patients
| Variables |
Univariate analysis |
Multivariate analysis |
||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| EDIL3 | 2.019 | 1.163-3.504 | 0.013 | 1.978 | 1.139-3.435 | 0.015 |
| Gender | 0.658 | 0.283-1.529 | 0.330 | |||
| Age | 0.817 | 0.487-1.370 | 0.443 | |||
| Tumor size | 1.662 | 1.133-2.437 | 0.009 | 1.492 | 0.980-2.270 | 0.062 |
| Histologic grade | 1.304 | 0.866-1.963 | 0.204 | |||
| Cirrhosis | 0.683 | 0.410-1.139 | 0.144 | |||
| HBsAg status | 1.244 | 0.565-2.738 | 0.588 | |||
| Serum AFP | 1.592 | 0.923-2.744 | 0.094 | |||
| Metastasis | 1.407 | 0.731-2.707 | 0.307 | |||
| Recurrence | 1.349 | 0.804-2.263 | 0.257 | |||
HR: Hazard ratio; CI: Confidence interval; EDIL3: Epidermal growth factor-like repeats and discoidin I-like domains 3; HBSAg: Hepatitis B surface antigen; AFP: α-fetoprotein.
DISCUSSION
HCC is one of the most malignant cancers with no effective chemotherapy available for it at present. Anti-angiogenic therapy is a novel systemic therapy for HCC by blocking the effect of angiogenic factors and inhibiting the proliferation of endothelial cells[18]. Further studies are needed to elucidate the mechanism underlying the angiogenesis of HCC in order to rationally use antiangiogenic agents in treatment of HCC. It has been shown that EDIL3 can enhance tumor angiogenesis by stimulating the proliferation of resting endothelial cells[14], indicating that EDIL3 may be a new target for antiangiogenic therapy and play an important role in the pathogenesis of HCC.
In the present study, EDIL3 was positively expressed in 94.06% of HCC patients and highly expressed in 48.5% of HCC patients, which is consistent with the reported data[14], indicating that human hepatocarcinoma cells can secrete EDIL3 involved in the angiogenesis of HCC.
In this study, the prognosis of HCC patients with a high expression level of EDIL3 was poor, and Cox regression analysis indicated that high expression level of EDIL3 was a significant prognostic factor for a poor overall survival rate of HCC patients, suggesting that EDIL3 may become a novel prognostic marker for HCC. However, the expression level of EDIL3 did not correlate with any clinicopathological parameters of HCC, which can be explained as follows. First, multivariate Cox regression analysis indicated that EDIL3 expression level was an independent predictor for the overall survival rate of HCC patients, and EDIL3 might have a direct effect on the prognosis of HCC patients rather than other factors. Second, some known risk factors for HCC, such as histological differentiation, presence of metastasis and disease recurrence, were not correlated with the prognosis of HCC.
Angiogenesis plays an important role in development and progression of HCC[19] that is the balanced result of the actions of multiple angiogenic and antiangiogenic factors from both tumor and host cells[18]. EDIL3 protein is involved in adhesion of HCC cells and HCC-derived endothelial cells, and initiates angiogenesis by binding to αvβ3 and αvβ5 in endothelial cells[8,16]. It has been confirmed that the expression level of αvβ3 and αvβ5 is higher in HCC-derived endothelial cells than in normal liver sinusoidal endothelial cells, suggesting that HCC cells with a high EDIL3 expression level can stimulate the growth of vascular endothelial cells and promote the angiogenesis in HCC. Furthermore, it has been shown that EDIL3 can prolong the survival time of endothelial cells by down-regulating their apoptosis-related gene expression[20], indicating that HCC cells with a high EDIL3 expression level can also promote angiogenesis by inhibiting the apoptosis of endothelial cells.
In conclusion, the prognosis of patients with a high expression level of the EDIL3 protein is poor, which may be attributed to the relation between EDIL3 and HCC angiogenesis. Current evidence identifies EDIL3 as a potential target of antiangiogenic therapy for HCC.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is a high malignancy with a poor prognosis. Angiogenesis plays a critical role in the development and progression of HCC. Epidermal growth factor (EGF)-like repeats and discoidin I-like domains 3 (EDIL3) can enhance tumor angiogenesis by stimulating the proliferation of resting endothelial cells and may be involved in HCC angiogenesis.
Research frontiers
EDIL3 is involved in tumor angiogenesis and plays an important role in interaction between HCC and endothelial cells. However, no report is available on the actual expression level of EDIL3 and its correlation with clinicopathologic features and prognosis of HCC patients. In this study, the EDIL3 protein expression profile in primary HCC was studied.
Innovations and breakthroughs
In this study, EDIL3 was expressed in most HCC patients and highly expressed in 48.5% of the HCC patients. Furthermore, this is the first study to report that high expression level of EDIL3 in HCC tissues was a reliable indicator for the poor prognosis of HCC patients. The prognosis of HCC patients with a high expression level of EDIL3 protein was poor, which may be attributed to the relation between EDIL3 and HCC angiogenesis.
Applications
High expression level of EDIL3 in HCC was a significant prognostic factor for a poor overall survival rate of HCC patients, indicating that EDIL3 can become a novel prognostic marker and a target of antiangiogenic therapy for HCC.
Terminology
EDIL3, an acronym for “EGF-like repeats and discoidin I-like domains 3”, contains a signal peptide, 3 epidermal growth factor-like repeats, and 2 discoidin I-like repeats. It is a glycoprotein secreted by endothelial cells.
Peer review
This is an interesting study. However, it is unclear why EDIL3 expression was associated with a poor prognosis, and factors known to have a negative impact on the outcome such as tumor size, histological differentiation, the presence of metastasis and disease recurrence. The authors should explain these findings in the DISCUSSION section.
Acknowledgments
The authors thank Professor Min-Shan Chen and Dr. Xiong-Hao Pang for their help in collecting tumor tissue samples.
Footnotes
Supported by National Natural Science Foundation of China, No. u0772002
Peer reviewer: Dr. BS Anand, Professor, Digestive Diseases Section (111D), VA Medical Center, 2002 Holcombe Blvd., Houston, TX 77030, United States
S- Editor Wang YR L- Editor Wang XL E- Editor Lin YP
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Poon D, Anderson BO, Chen LT, Tanaka K, Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 3.El-Assal ON, Yamanoi A, Soda Y, Yamaguchi M, Igarashi M, Yamamoto A, Nabika T, Nagasue N. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554–1562. doi: 10.1002/hep.510270613. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan ST, Wong J. Tumor microvessel density as a predictor of recurrence after resection of hepatocellular carcinoma: a prospective study. J Clin Oncol. 2002;20:1775–1785. doi: 10.1200/JCO.2002.07.089. [DOI] [PubMed] [Google Scholar]
- 5.Sun HC, Tang ZY, Li XM, Zhou YN, Sun BR, Ma ZC. Microvessel density of hepatocellular carcinoma: its relationship with prognosis. J Cancer Res Clin Oncol. 1999;125:419–426. doi: 10.1007/s004320050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto A, Dhar DK, El-Assal ON, Igarashi M, Tabara H, Nagasue N. Thymidine phosphorylase (platelet-derived endothelial cell growth factor), microvessel density and clinical outcome in hepatocellular carcinoma. J Hepatol. 1998;29:290–299. doi: 10.1016/s0168-8278(98)80015-9. [DOI] [PubMed] [Google Scholar]
- 7.Hidai C, Zupancic T, Penta K, Mikhail A, Kawana M, Quertermous EE, Aoka Y, Fukagawa M, Matsui Y, Platika D, et al. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev. 1998;12:21–33. doi: 10.1101/gad.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong J, Eliceiri B, Stupack D, Penta K, Sakamoto G, Quertermous T, Coleman M, Boudreau N, Varner JA. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest. 2003;112:30–41. doi: 10.1172/JCI17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hidai C, Kawana M, Habu K, Kazama H, Kawase Y, Iwata T, Suzuki H, Quertermous T, Kokubun S. Overexpression of the Del1 gene causes dendritic branching in the mouse mesentery. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1165–1175. doi: 10.1002/ar.a.20247. [DOI] [PubMed] [Google Scholar]
- 10.Fan Y, Zhu W, Yang M, Zhu Y, Shen F, Hao Q, Young WL, Yang GY, Chen Y. Del-1 gene transfer induces cerebral angiogenesis in mice. Brain Res. 2008;1219:1–7. doi: 10.1016/j.brainres.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman PM, Mendelsohn F, Henry TD, Hermiller JB, Litt M, Saucedo JF, Weiss RJ, Kandzari DE, Kleiman N, Anderson RD, et al. Results from a phase II multicenter, double-blind placebo-controlled study of Del-1 (VLTS-589) for intermittent claudication in subjects with peripheral arterial disease. Am Heart J. 2007;153:874–880. doi: 10.1016/j.ahj.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Quezada A, Larson J, French M, Ponce R, Perrard J, Durland R, Coleman M. Biodistribution and safety studies of hDel-1 plasmid-based gene therapy in mouse and rabbit models. J Pharm Pharmacol. 2004;56:177–185. doi: 10.1211/0022357022584. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan S, Olin JW, Young S, Erikson M, Grossman PM, Mendelsohn FO, Regensteiner JG, Hiatt WR, Annex BH. Design of the Del-1 for therapeutic angiogenesis trial (DELTA-1), a phase II multicenter, double-blind, placebo-controlled trial of VLTS-589 in subjects with intermittent claudication secondary to peripheral arterial disease. Hum Gene Ther. 2004;15:619–624. doi: 10.1089/104303404323142060. [DOI] [PubMed] [Google Scholar]
- 14.Aoka Y, Johnson FL, Penta K, Hirata Ki K, Hidai C, Schatzman R, Varner JA, Quertermous T. The embryonic angiogenic factor Del1 accelerates tumor growth by enhancing vascular formation. Microvasc Res. 2002;64:148–161. doi: 10.1006/mvre.2002.2414. [DOI] [PubMed] [Google Scholar]
- 15.Zou X, Qiao H, Jiang X, Dong X, Jiang H, Sun X. Downregulation of developmentally regulated endothelial cell locus-1 inhibits the growth of colon cancer. J Biomed Sci. 2008;16:33. doi: 10.1186/1423-0127-16-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu JX, Zhang WJ, Ye LY, Wu LQ, Zhu GJ, Yang ZH, Grau GE, Lou JN. The role of adhesion molecules, alpha v beta 3, alpha v beta 5 and their ligands in the tumor cell and endothelial cell adhesion. Eur J Cancer Prev. 2007;16:517–527. doi: 10.1097/CEJ.0b013e3280145c00. [DOI] [PubMed] [Google Scholar]
- 17.Luo JH, Ren B, Keryanov S, Tseng GC, Rao UN, Monga SP, Strom S, Demetris AJ, Nalesnik M, Yu YP, et al. Transcriptomic and genomic analysis of human hepatocellular carcinomas and hepatoblastomas. Hepatology. 2006;44:1012–1024. doi: 10.1002/hep.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151–167. doi: 10.1016/j.canlet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma. Anat Rec (Hoboken) 2008;291:721–734. doi: 10.1002/ar.20668. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Longaker MT, Yang GP. Anti-apoptotic effects of the angiogenic factor Del1 on endothelial cells. J Surg Res. 2006;130:237. [Google Scholar]