Abstract
During brain development, GABA and glycine switch from being depolarizing to being hyperpolarizing neurotransmitters. This conversion results from a gradual decrease in the chloride electrochemical equilibrium potential (ECl) of developing neurons, which correlates to an increase in the expression or activity of the potassium chloride cotransporter, KCC2. However, evidence as to whether KCC2 expression is sufficient, in and of itself, to induce this switch is lacking. In order to address this question, we used a gain-of-function approach by over-expressing human KCC2 (hKCC2) in immature cortical neurons, before endogenous up-regulation of KCC2. We found that premature expression of hKCC2 produced a substantial negative shift in the GABA reversal potential and decreased or abolished GABA-elicited calcium responses in cultured neurons. We conclude that KCC2 expression is not only necessary but is also sufficient for ending the depolarizing period of GABA in developing cortical neurons.
Keywords: Ca2+, cortex, depolarization, development
Introduction
GABA and glycine, the major inhibitory neurotransmitters in the central nervous system, activate ligand-gated anion channels that lead to membrane hyperpolarization in mature neurons (Bormann et al., 1987). In the immature brain, however, GABA and glycine, via activation of GABAA and glycine receptors, produce membrane depolarizations and, in many systems, are considered to be transiently excitatory (Ben Ari, 2002; Kandler et al., 2002). The developmental conversion from excitatory to inhibitory is caused by a decrease in the intracellular Cl− concentration ([Cl−]i; Owens et al., 1996; Ehrlich et al., 1999; Rivera et al., 1999). In immature neurons, the electrochemical equilibrium potential for chloride (ECl) lies above the resting membrane potential (Vrest) and, as a result, activation of GABAA or glycine receptors results in an net efflux of Cl− and membrane depolarization. During maturation, regulation of Cl− shifts ECl values negative to Vrest and activation of GABAA or glycine receptors produces Cl− influx and membrane hyperpolarization.
The neuron-specific potassium chloride cotransporter KCC2 (Payne et al., 1996; Payne, 1997) plays an important role in generating and maintaining an ECl below Vrest. This is supported by a number of studies that have demonstrated a correlation between the GABA reversal potential (EGABA) and the expression level (Clayton et al., 1998; Lu et al., 1999; Rivera et al., 1999; Coull et al., 2003; Galanopoulou et al., 2003; Shibata et al., 2004; Stein et al., 2004), membrane location (Balakrishnan et al., 2003), phosphorylation (Kelsch et al., 2001; Vale et al., 2003), and/ or activity of KCC2 (Woodin et al., 2003). Loss-of-function studies using antisense KCC2 RNA (Rivera et al., 1999), targeted gene knockout (Hubner et al., 2001), or gene knockdown (Woo et al., 2002; Zhu et al., 2004) have shown that KCC2 is necessary for creating and maintaining a low [Cl−]i. However, it has yet to be established whether KCC2 is also sufficient, in and of itself, for decreasing ECl. Here, we have addressed this issue by prematurely over-expressing human KCC2, hKCC2 (Song et al., 2002) in tissue culture of embryonic cortical neurons which, lack or have a very low level of KCC2. Parts of the data presented here have been published previously in abstract form (Lee et al., 2003).
Materials and methods
Subcloning of hKCC2
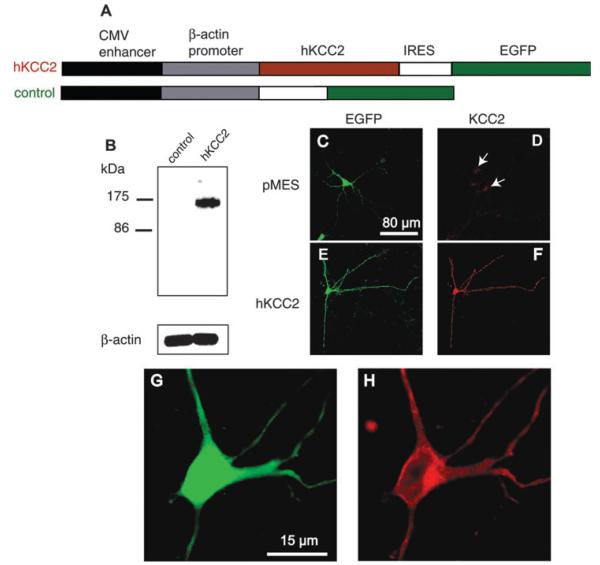
hKCC2 was subcloned into pMES vector, which contains a cytomegalovirus enhancer, a β-actin promoter (Swartz et al., 2001) and an internal ribosome entry site (IRES), followed by an enhanced green fluorescence protein (EGFP) sequence (Fig. 1).
Fig. 1.
Heterologous expression of hKCC2. (A) pMES vector (control) and hKCC2 expression vector used in this study. (B) Western blotting reveals a protein of approximately 150 kDa molecular weight in hKCC2 transfected COS7 cells but not in vector-transfected cells. β-actin (42 kDa) was detected using a different blot from the same membrane preparation. (C) Embryonic cortical neurons transfected with control vector (pMES) showed high EGFP-expression (green) but only very low levels of KCC2 expression (red) that were detectable only when drastically increasing the gain of the photomultiplier tube (D). (E and F) KCC2-immunoreactivity was high in KCC2 transfected cells. (G and H) Higher magnification shows that KCC2 appeared to be concentrated in or near the cell membrane (H).
Transfection of COS7 cells and cortical neurons
COS7 cells were maintained in 5% CO2 at 37 °C in Dulbecco’s modified Eagle’s-H21 medium with 10% Cosmic calf serum containing penicillin and streptomycin. For transfection, cells were electroporated (0.4 kV; 1070 microfarads) with 10 μg of DNA per 15-cm plate. Cortical primary cultures were prepared from embryonic day 17 rat fetuses as previously described (Hartnett et al., 1997). Rat fetuses were obtained from timed-pregnant Sprague-Dawley rats, killed by CO2 inhalation following procedures in accordance with USA National Institute of Health, guidelines and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. After three days in culture, cells were transfected with 1.5 μg of plasmid DNA per well using LipofectAMINE 2000 (Life Technologies, Inc., Grand Island, NY, USA) as previously described (Pal et al., 2003).
Immunoblotting
Two days following transfection, COS7 cells were fractionized by sonification and the non-nuclear fraction was centrifuged (35 000 r.p.m., SW 55 Ti rotor, Beckman for 35 min, 4 °C). The membrane pellet was re-suspended in SDS sample buffer (New England Biolabs, Beverly, MA, USA), and 50 μg of protein was separated by electrophoresis through 8.5% SDS-polyacrylamide gel. Proteins were transferred to nitrocellulose membrane. KCC2 protein was visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA) using affinity purified polyclonal anti-rat KCC2 [1 : 2000, 2 h at room temperature (RT)] (Upstate, NY, USA; Williams et al., 1999) and horseradish peroxidase-conjugated rabbit secondary antibody (1 : 4000, 2 h at RT) (Amersham, Arlington Heights, IL, USA).
Immunocytochemistry
Cells were fixed (4% paraformaldehyde in 0.1 m PBS, pH 7.2, RT for 40 min) and permeabilized for 3 h in blocking solution containing 0.02% saponin, 2% bovine serum albumin, and 1% fish skin gelatin in PBS buffer. Cells were incubated with KCC2 antibody (1 : 200; 3 h at RT; Williams et al., 1999), rinsed with PBS, incubated with Cy3–labelled secondary antibodies (1 : 1000 for 2 h at RT; Jackson Immuno Laboratory, ME, USA), rinsed again and coversliped with Slowfade (Molecular Probes, Eugene, OR, USA). Analysis was performed on an epifluorescent microscope (Axiophot, Zeiss, Germany) using 40× objective (Neofluar, NA 0.75) or on a laser scanning confocal microscope (Olympus Fluoview, USA) at 60× (PlanApo, NA 1.4).
Gramicidin-perforated patch clamp recordings
Cell plates were moved into a recoding chamber mounted to an inverted epifluorescent microscope (Zeiss IM 35) and superfused with a solution containing (in mm): NaCl 140, KCl 5, MgCl2·7H2O 1, d-glucose 24, CaCl2 2, Hepes 10, pH adjusted to 7.2 with NaOH. Patch pipettes (3–5 MΩ) contained (in mm): K2SO4 77, KCl 5, CaCl2 0.5, EGTA 5, Hepes 10, pH adjusted to 7.3 with KOH (Kyrozis & Reichling, 1995). Gramicidin (10 mg/mL in DMSO) was prepared fresh every 2 h and added to the pipette solution resulting in a final concentration of 100 ng/mL. Series resistance was approximately 40–50 MΩ and was not compensated. Off-line correction of series resistance did not change the estimated value of EGABA (P > 0.6 for both KCC2-transfected and control neurons). In voltage clamp experiments, voltage gated calcium channels and fast sodium channels were blocked with lanthanum (30 μm) and TTX (1 μm; Owens et al., 1996).
Liquid junction potential with a 2 m KCl agar bridge as the reference electrode was less than 2 mV. As the same extracellular solution was used for recordings from transfected and non-transfected cells, membrane potential values were left uncorrected for the small liquid junction potential.
GABA was applied to the soma of neurons using a multibarrel fast perfusion system (Warner Instruments, Hamden, CT, USA). All recordings were performed at 23–26 °C. Data were filtered at 0.5–1 kHz and digitized at 1–2 kHz using Axopatch 1C Amplifier, Digidata 1200 Interface, and Clampex 8.0 (Axon Instruments, Union City, CA, USA).
Calcium imaging
Cells were incubated for 0.5–2 h in Fura-2-containing (10 μm, TEF labs, Austin, TX, USA) artificial cerebrospinal fluid (ACSF) composition in mm: NaCl 124, NaHCO3 26, MgSO4·7H2O 1.3, KCl 5.0, KH2PO4 1.25, dextrose 10, CaCl2 2.0, kynurenic acid 1.0; pH 7.4 when gassed with 5% CO2 95% O2 37 °C). Cover slips were placed in a submerged-type recording chamber mounted on an inverted epifluorescence microscope (Nikon Eclipse TE200) equipped with 10× (NA 0.5) and 20× air objectives (NA 0.75) and were superfused with oxygenated ACSF without kynurenic acid. All drugs were applied through a superfusion system. Before recording calcium responses, EGFP expression was identified with 480 nm excitation light. Ratiometric imaging (340 nm/380 nm) was performed as described elsewhere (Kullmann et al., 2002) using a computer-controlled monochromator (Polychrome II, TILL Photonics, Martinsried, Germany) and a CCD camera (IMAGO, TILL Photonics). 340/380 nm image pairs were acquired at 0.1 Hz using TILLvisION v 4.0. Digital images were low pass filtered with a Gaussian 3 × 3 kernel and background fluorescence was subtracted as described previously (Ene et al., 2003). GABA was applied through a superfusion system. Only cells that responded to 60 mm KCl were analysed. 340/380 nm fluorescent ratios (R) were measured from the cell body.
Data were analysed and plotted using OriginPro 7.0 SR4 [v7.0552 (B552)] (OriginLab Corporation, MA, USA). For the calcium imaging experiment, data analysis was performed while the experimenter was blinded with regards to the transfection history of cells.
Statistical analysis
Statistical significance was tested using Fisher’s exact test and Student’s t-test. Throughout the text, values are expressed as arithmetic mean ± standard error of mean (SEM).
Results
Expression of hKCC2
COS7 cells were transfected with hKCC2 cDNA or control EGFP vectors (Fig. 1A). In Western blots prepared from crude membrane fractions of hKCC2 transfected cells, anti-rat KCC2 recognized a protein with a molecular weight of ≈150 kDa (Fig. 1B) similar to the molecular weight of KCC2 extracted from mouse and rat brain (Lu et al., 1999; Williams et al., 1999; Stein et al., 2004). No corresponding signal or other non-specific bands were present in Western blots prepared from cells that had been transfected with the control EGFP vector.
We next transfected embryonic primary cortical neurons (E17 + 3 DIV) in which the expression of endogenous KCC2 is very low (Fig. 1D; Lu et al., 1999; Rivera et al., 1999; Balakrishnan et al., 2003; Stein et al., 2004; Zhu et al., 2004). Two days after transfection, hKCC2-transfected, but not pMES-transfected, neurons showed strong immunoreactivity for KCC2 in cell bodies and neurites (Fig. 1F). Similar to endogenous KCC2 expression in mature neurons (Williams et al., 1999; Balakrishnan et al., 2003; Zhu et al., 2004), KCC2 immunoreactivity was highest close to the plasma membrane (Fig. 1H). In contrast to KCC2, bicistronically expressed EGFP was homogenously distributed throughout the cell body and processes (Fig. 1G). In the absence of primary antibody, no signal was detected (data not show).
Effect of KCC2 expression on EGABA
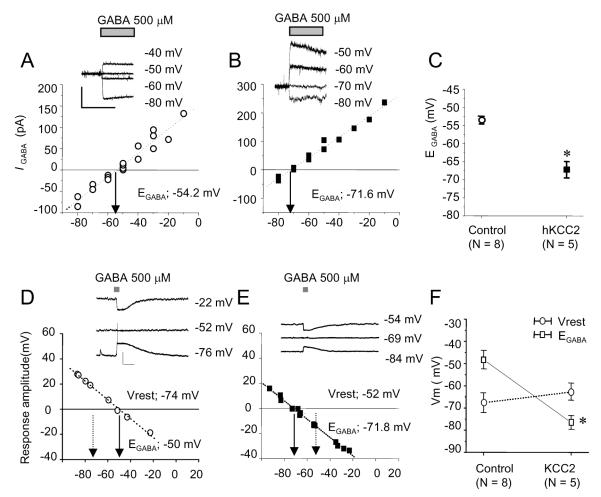
To test whether the premature expression of hKCC2 resulted in a functional chloride outward transporter, we examined the membrane potential response to GABA using the gramicidin-perforate patch clamp method, which leaves the intracellular chloride concentration intact (Kyrozis & Reichling, 1995). In voltage clamp recordings and while voltage-gated sodium and calcium currents were blocked by lanthanum (30 μm) and TTX (1 μm; Owens et al., 1996), short pulses of GABA (0.5 mm, 200 ms) elicited inward currents in control neurons (holding potential −60 mV, Fig. 2A), but outward currents in KCC2-transfected neurons (Fig. 2B). In control cells, EGABA was −53.5 ± 1.1 mV (non-transfected −53.7 ± 2.2 mV, n = 4; pMES-transfected −53.3 ± 0.7 mV, n = 4, P > 0.1, unpaired Student’s t-test). This value is very similar to EGABA measured in acute cortical slices at the corresponding age (corrected for differences in extracellular [Cl−]; Owens et al., 1996). In contrast, in hKCC2 expressing neurons, EGABA was −67.3 ± 2.2 mV (n = 5; Fig. 2C), significantly more negative than EGABA in control neurons (P < 0.001, unpaired Student’s t-test).
Fig. 2.
Overexpression of hKCC2 produces a negative shift in EGABA in immature cortical neurons. Gramicidin-perforated patch clamp recordings from cortical neurons in voltage clamp (A–C) and current clamp (D–F). Voltage–current relationship of membrane potential and GABA-elicited (0.5 mm, 200 ms) currents in a control neuron (A) and a KCC2-transfected neuron (B). (C) Summary data. (D) GABA-elicited membrane potential responses in a control neuron (D) and a hKCC2-transfected neuron. (F) Summary data. *P < 0.001, Student’s t-test. Scale bars, 200 ms, 50 pA (A and B); 1 s, 20 mV (D and E).
In current clamp recordings, without the presence of lanthanum and TTX, GABA induced depolarizing responses with action potential firing in control neurons while generating hyperpolarizations in KCC2-transfected neurons (Fig. 2D–F). In control neurons, EGABA was −48.3 ± 4.1 mV (n = 8) and in hKCC2 transfected neurons EGABA was significantly more negative at −76.5 ± 3.1 mV (n = 5; P < 0.005). The resting membrane potential (Vrest) was not significantly different between both groups (control −67.6 ± 4.5 mV, n = 8; hKCC2-transfected neurons −62.8 ± 3.7 mV, n = 5; P > 0.1), resulting in negative shift of the electrochemical potential (EGABA – resting membrane potentials) from +19.3 ± 4.9 mV in control neurons to −12.2 ± 4.3 mV in KCC2 transfected neurons.
The larger negative shift of EGABA in current clamp (−28.2 mV) compared to voltage clamp (13.8 mV) most likely reflects the contribution of voltage-gated sodium and calcium conductances in current clamp recordings.
Effect of KCC2 expression on GABAergic calcium responses
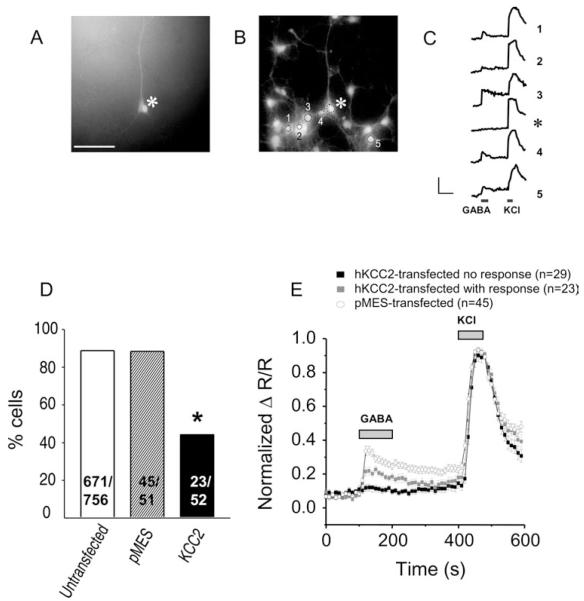
In immature cortical neurons, depolarizing GABA increases the intracellular calcium concentration ([Ca2+]i) by activating voltagegated calcium channels (Connor et al., 1987; Yuste & Katz, 1991; Owens et al., 1996) or by removing the magnesium block from NMDA receptors (Ben Ari et al., 1997). We therefore tested whether, and to what extent, the hKCC2 induced negative shift of EGABA and was correlated with a loss of the capacity of GABA to elevate intracellular Ca2+ levels. GABA (1 mm) elicited a transient increase in [Ca2+]i in 88% of control cells (716/807 cells; non-transfected cells 671/756, control vector-transfected cells 45/51; Fig. 3D) but in only 44% of hKCC2-transfected cells (23/52 cells; P < 0.01, Fisher’s exact test). In hKCC2 expressing neurons that showed a GABAergic calcium response, peak amplitudes were significantly smaller than in control neurons (38% reduction, P < 0.05, unpaired Student’s t-test, Fig. 3E). Response amplitudes to KCl-induced depolarization were not affected by KCC2 expression. Peak amplitudes (ΔR/R) in control neurons were 1.36 ± 0.03 (n = 45), in non-GABA responding KCC2-transfected cells 1.41 ± 0.05 (n = 29), and 1.46 ± 0.05 in GABA responding KCC2 transfected cells (n = 23; P > 0.05, two-tailed Student’s t-test).
Fig. 3.
KCC2 expression decreases GABA-elicited calcium responses. (A) Photomicrograph of EGFP fluorescence (488 nm excitation) of an hKCC2-transfected neuron. (B) Same field showing Fura-2 labelling (380 nm excitation). (C) Calcium responses to GABA (1 mm) and KCl (60 mm) of cells numbered in (B). The hKCC2 transfected cell (asterisk) did respond to KCl but not to GABA (arrow). Calibration: 0.4 ΔR/R, 100 s. (D) Percentage of KCl responding cells that also responded to GABA (*P < 0.01, Fisher’s exact test). (E) Calcium responses (ΔR/R normalized to KCl response) of hKCC2-transfected, GABA responding and GABA non-responding cells. Scale bar, 100 μm (A and B).
The GABAA receptor agonist muscimol (30 μm) elicited calcium-responses in the same fraction of cells as did GABA [500 μm; muscimol 90% (n = 112/124), GABA 87% (n = 108/124), P > 0.1]. The amplitude of 30 μm muscimol-elicited responses was slightly larger than those elicited by 500 μm GABA (muscimol 117% of GABA). This might reflect GABA uptake by glial cells or might reflect smaller membrane depolarizations by the additional activation of GABAB receptors. In the presence of bicuculline (100 μm) the percentage of cells responding to GABA was decreased by 90% (n = 90 cells) and the percentage of cells responding to muscimol was decreased by 71% (n = 56). The amplitudes of responses in the remaining cells were reduced by 86.2% (n = 16). Bicuculline did not reduce KCl-elicted responses (change 96.4%, n = 32, P = 0.2).
Discussion
The results presented here demonstrate that exogenous expression of hKCC2 in embryonic cortical neurons induces a negative shift of EGABA, and as a consequence, abolishes or significantly decreases GABA-elicited calcium responses. These results provide the first evidence that KCC2 is not only necessary, but also sufficient to induce the end of the depolarizing and excitatory period of GABA during cortical development.
In KCC2 overexpressing neurons, EGABA was 13 mV more negative than in age-matched control neurons. As GABAergic currents in early neonatal cortical neurons are carried primarily by GABAA receptors and chloride flux (Luhmann & Prince, 1991; Owens et al., 1996) a shift of EGABA from −54 mV to −67 mV corresponds to an estimated decrease in [Cl−]i from ≈18 mm to ≈11 mm, calculated with the Nernst equation. Interestingly, the 7 mm decrease in [Cl−]i observed here in vitro closely matches the 6–8 mm decrease that occurs during the first two postnatal weeks in rat cortex development in vivo (P0–P4 18–20 mm, P16 11.7 mm; Owens et al., 1996). This result was somewhat unexpected as it is unlikely that hKCC2 transfected cultured neurons, in which hKCC2 transcription is driven by the beta-actin promotor, express similar levels of KCC2 protein as cortical neurons in vivo, in which KCC2 expression is under normal endogenous control. It is possible, however, that both in vitro and in vivo, KCC2 activity was high enough to establish a thermodynamic equilibrium at the given extra- and intracellular K+ and Cl− concentrations, or that both in vitro and in vivo, KCC2 activity is similarly regulated by [Cl−]i (Breitwieser et al., 1990; Schomberg et al., 2003). In addition, it is possible that phosphorylation of KCC2 (LoTurco et al., 1995; Kelsch et al., 2001; Vale et al., 2003; Stein et al., 2004) or availability of membrane anchoring proteins could act as functional limiting factors, regardless of the expression levels of the transporter.
Our studies cannot exclude the possibility that expression of hKCC2 induces the down-regulation of chloride inward transporters, such as the sodium potassium chloride cotransporter (NKCC) that is present in immature cortical neurons (Sun & Murali, 1999). This possibility, however, seems unlikely because NKCC activity is increased rather than decreased by decreasing [Cl−]i (Breitwieser et al., 1990; Schomberg et al., 2003). In addition, in developing auditory neurons, up-regulation of KCC2 activity and the negative shift of ECl is paralleled by an up-regulation of NKCC expression. This indicates that up-regulation of KCC2 is not negatively coupled to NKCC expression and that KCC2-mediated outward Cl− transport can overcome NKCC-mediated inward Cl− transport (Balakrishnan et al., 2003). On the other hand, NKCC2 activity is up-regulated by an increase in [Ca2+]i (Sun & Murali, 1998; Schomberg et al., 2001), a situation which is less likely to occur in hKCC2-transfected neurons (Fig. 3). Clearly, additional studies are necessary to investigate in detail the possible direct and indirect interactions between KCC2 and other chloride transporters.
Because premature expression of KCC2 protein resulted in a functional chloride transporter, the mechanisms for membrane trafficking and post-translational modifications that are required for KCC2 activation (Payne, 1997; Strange et al., 2000; Kelsch et al., 2001; Balakrishnan et al., 2003; Stein et al., 2004) already have to be present before developing cortical neurons endogenously up-regulate KCC2 expression. This conclusion is supported by a recent in vivo study, which demonstrated phosphorylation of KCC2 in cortex even before the steep increase in KCC2 expression levels, suggesting that the developmental negative shift in EGABA is primarily caused by transcriptional up-regulation of KCC2 expression (Stein et al., 2004). As such, the developmental regulation of KCC2 activity in cortical neurons seems to differ from other brain areas in which the developmental increase in KCC2 activity is regulated primarily by post-translational mechanisms (Kelsch et al., 2001; Vale et al., 2003), and/or subcellular translocation (Balakrishnan et al., 2003). None-theless, our results clearly indicate that KCC2 function is the determining factor in converting a GABAergic response from excitatory to inhibitory during brain development.
Acknowledgements
We thank D.B. Mount and E. Delpire for hKCC2 and Dr Cathy Krull for pMES vector. Natalie Almann and Karen Hartnett provided expert technical support. This work was supported by NIDCD 04199 (KK), NS43277 and NS29365 (EA).
Abbreviations
- [Ca 2+]i
intracellular calcium concentration
- [Cl−]i
intracellular chloride concentration
- ECl
chloride electrochemical equilibrium potential
- EGABA
GABA reversal potential
- EGFP
enhanced green fluorescence protein
- KCC2
potassium chloride cotransporter
- hKCC2
human potassium chloride cotransporter 2
- NKCC
sodium potassium chloride cotransporter
- Vrest
resting membrane potential
- ΔR/R
340/380 fluorescent ratio.
References
- Balakrishnan V, Becker M, Lohrke S, Nothwang HG, Guresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J. Neurosci. 2003;23:4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. TINS. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. (Lond.) 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser GE, Altamirano AA, Russell JM. Osmotic stimulation of Na(+)-K(+)-Cl− cotransport in squid giant axon is [Cl−]i dependent. Am. J. Physiol. 1990;258:C749–C753. doi: 10.1152/ajpcell.1990.258.4.C749. [DOI] [PubMed] [Google Scholar]
- Clayton GH, Owens GC, Wolff JS, Smith RL. Ontogeny of cation-Cl− cotransporter expression in rat neocortex. Brain Res. Dev. Brain Res. 1998;109:281–292. doi: 10.1016/s0165-3806(98)00078-9. [DOI] [PubMed] [Google Scholar]
- Connor JA, Tseng HY, Hockberger PE. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J. Neurosci. 1987;7:1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JAM, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Lohrke S, Friauf E. Shift from depolarizing to hyperpolarizing glycine action in rat auditory neurones is due to age-dependent Cl− regulation. J. Physiol. (Lond.) 1999;520:121–137. doi: 10.1111/j.1469-7793.1999.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene FA, Kullmann PHM, Gillespie DC, Kandler K. Glutamatergic calcium responses in the developing lateral superior olive: Receptor types and their specific activation by synaptic activity patterns. J. Neurophysiol. 2003;90:2581–2591. doi: 10.1152/jn.00238.2003. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshe SL. Sex-specific KCC2 expression and GABA(A) receptor function in rat substantia nigra. Exp. Neurol. 2003;183:628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Hartnett KA, Stout AK, Rajdev S, Rosenberg PA, Reynolds IJ, Aizenman E. NMDA receptor-mediated neurotoxicity: a paradoxical requirement for extracellular Mg2+ in Na+/Ca2+-free solutions in rat cortical neurons in vitro. J. Neurochem. 1997;68:1836–1845. doi: 10.1046/j.1471-4159.1997.68051836.x. [DOI] [PubMed] [Google Scholar]
- Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Kandler K, Kullmann PHM, Ene FA, Kim G. Excitatory action of an immature glycinergic/GABAergic sound localization pathway. Physiol. Behav. 2002;77:583–587. doi: 10.1016/s0031-9384(02)00905-8. [DOI] [PubMed] [Google Scholar]
- Kelsch W, Hormuzdi S, Straube E, Lewen A, Monyer H, Misgeld U. Insulin-like growth factor 1 and a cytosolic tyrosine kinase activate chloride outward transport during maturation of hippocampal neurons. J. Neurosci. 2001;21:8339–8347. doi: 10.1523/JNEUROSCI.21-21-08339.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann PHM, Ene FA, Kandler K. Glycinergic and GABAergic calcium responses in the developing lateral superior olive. Eur. J. Neurosci. 2002;15:1093–1104. doi: 10.1046/j.1460-9568.2002.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J. Neurosci. Meth. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Lee H, Chen CXQ, Liu Y, Aizenman E, Kandler K. KCC2 expression is sufficient for decreasing GABA-mediated excitation in developing cortical neurons. Soc. Neurosci. Abstr. 2003;374:1. [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJS, Davis MBE, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J. Neurobiol. 1999;39:558–568. [PubMed] [Google Scholar]
- Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J. Neurophysiol. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, Davis MBE, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J. Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E. Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. J. Neurosci. 2003;23:4798–4802. doi: 10.1523/JNEUROSCI.23-12-04798.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am. J. Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J. Biol. Chem. 1996;271:16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Schomberg SL, Bauer J, Kintner DB, Su G, Flemmer A, Forbush B, Sun D. Cross talk between the GABA(A) receptor and the Na-K-Cl cotransporter is mediated by intracellular Cl−. J. Neurophysiol. 2003;89:159–167. doi: 10.1152/jn.00229.2002. [DOI] [PubMed] [Google Scholar]
- Schomberg SL, Su G, Haworth RA, Sun D. Stimulation of Na-K-2Cl cotransporter in neurons by activation of non-NMDA ionotropic receptor and group-I mGluRs. J. Neurophysiol. 2001;85:2563–2575. doi: 10.1152/jn.2001.85.6.2563. [DOI] [PubMed] [Google Scholar]
- Shibata S, Kakazu Y, Okabe A, Fukuda A, Nabekura J. Experience-dependent changes in intracellular Cl− regulation in developing auditory neurons. Neurosci. Res. 2004;48:211–220. doi: 10.1016/j.neures.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Song L, Mercado A, Vazquez N, Xie Q, Desai R, George AL, Jr, Gamba G, Mount DB. Molecular, functional, and genomic characterization of human KCC2, the neuronal K-Cl cotransporter. Brain Res. Mol. Brain Res. 2002;103:91–105. doi: 10.1016/s0169-328x(02)00190-0. [DOI] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J. Comp. Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Strange K, Singer TD, Morrison R, Delpire E. Dependence of KCC2 K-Cl cotransporter activity on a conserved carboxy terminus tyrosine residue. Am. J. Physiol. Cell Physiol. 2000;279:C860–C867. doi: 10.1152/ajpcell.2000.279.3.C860. [DOI] [PubMed] [Google Scholar]
- Sun D, Murali SG. Stimulation of Na+-K+-2Cl− cotransporter in neuronal cells by excitatory neurotransmitter glutamate. Am. J. Physiol. 1998;275:C772–C779. doi: 10.1152/ajpcell.1998.275.3.C772. [DOI] [PubMed] [Google Scholar]
- Sun DD, Murali SG. Na+-K+-2Cl(−) cotransporter in immature cortical neurons: a role in intracellular Cl− regulation. J. Neurophysiol. 1999;81:1939–1948. doi: 10.1152/jn.1999.81.4.1939. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Eberhart J, Pasquale EB, Krull CE. EphA4/ephrin-A5 interactions in muscle precursor cell migration in the avian forelimb. Development. 2001;128:4669–4680. doi: 10.1242/dev.128.23.4669. [DOI] [PubMed] [Google Scholar]
- Vale C, Schoorlemmer J, Sanes DH. Deafness disrupts chloride transporter function and inhibitory synaptic transmission. J. Neurosci. 2003;23:7516–7524. doi: 10.1523/JNEUROSCI.23-20-07516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Sharp JW, Kumari VG, Wilson M, Payne JA. The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J. Biol. Chem. 1999;274:12656–12664. doi: 10.1074/jbc.274.18.12656. [DOI] [PubMed] [Google Scholar]
- Woo NS, Lu J, England R, McClellan R, Dufour S, Mount DB, Deutch AY, Lovinger DM, Delpire E. Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus. 2002;12:258–268. doi: 10.1002/hipo.10014. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl− transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- Yuste R, Katz LC. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- Zhu L, Lovinger D, Delpire E. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J. Neurophysiol. 2004;93:1557–1568. doi: 10.1152/jn.00616.2004. [DOI] [PubMed] [Google Scholar]