Abstract
Object
Many patients with Cushing disease still have active or recurrent disease after pituitary surgery. The histological pseudocapsule of a pituitary adenoma is a layer of compressed normal anterior lobe that surrounds the adenoma and can be used during surgery to identify and guide removal of the tumor. In this study the authors examined the results of using the pseudocapsule as a surgical capsule in the resection of adenomas in patients with Cushing disease.
Methods
The authors reviewed a prospective database of data obtained in patients with Cushing disease who underwent surgery. The analysis included all cases in which a lesion was identified during surgery and in which the lesion was believed to be confined to the pituitary gland in patients with Cushing disease between January 1990 and March 2007. Since the objective was to determine the success of using the pseudocapsule as a surgical capsule, patients with invasive tumors and patients in whom no lesion was identified during surgery—challenging cases for surgical success—were excluded from analysis.
Results
In 261 patients an encapsulated adenoma was identified at surgery. Tumor was visible on MR imaging in 135 patients (52%); in 126 patients (48%) MR imaging detected no tumor. The range of tumor size overlapped considerably in the groups with positive and negative MR imaging results, indicating that in addition to size other features of the adenoma influence the results of MR imaging. In 252 patients hypercortisolism resolved after the first operation, whereas in 9 patients (3 with positive MR imaging and 6 with negative MR imaging) early reoperation was required. Hypercortisolism resolved in all 261 patients (256 with hypocortisolism and 5 with eucortisolism) before hospital discharge. Forty-six patients (18%) had postoperative electrolyte abnormalities (30 with hyponatremia and 16 with diabetes insipidus), but only 2 patients required treatment at discharge. The mean clinical follow-up duration was 84 months (range 12–215 months). Six patients (2%) had recurrence of hypercortisolism, all of whom were treated successfully with reoperation.
Conclusions
Because of their small size, adenomas can be challenging to identify in patients with Cushing disease. Use of the histological pseudocapsule of an adenoma allows accurate identification of the tumor and helps guide its complete excision. With this approach the overall remission rate is high and the rate of complications is low.
Keywords: Cushing disease, extracapsular tumor, histological pseudocapsule, outcomes, pituitary adenoma, surgery
Over the past 40 years numerous authors have described the efficacy of the transsphenoidal approach in exposing the sella.5,6,13–15 However, few papers have discussed the details of tumor removal, although the technique commonly used is to enter the tumor and remove it from within. This is particularly relevant in Cushing disease, as the adenomas tend to be small and often are not identified by MR imaging.5,11,31 Even in the hands of an experienced medical, radiological, and surgical team, 25–35% of patients with Cushing disease are not in remission following surgical exploration of the sella turcica.16,20,22,26,28,30
The presence of a histological pseudocapsule associated with pituitary tumors was initially noted by Costello in the early 1930s.7 This capsule, formed by layers of compressed pituitary tissue surrounding the adenoma, histologically and physically separates the adenoma from the normal pituitary gland. An earlier report presented the development of this histological pseudocapsule and the technical aspects of its use in the excision of pituitary adenomas.23 In the present study we examined the utility of this extracapsular approach in the surgical management of Cushing disease. Specifically, we sought to define: 1) the utility of using the pseudocapsule to identify tumors in patients with positive and negative MR imaging; and 2) the effectiveness of this technique in inducing endocrine remission in patients with intrasellar tumors.
As secondary objectives, we compared the size of adenomas as seen on MR imaging with that of the encapsulated adenoma observed intraoperatively; compared the sizes of the adenomas in patients with positive and negative MR imaging; examined the incidence of remission in patients with positive and negative MR imaging; and assessed the long-term remission rates after removal of adenomas within the envelope of compressed anterior lobe provided by the pseudocapsule.
Methods
Study Design
All patients were enrolled in institutional review board–approved protocols at the NIH. Included in this study were patients who underwent transsphenoidal exploration for Cushing disease at the NIH between January 1990 and March 2007, and in whom a lesion considered to be an adenoma confined to the anterior lobe of the pituitary gland was found at surgery. Since the objective of the study was to document the success of using the pseudocapsule as a surgical capsule, patients with prior surgery, patients with invasive and ectopic pituitary adenomas, and patients in whom no lesion considered to be an adenoma was identified during exploration of the pituitary were excluded from the analysis. Magnetic resonance imaging findings, intraoperative findings, operative complications, and presence/absence of remission were prospectively documented. Patient charts were reviewed retrospectively to identify postoperative complications, postoperative endocrinopathy, and recurrence of Cushing disease. Follow-up information was obtained from the patients.
Diagnosis of Cushing Disease
Before surgery all patients underwent a comprehensive endocrine evaluation including measurements of plasma ACTH, diurnal serum cortisol levels, and 24-hour UFC levels to determine the presence of ACTH-dependent Cushing syndrome, and dexamethasone suppression testing and corticotropin-releasing hormone stimulation testing to identify its cause. Sellar spin echo T1-weighted MR imaging and SPGR MR imaging were used to define the presence and location of an adenoma (as interpreted by the clinical neuroradiologist's report). Inferior petrosal sinus sampling was performed if MR imaging was negative or if the noninvasive endocrine test results were conflicting.
Surgical Technique
Using a sublabial transsphenoidal approach, which is required for a broad, complete exposure of the sella for microsurgery, the anterior face of the sella turcica is opened. The dura mater is opened and separated from the pituitary capsule to expose the entire anterior surface and much of the inferior surface of the pituitary gland with an intact pituitary capsule.
In patients with surface presentation of the tumor, a curvilinear incision is made through the pituitary capsule just beyond the point at which the most superficial dome of the tumor reaches the surface of the gland (Fig. 1) so that a thin layer of normal gland is traversed before reaching the surgical capsule of the adenoma, allowing its identification. In patients with no adenoma surface presentation, a series of vertical incisions is carried deep in stages until the capsule of the adenoma is identified. A surgical plane is then created at the interface of the tumor and the normal gland. Gentle dissection of this interface then continues. After the margins of the tumor are completely defined and dissected, the tumor is removed, as previously described.23
Fig. 1.
Illustrations of the surgical technique. A: A curvilinear incision is made through the pituitary capsule just beyond the point at which the most superficial dome of the tumor reaches the surface of the gland (left image). This permits a thin layer of normal gland to be traversed before reaching the surgical capsule of the adenoma (left inset), allowing easy identification of the surgical capsule and the creation of a surgical plane of dissection at the margin of the tumor, at the interface of the normal gland and the surgical capsule of the adenoma. This interface is further defined using the tips of the bipolar forceps in a series of movements parallel to the surface of the adenoma and in the crevice between the gland and adenoma (right image and center and right insets). Gentle dissection of the interface between the adenoma and gland is continued following the curvilinear margin of the adenoma. B: After the most superficial portion of the tumor has been defined circumferentially, the deeper adenoma margins are defined and dissected in a similar fashion; the posterior margin of the adenoma often requires dissection using a disc dissector and a small and/or medium ring curette (left image and inset). After the margins of the tumor have been completely dissected, to prevent rupture of the tumor the last remaining connection between the pseudocapsule of the specimen and the pituitary capsule is grasped with a small cup forceps and the tumor is removed (right image). In cases of tumors that are ≤ 8–10 mm in diameter, the entire tumor can usually be shelled out of its bed in the anterior lobe as an intact specimen. Successful and complete removal leaves a smoothly lined hemispherical tissue void in the anterior lobe. Modified from J Neurosurg 104:7–19, 2006.
Postoperative Evaluation and Remission
Starting on postoperative Day 3 we ceased administering all steroid medications for 3 days, and a series of 24-hour UFC levels and early morning serum cortisol levels was obtained for 3 consecutive days. Remission of Cushing disease was defined as postoperative hypocortisolism or eucortisolism. Hypocortisolism was defined as a 24-hour UFC level of < 20 μg/24 hours or an early morning serum cortisol of < 5 μg/dl in all, or the majority of, samples. Eucortisolism was defined as 24-hour UFC excretion within the normal range.
Clinical Follow-Up
All patients had at least 12 months of clinical follow-up data. Clinical follow-up was obtained through clinic records at the NIH, by a formatted letter requesting clinical information, current medications, evidence of clinical recurrence, and requirement of additional treatment, and through conversations with patients, an approach similar to that used in prior reports.3,12,19 Patients were asked whether they currently had signs or symptoms of Cushing syndrome, if they had required additional treatment for Cushing disease after transsphenoidal surgery, and to list their current endocrine medications.
Results
General Demographics
Between January 1990 and March 2007, 483 patients with Cushing disease without prior surgery underwent transsphenoidal exploration performed by the senior author (E.H.O.). Of these 483 patients, 261 (54%) were considered intraoperatively to have an encapsulated adenoma confined to the anterior lobe of the pituitary gland; 166 (34%) had tumors invading the dura surrounding the pituitary; 11 (2%) had tumors within the posterior lobe;32 and in 45 (9%) no tumor could be identified at surgery. During hospitalization for surgery, 453 (94%) of the 483 patients experienced remission of hypercortisolism (of the 453 patients, 441, [97%] had hypocortisolism and 12 [3%] had eucortisolism). Remission occurred in 147 (89%) of the 166 patients with invasive tumors (94% of the 147 patients with remission had postoperative hypocortisolism and 6% had eucortisolism), all 11 patients with posterior lobe tumors (all had hypocortisolism), and 34 (76%) of the 45 patients in whom no tumor could be found at surgery—and a portion of the anterior lobe (30–100%) was removed (91% hypocortisolism, 9% eucortisolism). Because factors other than the surgical envelope provided by the pseudocapsule, factors such as parasellar invasion and the inability to find an adenoma at surgery influenced the success of surgery, patients with these features were excluded from this analysis, since inclusion of them would introduce variables other than the use of the histological pseudocapsule as a surgical capsule, the focus of the study, in the results.
It is the experience of one of us (E.H.O.) that, until they reach a very large size, all pituitary tumors have a distinct pseudocapsule, which may or may not begin to be difficult to define completely around the margin of the tumor when the tumor exceeds a size of 2–3 cm. As tumors develop and enlarge they may invade structures adjacent to the pituitary, but the portion of the tumor within the gland still retains a distinct pseudocapsule that can be used to identify the tumor margin and for dissection between the anterior lobe and the tumor. Dissection of this interface is also useful to identify sites in which the adenoma leaves the pituitary capsule to invade the contiguous dura. However, because the goal of the study was to examine the utility of excising adenomas by dissection around the margin of the histological pseudocapsule as a surgical capsule, the 261 patients considered at surgery to have encapsulated adenomas contained within the anterior lobe of the pituitary form the basis of this study. Of these, 199 patients were female (76%). The mean age of patients at surgery was 32.5 years (range 4.7–77 years). Eighty-five patients were 21 years of age or younger.
Effectiveness of the Histological Pseudocapsule in Identifying and Facilitating Removal of Adenoma at Surgery
Resolution of hypercortisolism was experienced in all 261 patients before hospital discharge, 135 patients with positive and 126 patients with negative MR imaging findings.
Patients With Positive Preoperative MR Imaging Findings
In all 135 patients in whom MR imaging identified a tumor, an encapsulated lesion was identified at surgery at the same site as the lesion identified by MR imaging (Fig. 2). In 133 patients, the lesion proved to be an ACTH-staining adenoma. In 1 patient, pathological examination demonstrated a prolactinoma. The final patient suffered hypocortisolemia (follow-up duration 74 months) after selective excision of the encapsulated lesion, but no adenoma was identified in the specimen.
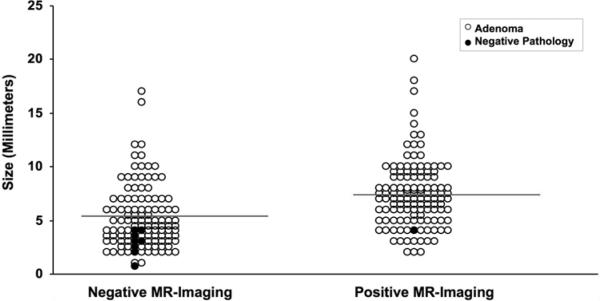
Fig. 2.
Graph showing disease in patients with negative and positive MR imaging. Mean tumor volume is depicted by the gray lines. Cases in which the encapsulated lesion removed at surgery proved to be an ACTH-staining adenoma are represented by open circles. Black circles represent patients in whom the encapsulated lesion removed at surgery did not contain an ACTH-staining adenoma. Most surgical specimens with negative pathology occurred in patients with negative MR imaging, and all were smaller than the mean size of those in the MR imaging–negative group.
In 132 patients (98%) hypercortisolism resolved after the first operation (131 hypocortisolism, 1 eucortisolism; the patient with eucortisolism remains disease free at 89 months after treatment). Three patients required early reoperation within 9 days for persistent hypercortisolism. In 2 patients with corticotropinomas partially removed at the initial surgery, residual encapsulated adenoma and a thin rim of anterior lobe were removed adjacent to the initial surgical cavity. In the patient with the prolactinoma, at reoperation an ACTH-positive adenoma was identified and resected from the opposite side of the anterior lobe. All 3 patients had hypocortisolemia following reoperation.
Patients With Negative Preoperative MR Imaging Findings
Of the 126 patients with negative MR imaging, 120 (95%) had resolution of hypercortisolism (115 with hypocortisolism and 5 with eucortisolism, 4 of whom had low normal UFC excretion, 1 had cortisol suppression to < 1 μg/dl after overnight low-dose dexamethasone) after selective removal of the encapsulated lesion identified as adenoma at surgery.
Six patients (5%) with persistent hypercortisolism after removal of a lesion, which proved not to be adenoma at pathological examination, underwent early repeat surgery (mean 5.2 days postoperatively, range 3–8 days postoperatively). On reoperation an adenoma was identified and removed in 4 of these patients, all of whom had hypocortisolism postoperatively (3 had a microadenoma above the diaphragma sella and adjacent to the pituitary stalk, 1 was outside the pituitary adjacent to the cavernous sinus). In 2 patients, no pathologically confirmed tumor was identified intraoperatively, and 50–75% of the remaining anterior lobe was removed. Both patients developed hypocortisolemia immediately after surgery and remained in remission 12 and 71 months later.
Accuracy of the Surgical Capsule in Identifying Histological Adenoma
An encapsulated tumor was identified by the surgeon during initial surgery in all patients but was lost to suction in 1, leaving 260 patients with lesions that were submitted for pathological examination.
Patients With Positive Preoperative MR Imaging Findings
As described above, 134 (99%) of 135 patients in whom MR imaging was positive harbored a histologically confirmed adenoma at their first operation. In 133 (99%) the adenoma was an ACTH-secreting adenoma; 1 adenoma stained for prolactin.
Patients With Negative Preoperative MR Imaging Findings
In 113 (90%) of the 125 patients in whom MR imaging was negative and for whom a lesion was available for histological assessment (in 1 of the 126 patients with negative MR imaging a small lesion was lost by suction at surgery), the surgical specimen contained an adenoma at histological assessment; 112 of the adenomas stained for ACTH, and 1 adenoma proved to be a prolactinoma. In the remaining 12 cases (10%), histological examination of the specimen did not identify an adenoma. Despite this, 6 of these patients, in whom the only tissue removed at surgery was an encapsulated lesion 1.5–4 mm in diameter, had profound hypocortisolism and remission of Cushing syndrome after the first surgery. The other 6 patients had hypercortisolemia after the first operation and had early repeat pituitary exploration, at which an ACTH-staining adenoma was removed in 4 patients (see above).
Correlation of MR Imaging Characteristics of Tumor Size and Surgical Findings
The mean maximum tumor diameter on MR imaging was 6 mm (range 3–22 mm). Among the 135 patients in whom MR imaging results were interpreted to be positive, 115 (85%) had microadenomas and 20 patients (15%) had macroadenomas (> 10 mm), as measured on the MR images. The mean maximum tumor diameter, as measured at surgery, for these tumors was 6 mm (range 1–23 mm). Compared with the estimate on MR imaging, the size of the tumor measured at surgery was the same in 74 patients (55%), larger in 50 patients (37%), and smaller in 11 patients (8%) (Fig. 3). In 127 patients (94%) the surgical and MR imaging estimates of tumor diameter were within 2 mm.
Fig. 3.
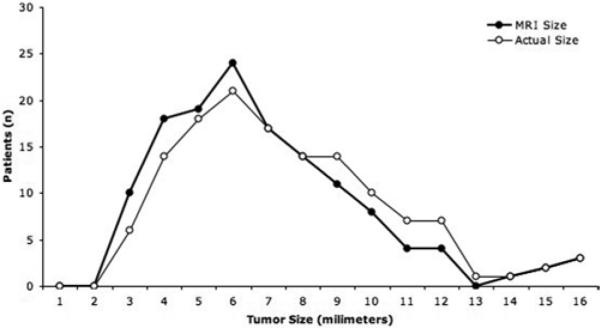
Graphic representation of tumor size on MR imaging (bold black line) compared with the tumor size at resection. Magnetic resonance imaging–based measurement was within 2 mm of the tumor size at surgery in most cases, although there was an overall tendency to underestimate tumor size on MR imaging, a factor that is probably related to the resection of the pseudocapsule with the adenoma.
Comparison of Adenoma Size in Patients With and Without Positive MR Imaging
In patients in whom MR imaging was negative, the mean maximum tumor diameter at surgery was 5 mm (range 1–23 mm). Although patients with negative MR imaging tended to have smaller tumors than those with positive imaging studies, some patients had large tumors (including 8 macroadenomas) that were not detected on preoperative MR imaging (Fig. 4). In the majority of the 8 macroadenoma cases, the tumor involved the entire pituitary gland and was not discernible as abnormal on MR imaging.
Fig. 4.
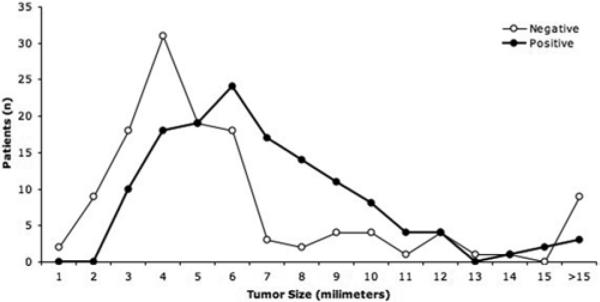
Graphic representation of tumor size in patients with negative MR imaging compared with those in patients with positive MR imaging (closed circles). The patients with negative MR imaging (open circles) tended to have smaller tumors, although there is considerable overlap in the range of tumor size with and without detection on MR imaging.
Complications
Surgical Approach
Thirteen patients (5%) had a total of 14 postoperative complications. In most instances these were minor and were related to the surgical approach. One patient (0.4%) had postoperative epistaxis requiring cauterization Four patients (1.5%) required antibiotics for postoperative sinusitis. Four patients (1.5%) had postoperative CSF leakage that was effectively treated using lumbar drainage. Four patients (1.5%) had postoperative maxillary numbness associated with a unilateral maxillary fracture during the wide exposure. The numbness resolved over the next several months in all 4. One patient (0.4%) developed postoperative meningitis, which responded to antibiotic treatment.
Hyponatremia
Thirty patients (11%) had hyponatremia postoperatively. In 12 (40%) of these patients preoperative MR imaging had been negative, and in 18 (60%) it had been positive. The median time to diagnosis of hyponatremia was 5 days (range 2–9 days). In all cases syndrome of inappropriate antidiuretic hormone resolved with fluid restriction before discharge.
Diabetes Insipidus
Sixteen patients (6%) had diabetes insipidus during their early postoperative course. Nine of these patients (56%) had negative preoperative MR imaging, and 7 patients had positive MR imaging. Two pa tients (22%), both with negative MR imaging, were discharged from the hospital on desmopressin. At 1-year follow-up only 1 of these patients was still receiving desmopressin for persistent diabetes insipidus.
Follow-Up Data
The mean follow-up duration was 84 months (range 12–215 months; median, 69 months). At most recent follow-up examination, 260 patients were alive (1 patient died of complications related to hepatic cirrhosis 15 months after surgery). In 260 patients (99.6%) the physical stigmata of Cushing disease completely disappeared. One patient noted no change in weight or headaches, but laboratory and imaging studies were normal 110 months after surgery.
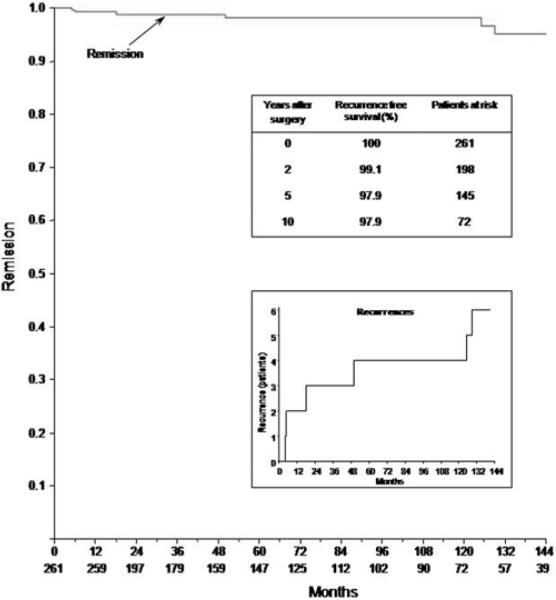
During the follow-up period, 6 patients (2.3%) had recurrence of Cushing disease (Fig. 5), and the senior author (E.H.O.) performed repeat surgery in all cases. The mean time to recurrence was 56 months (range 5–129 months). The Kaplan-Meier estimates of recurrence-free survival are shown in Fig. 5.
Fig. 5.
Kaplan-Meier plots showing the cumulative remission-free survival of Cushing disease. The numbers in the bottom-most row indicate the number of patients at risk at various intervals. Recurrences are shown in the lower inset.
Recurrences fell into 2 categories. In 2 patients postoperative UFC levels dropped to < 1 μg/24 hours despite the fact that the lesion that appeared to be an encapsulated adenoma at surgery and removed proved not to be an adenoma at histological examination. Hypercortisolism recurred 5 and 18 months later, and in each patient an encapsulated ACTH-staining adenoma was found and removed from the opposite side of the anterior lobe at repeat surgery, resulting in hypocortisolism after surgery. In each of the other 4 patients, a well-defined ACTH-positive adenoma was found with one margin contiguous to the surface of the pituitary and removed, resulting in profound hypocortisolism with morning cortisol levels of < 1 μg/dl and UFC levels < 10 μg/24 hours. At surgery for recurrent hypercortisolism, which occurred 6, 50, 125, and 129 months after the initial surgery, an ACTH-staining adenoma was found embedded in the dura mater adjacent to the site at which the adenoma had been removed at initial surgery. Excision of the involved dura resulted in hypocortisolism after surgery in each patient.
Discussion
The surgical technique for using the histological pseudocapsule as a surgical capsule in the excision of pituitary adenomas has recently been described.23 No information exists on the efficacy of using this technique to identify and remove adenomas or to induce remission in patients with pituitary tumors. We examined the utility of using this approach in a large series of patients with Cushing disease. Because the objective was to determine the success of using the pseudocapsule as a surgical capsule, patients with invasive tumors and patients in whom we identified no lesion considered to be an adenoma during pituitary exploration were excluded from the analysis.
Utility of Using the Pseudocapsule to Identify and Remove Pituitary Adenomas
Patients With Positive MR Imaging Findings
The histological pseudocapsule was highly reliable for identification and resection of adenomas in patients with positive MR imaging, as an encapsulated lesion was identified at surgery at the location predicted by the MR imaging in 135 of 135 patients and contained an ACTH-staining adenoma in 99% of these cases. This compares favorably with the results of other experienced pituitary groups, for which the rate of adenoma identification at surgery is 75–90% in patients with Cushing disease and positive MR imaging.17,21,27,29 The histological pseudocapsule can be identified easily at the site of the abnormality on the MR images and can be used to definitively dissect the tumor from the normal anterior lobe. Resolution of hypercortisolism was nearly universal following the first operation, occurring in 132 patients (98%). Additional surgery a few days later in the other 3 patients led to resolution of hypercortisolism in all 135 patients at hospital discharge.
Patients With Negative MR Imaging Findings
Because the NIH is a tertiary referral center, many patients referred with Cushing disease have negative MR imaging. In 126 patients (48%), the findings on the MR images were interpreted as normal or indeterminate by the neuroradiologist.
In patients with negative or ambiguous preoperative MR imaging findings, a thorough exploration of the pituitary gland is required. This requires a broad removal of bone and wide exposure of the gland combined with a large dural opening that allows direct visualization of the lateral margin of the gland on each side. Intraoperative ultrasonography is occasionally useful as an adjunct to MR imaging, as dissection of the pituitary gland can be directed to areas of clear abnormality on the ultrasound image.31
The use of the pseudocapsule to identify accurately the adenoma during the exploration of the pituitary gland was an effective approach compared with that used in other series of patients in whom MR imaging was negative. For instance, Salenave et al.27 reported that only 10 (71%) of 14 lesions removed as adenomas at surgery in patients with negative MR imaging proved to be adenomas at pathological examination. Overall, the extracapsular approach was highly effective in the identification and removal of the lesion causing Cushing disease in patients with negative MR imaging, as 120 (95%) of the 126 patients had resolution of hypercortisolism immediately after selective removal of an encapsulated lesion at initial surgery, and all patients were discharged from the hospital with resolution of hypercortisolism, 6 of them after early repeat surgery.
It is not surprising that 6 (86%) of the 7 patients in whom a lesion was considered an encapsulated adenoma at surgery—removal of which did not induce remission of hypercortisolism, and which proved to be either an incidental adenoma (1 patient, positive MR imaging) or normal anterior lobe (6 patients with negative MR imaging)—were patients with negative MR imaging findings (Fig. 4). As exploration of the anterior lobe proceeds, because one cannot go directly to the site of an abnormality on MR imaging, the likelihood of encountering a small incidental adenoma increases and, in the absence of finding a well-encapsulated lesion, the acceptance of a less-defined lesion as the encapsulated adenoma and removal of it increases.
This rate of success is, to a certain extent, a product of excluding patients with invasive tumors and patients in whom no tumor could be identified at surgery, the most challenging patients for curative treatment, from the analysis. Nonetheless, the consistency of success compares favorably with the results of other recent reports. For instance, Patil et al.24 reported that 85% of 215 patients had remission of hypercortisolism after surgery, despite the fact that, unlike the current study, cases involving macroadenomas, the tumors with the highest rate of invasion, were excluded from the analysis.
Sustained Remission of Cushing Disease After Extracapsular Dissection
The reported recurrence rate for Cushing disease after adenomectomy ranges from 10 to 48%, with diminishing success as patients are followed for > 5 years after their initial surgery.1,4,17,24,33 Only 1 of our 6 patients with eucortisolism (with low-normal UFC excretion) had recurrent Cushing disease. In the other 5 patients with eucortisolism after the initial surgery, remission of Cushing disease persisted at last follow-up (median 89 months, range 36–168 months).
Our experience indicates that the overall recurrence rate associated with using the histological pseudocapsule for tumors confined to the pituitary gland (2.3% with mean follow-up of 84 months, 5.5% of the 72 patients followed until recurrence or until a minimum of 10 years) is significantly lower than that in other studies.1,20,24,33 Even in pediatric patients with Cushing disease, in whom a higher recurrence rate than adults has been noted,18 persistent remission was achieved. Although the low recurrence rate for the current series is in part a result of the exclusion from the analysis of invasive tumors, it is also related to the fact that the tumor resection was performed from outside the pseudocapsule, which allows a clear margin between the tumor and pituitary gland, ensuring complete tumor removal within a thin envelope of compressed normal anterior lobe containing the most peripheral margins of the adenoma. In contrast, the general practice is to remove adenoma from within, by initially removing the soft interior of the tumor and then stripping residual portions of the pseudocapsule that remain in the tumor cavity. In this method the surgeon attempts to identify the periphery of the tumor from within, rather than at the interface of a compressed envelope of normal gland that contains the tumor. Removal of the tumor from within also requires identification and excision of all the fragments of the now-fragmented pseudocapsule, potentially leaving tumor cells behind and resulting in recurrence. It is possible that working outside the capsule may result in the removal of a small portion of the normal pituitary gland, which could cause late endocrinopathy. This underscores the importance of long-term endocrine follow-up in patients who undergo surgery.
Recurrence is a result of incomplete tumor removal during the initial surgery, as evidenced by all recurrent adenomas appearing at the same location as the original tumor, often by unnoticed tumor invasion of the adjacent dura.10,21 The 6 patients with recurrent hypercortisolism in this series are explained by either misinterpretation of a small region of tissue heterogeneity within the anterior lobe as an adenoma (2 [0.8%] of the 261 patients), or by the failure to detect a small area of dural invasion at surgery in patients with tumors with an edge next to the margin of the anterior lobe and recurrence in the contiguous dura, as occurred in 4 patients (1.5%).
Size of Adenomas on MR Imaging and at Surgery
At the NIH, in addition to the conventional techniques, SPGR MR imaging has been included as part of the standard pituitary imaging regimen since 1997. Unlike conventional T1-weighted spin echo imaging, SPGR MR imaging can be performed in sections of 1-mm thickness, providing substantial enhancement of spatial resolution compared with standard MR imaging.2,25
In patients with positive MR imaging, the fact that the tumor visualized on MR imaging tended to be smaller than the adenoma measured intraoperatively reflects the contribution of the histological pseudocapsule, which we attempt to keep intact during the dissection at the margin of the adenoma (Fig. 1).
Comparison of Size of Adenoma in Patients With Positive and Negative MR Imaging
Magnetic imaging of the sella is the radiological modality of choice in patients with pituitary adenomas. Factors that influence visualization on MR imaging include the size, vascularity, and structure of the adenoma, with the majority of MR imaging–positive tumors being 3 mm or greater in diameter. The use of SPGR MR imaging compared with spin echo MR imaging in Cushing disease has been reported to increase the diagnostic accuracy of tumor identification in adults from 48% in the former to 74% in the latter, and from 18 to 68%, respectively, in children with microadenomas.2,25 Because SPGR MR imaging, which has greater sensitivity in detecting small adenomas,2,25 was used in most of the patients included in the current series, the use of conventional spin echo MR imaging only, the practice in many centers, would have produced greater representation of larger adenomas in patients with negative MR imaging and would have resulted in even greater average tumor size in patients with negative MR imaging findings. This also suggests that many patients explored with negative MR imaging results in centers using only spin echo imaging would likely have visible tumor if the more sensitive screening technique of SPGR imaging were used; it also suggests that in those centers the incidence of accurately identifying an encapsulated adenoma using the intraoperative approach described here in patients with negative MR imaging should be even greater.
Successful surgery for Cushing disease in patients with negative MR imaging has always been challenging, and the authors of published series describe lower remission rates in patients with negative preoperative MR imaging.2,20,30,31 Several factors may explain why tumors may not be visible in some patients. First, ACTH-secreting tumors tend to be microadenomas at diagnosis, and many are so small (3 mm) that they escape the limits of detection on MR imaging. Second, some of the larger tumors occupy the entire sella and displace the anterior lobe symmetrically to the margins of the gland in all directions, reducing the clear contrast in the image between the adenoma and the anterior lobe, as occurred in several patients in the current series. Our study clearly demonstrates that negative MR imaging is not necessarily predictive of very small tumor size, as many adenomas > 3 mm in diameter were not evident on MR imaging, and occasionally large nonenhancing tumors that occupy the entire gland cannot be distinguished from normal pituitary gland. Therefore, as yet unidentified biological characteristics of some tumors result in their exhibiting similar signal and enhancing characteristics as the normal gland.
Future improvements in imaging technology and our understanding of features of pituitary tumor biology that influence MR imaging should allow more sensitive methods of detecting tumors that are currently MR imaging negative.2,9,25
Conclusions
The focus on the use of the histological pseudocapsule as a surgical capsule is a safe and effective technique which one of the authors (E.H.O.) has used for successful removal of pituitary adenomas of any type (Cushing disease, acromegaly, and nonsecreting tumors) that are < 2–3 cm in diameter. This technique facilitates the identification and selective removal of adenomas in patients with negative MR imaging. The overall rate of surgical remission is high and the complications associated with the approach are uncommon and minor. Furthermore, the findings described here indicate that biological features of the tumor other than small size underlie negative MR imaging in many patients.
The results attained are also attributable to the wide exposure achieved by the use of the sublabial submucosal approach. This approach provides a direct midline orientation to the sella turcica and permits wide opening of the self-retaining nasal speculum, which is necessary to expose the entire pituitary. An analysis of various approaches in studies of cadavers has demonstrated that a substantially wider exposure (wider by 60%) of the face of the sella turcica is obtained using the sublabial approach than using intranasal approaches.8 Further, in spite of numerous descriptions of the endonasal approaches as being “less invasive” approaches, most surgeons using these approaches remove the nasal mucosa along the posterior margin of the nasal cavity, the nasal mucosa covering the anterior face of the sphenoid bone. In contrast, the sublabial approach leaves all the nasal mucosa intact and in place when the procedure is completed.
Finally, because all types of pituitary adenomas enlarge slowly and compress the surrounding tissue, producing a histological pseudocapsule that can be used as a surgical capsule (E.H.O., personal experience), the same surgical approach shown here for Cushing disease should be useful for other types of pituitary tumors.
Acknowledgments
Disclosure This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, NIH.
Abbreviations used in this paper
- ACTH
adrenocorticotropic hormone
- NIH
National Institutes of Health
- SPGR
spoiled gradient recalled acquisition in the steady state
- UFC
urine free cortisol
References
- 1.Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B. Long-term remission rates after pituitary surgery for Cushing's disease: the need for long-term surveillance. Clin Endocrinol (Oxf) 2005;63:549–559. doi: 10.1111/j.1365-2265.2005.02380.x. [DOI] [PubMed] [Google Scholar]
- 2.Batista D, Courkoutsakis NA, Oldfield EH, Griffin KJ, Keil M, Patronas NJ, et al. Detection of adrenocorticotropin-secreting pituitary adenomas by magnetic resonance imaging in children and adolescents with cushing disease. J Clin Endocrinol Metab. 2005;90:5134–5140. doi: 10.1210/jc.2004-1778. [DOI] [PubMed] [Google Scholar]
- 3.Blevins LS, Jr, Christy JH, Khajavi M, Tindall GT. Outcomes of therapy for Cushing's disease due to adrenocorticotropin-secreting pituitary macroadenomas. J Clin Endocrinol Metab. 1998;83:63–67. doi: 10.1210/jcem.83.1.4525. [DOI] [PubMed] [Google Scholar]
- 4.Chee GH, Mathias DB, James RA, Kendall-Taylor P. Transsphenoidal pituitary surgery in Cushing's disease: can we predict outcome? Clin Endocrinol (Oxf) 2001;54:617–626. doi: 10.1046/j.1365-2265.2001.01261.x. [DOI] [PubMed] [Google Scholar]
- 5.Ciric I. Pituitary tumors. Neurol Clin. 1985;3:751–768. [PubMed] [Google Scholar]
- 6.Cohen-Gadol AA, Liu JK, Laws ER., Jr Cushing's first case of transsphenoidal surgery: the launch of the pituitary surgery era. J Neurosurg. 2005;103:570–574. doi: 10.3171/jns.2005.103.3.0570. [DOI] [PubMed] [Google Scholar]
- 7.Costello R. Subclinical adenoma of the pituitary gland. Am J Pathol. 1936;12:205–214. [PMC free article] [PubMed] [Google Scholar]
- 8.Das K, Spencer W, Nwagwu CI, Schaeffer S, Wenk E, Weiss MH, et al. Approaches to the sellar and parasellar region: anatomic comparison of endonasal-transsphenoidal, sublabial-transsphenoidal, and transethmoidal approaches. Neurol Res. 2001;23:51–54. doi: 10.1179/016164101101198280. [DOI] [PubMed] [Google Scholar]
- 9.Davis WL, Lee JN, King BD, Harnsberger HR. Dynamic contrast-enhanced MR imaging of the pituitary gland with fast spin-echo technique. J Magn Reson Imaging. 1994;4:509–511. doi: 10.1002/jmri.1880040345. [DOI] [PubMed] [Google Scholar]
- 10.Dickerman RD, Oldfield EH. Basis of persistent and recurrent Cushing disease: an analysis of findings at repeated pituitary surgery. J Neurosurg. 2002;97:1343–1349. doi: 10.3171/jns.2002.97.6.1343. [DOI] [PubMed] [Google Scholar]
- 11.Gierach M, Pufal J, Pilecki S, Junik R. The case of Cushing's disease imaging by SPECT examination without manifestation of pituitary adenoma in MRI examination. Nucl Med Rev Cent East Eur. 2005;8:137–139. [PubMed] [Google Scholar]
- 12.Hammer GD, Tyrrell JB, Lamborn KR, Applebury CB, Hannegan ET, Bell S, et al. Transsphenoidal microsurgery for Cushing's disease: initial outcome and long-term results. J Clin Endocrinol Metab. 2004;89:6348–6357. doi: 10.1210/jc.2003-032180. [DOI] [PubMed] [Google Scholar]
- 13.Hardy J. Transsphenoidal hypophysectomy. J Neurosurg. 1971;34:582–594. doi: 10.3171/jns.1971.34.4.0582. [DOI] [PubMed] [Google Scholar]
- 14.Hardy J. Transsphenoidal surgery. Surg Neurol. 1976;5:160. Letter. [PubMed] [Google Scholar]
- 15.Hardy J, Vezina JL. Transsphenoidal neurosurgery of intracranial neoplasm. Adv Neurol. 1976;15:261–273. [PubMed] [Google Scholar]
- 16.Hofmann BM, Hlavac M, Martinez R, Buchfelder M, Muller OA, Fahlbusch R. Long-term results after microsurgery for Cushing disease: experience with 426 primary operations over 35 years. J Neurosurg. 2008;108:9–18. doi: 10.3171/JNS/2008/108/01/0009. [DOI] [PubMed] [Google Scholar]
- 17.Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F. Diagnosis and management of Cushing's syndrome: results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J Clin Endocrinol Metab. 1999;84:440–448. doi: 10.1210/jcem.84.2.5465. [DOI] [PubMed] [Google Scholar]
- 18.Kanter AS, Dumont AS, Asthagiri AR, Oskouian RJ, Jane JA., Jr Laws ER Jr: The transsphenoidal approach. A historical perspective. Neurosurg Focus. 2005;18(4):E6. doi: 10.3171/foc.2005.18.4.7. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay JR, Nansel T, Baid S, Gumowski J, Nieman LK. Long-term impaired quality of life in Cushing's syndrome despite initial improvement after surgical remission. J Clin Endocrinol Metab. 2006;91:447–453. doi: 10.1210/jc.2005-1058. [DOI] [PubMed] [Google Scholar]
- 20.Mampalam TJ, Tyrrell JB, Wilson CB. Transsphenoidal microsurgery for Cushing disease. A report of 216 cases. Ann Intern Med. 1988;109:487–493. doi: 10.7326/0003-4819-109-6-487. [DOI] [PubMed] [Google Scholar]
- 21.Nakane T, Kuwayama A, Watanabe M, Takahashi T, Kato T, Ichihara K, et al. Long term results of transsphenoidal adenomectomy in patients with Cushing's disease. Neurosurgery. 1987;21:218–222. doi: 10.1227/00006123-198708000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Nieman LK. Medical therapy of Cushing's disease. Pituitary. 2002;5:77–82. doi: 10.1023/a:1022308429992. [DOI] [PubMed] [Google Scholar]
- 23.Oldfield EH, Vortmeyer AO. Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg. 2006;104:7–19. doi: 10.3171/jns.2006.104.1.7. [DOI] [PubMed] [Google Scholar]
- 24.Patil CG, Prevedello DM, Lad SP, Vance ML, Thorner MO, Katznelson L, et al. Late recurrences of Cushing's disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab. 2008;93:358–362. doi: 10.1210/jc.2007-2013. [DOI] [PubMed] [Google Scholar]
- 25.Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, et al. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional post-contrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab. 2003;88:1565–1569. doi: 10.1210/jc.2002-021438. [DOI] [PubMed] [Google Scholar]
- 26.Ram Z, Nieman LK, Cutler GB, Jr, Chrousos GP, Doppman JL, Oldfield EH. Early repeat surgery for persistent Cushing's disease. J Neurosurg. 1994;80:37–45. doi: 10.3171/jns.1994.80.1.0037. [DOI] [PubMed] [Google Scholar]
- 27.Salenave S, Gatta B, Pecheur S, San-Galli F, Visot A, Lasjaunias P, et al. Pituitary magnetic resonance imaging findings do not influence surgical outcome in adrenocorticotropin-secreting microadenomas. J Clin Endocrinol Metab. 2004;89:3371–3376. doi: 10.1210/jc.2003-031908. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan JM, Lopes MB, Sheehan JP, Ellegala D, Webb KM, Laws ER., Jr Results of transsphenoidal surgery for Cushing's disease in patients with no histologically confirmed tumor. Neurosurgery. 2000;47:33–39. doi: 10.1097/00006123-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Testa RM, Albiger N, Occhi G, Sanguin F, Scanarini M, Berlucchi S, et al. The usefulness of combined biochemical tests in the diagnosis of Cushing's disease with negative pituitary magnetic resonance imaging. Eur J Endocrinol. 2007;156:241–248. doi: 10.1530/eje.1.02332. [DOI] [PubMed] [Google Scholar]
- 30.Thapar K, Laws ER., Jr . Pituitary tumors. In: Kaye AH, Laws ER Jr, editors. Brain Tumors. Churchill Livingstone; London: 2001. pp. 803–854. [Google Scholar]
- 31.Watson JC, Shawker TH, Nieman LK, DeVroom HL, Doppman JL, Oldfield EH. Localization of pituitary adenomas by using intraoperative ultrasound in patients with Cushing's disease and no demonstrable pituitary tumor on magnetic resonance imaging. J Neurosurg. 1998;89:927–932. doi: 10.3171/jns.1998.89.6.0927. [DOI] [PubMed] [Google Scholar]
- 32.Weil RJ, Vortmeyer AO, Nieman LK, Devroom HL, Wanebo J, Oldfield EH. Surgical remission of pituitary adenomas confined to the neurohypophysis in Cushing's disease. J Clin Endocrinol Metab. 2006;91:2656–2664. doi: 10.1210/jc.2006-0277. [DOI] [PubMed] [Google Scholar]
- 33.Yap LB, Turner HE, Adams CB, Wass JA. Undetectable postoperative cortisol does not always predict long-term remission in Cushing's disease: a single centre audit. Clin Endocrinol (Oxf) 2002;56:25–31. doi: 10.1046/j.0300-0664.2001.01444.x. [DOI] [PubMed] [Google Scholar]