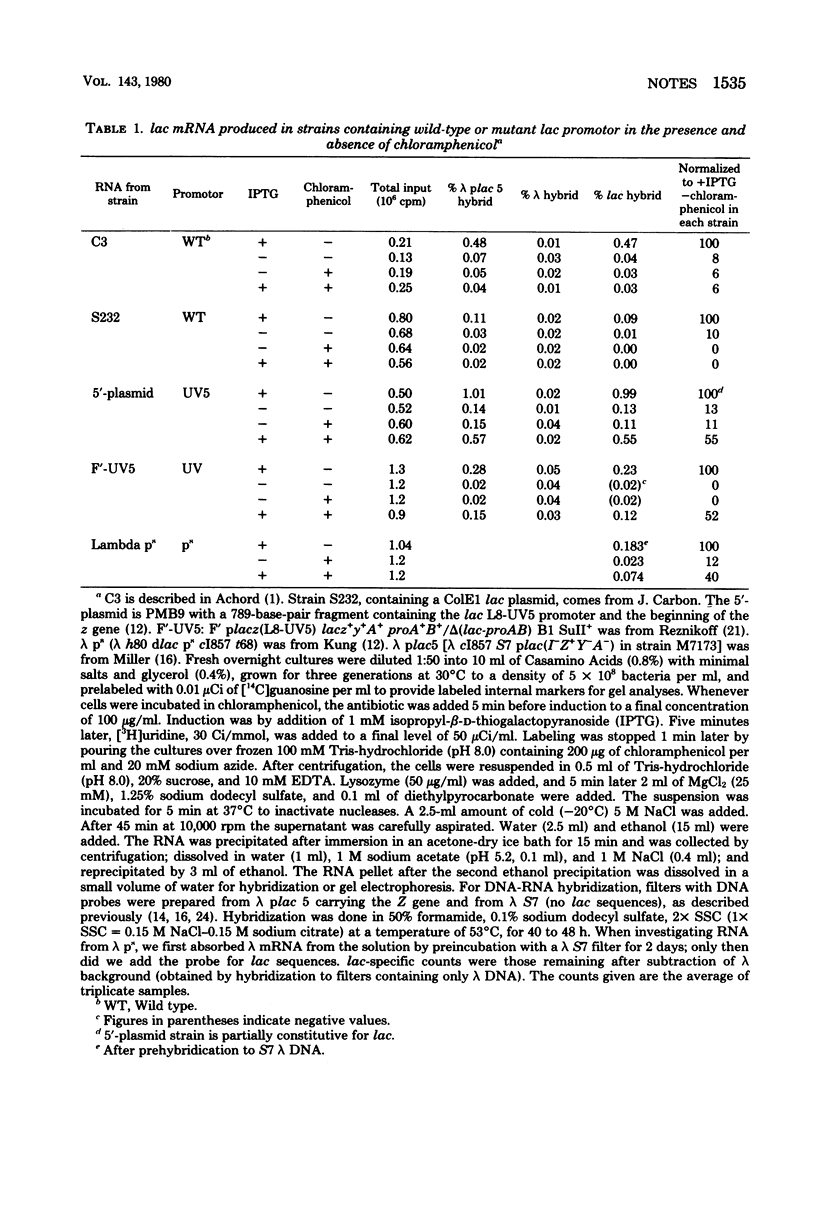
Abstract
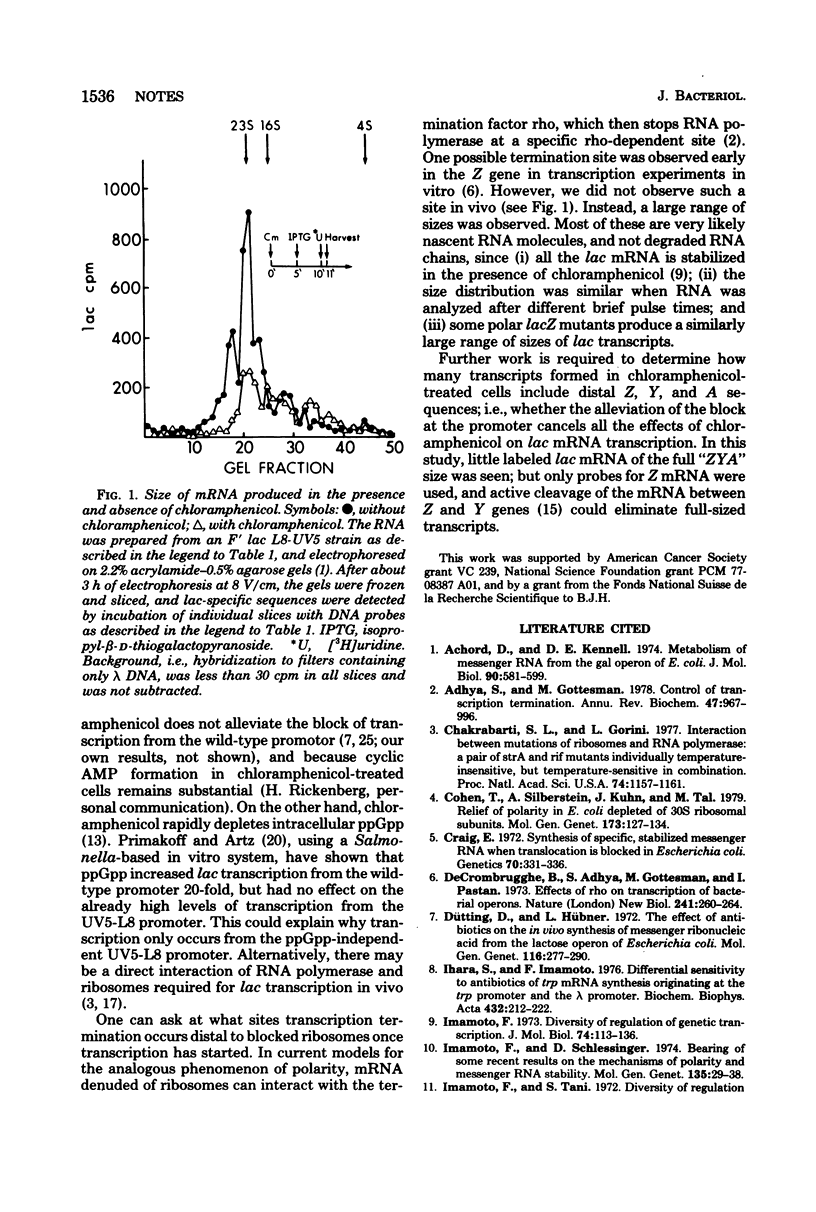
In cells treated with chloramphenicol and the inducer isopropyl-beta-D-thiogalacto-pyranoside, messenger ribonucleic acid transcription from the wild-type lac promoter was not detected. Transcription occurred from the mutant UV5-L8 promoter. The transcripts were of variable length; some included the whole Z gene. No major site of transcription arrest within the Z gene was apparent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D., Kennell D. Metabolism of messenger RNA from the gal operon of Escherichia coli. J Mol Biol. 1974 Dec 15;90(3):581–599. doi: 10.1016/0022-2836(74)90236-8. [DOI] [PubMed] [Google Scholar]
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. Interaction between mutations of ribosomes and RNA polymerase: a pair of strA and rif mutants individually temperature-insensitive but temperature-sensitive in combination. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1157–1161. doi: 10.1073/pnas.74.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen T., Silberstein A., Kuhn J., Tal M. Relief of polarity in E. coli depleted of 30S ribosomal subunits. Mol Gen Genet. 1979 Jun 7;173(2):127–134. doi: 10.1007/BF00330302. [DOI] [PubMed] [Google Scholar]
- Craig E. Synthesis of Specific, Stabilized Messenger RNA When Translocation Is Blocked in ESCHERICHIA COLI. Genetics. 1972 Feb;70(2):331–336. doi: 10.1093/genetics/70.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Adhya S., Gottesman M., Pastan I. Effect of Rho on transcription of bacterial operons. Nat New Biol. 1973 Feb 28;241(113):260–264. doi: 10.1038/newbio241260a0. [DOI] [PubMed] [Google Scholar]
- Dütting D., Hübner L. The effect of antibiotics on the in vivo synthesis of messenger ribonucleic acid from the lactose operon of Escherichia coli. Mol Gen Genet. 1972;116(3):277–290. doi: 10.1007/BF00269771. [DOI] [PubMed] [Google Scholar]
- Gallant J., Margason G., Finch B. On the turnover of ppGpp in Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6055–6058. [PubMed] [Google Scholar]
- Goldberg A. R., Howe M. New mutations in the S cistron of bacteriophage lambda affecting host cell lysis. Virology. 1969 May;38(1):200–202. doi: 10.1016/0042-6822(69)90148-2. [DOI] [PubMed] [Google Scholar]
- Ihara S., Imamoto F. Differential sensitivity to antibiotics of trp mRNA synthesis originating at the trp promoter and the lambda promoter. Biochim Biophys Acta. 1976 May 3;432(2):212–222. doi: 10.1016/0005-2787(76)90163-5. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Schlessinger D. Bearing of some recent results on the mechanisms of polarity and messenger RNA stability. Mol Gen Genet. 1974;135(1):29–38. doi: 10.1007/BF00433898. [DOI] [PubMed] [Google Scholar]
- Kung H., Tainsky M., Weissbach H. Regulation of the in vitro synthesis of the alpha-peptide of beta-galactosidase directed by a restriction fragment of the lactose operon. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1000–1010. doi: 10.1016/0006-291x(78)91450-x. [DOI] [PubMed] [Google Scholar]
- Lim L. W., Kennell D. Models for decay of Escherichia coli lac messenger RNA and evidence for inactivating cleavages between its messages. J Mol Biol. 1979 Dec 5;135(2):369–390. doi: 10.1016/0022-2836(79)90442-x. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Kano Y., Schlessinger D., Imamoto F., McPartland A., Somerville R. L. Translation-uncoupled transcription of promoter-proximal DNA sequences in E. coli strains harboring mutationally-generated constitutive promoters within genes of the trp operon. Mol Gen Genet. 1979 May 4;172(2):127–136. doi: 10.1007/BF00268273. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Artz S. W. Positive control of lac operon expression in vitro by guanosine 5'-diphosphate 3'-diphosphate. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa T., Imamoto F. Diversity of regulation of genetic transcription. II. Specific relaxation of polarity in read-through transcription of the translocated trp operon in bacteriophage lambda trp. J Mol Biol. 1974 Aug 25;87(4):741–754. doi: 10.1016/0022-2836(74)90082-5. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in antibiotic-treated E. coli. Nat New Biol. 1971 Mar 10;230(10):41–44. doi: 10.1038/newbio230041a0. [DOI] [PubMed] [Google Scholar]



