Abstract
Activation of NF-κB transcription factors by locally produced angiotensin II (Ang II) is proposed to be involved in chronic inflammatory reactions leading to atherosclerosis development. However, a clear understanding of the signaling cascades coupling the Ang II AT1 receptors to the activation of NF-κB transcription factors is still lacking. Using primary cultured aortic vascular smooth muscle cells, we show that activation of the IKK complex and NF-κB transcription factors by Ang II is regulated by phosphorylation of the catalytic subunit IKKβ on serine residues 177 and 181 in the activation T-loop. The use of pharmacological inhibitors against conventional protein kinases C (PKCs), mitogen-activated/extracellular signal-regulated kinase (MEK) 1/2, ribosomal S6 kinase (RSK), and silencing RNA technology targeting PKCα, IKKβ subunit, tumor growth factor β-activating kinase-1 (TAK1), the E3 ubiquitin ligase tumor necrosis factor receptor-associated factor-6 (TRAF6), and RSK isoforms, demonstrates the requirement of two distinct signaling pathway for the phosphorylation of IKKβ and the activation of the IKK complex by Ang II. Rapid phosphorylation of IKKβ requires a second messenger-dependent pathway composed of PKCα-TRAF6-TAK1, whereas sustained phosphorylation and activation of IKKβ requires the MEK1/2-ERK1/2-RSK pathway. Importantly, simultaneously targeting components of these two pathways completely blunts the phosphorylation of IKKβ and the proinflammatory effect of the octapeptide. This is the first report demonstrating activation of TAK1 by the AT1R. We propose a model whereby TRAF6-TAK1 and ERK-RSK intracellular pathways independently and sequentially converge to the T-loop phosphorylation for full activation of IKKβ, which is an essential step in the proinflammatory activity of Ang II.
Keywords: G Protein-coupled Receptors (GPCR), Inflammation, NF-κB Transcription Factor, Signal Transduction, Ubiquitin Ligase, IKK Complex, TAK1, TRAF6, Angiotensin II, Inflammation
Introduction
The hormone angiotensin II (Ang II)3 is the effector of the circulating renin angiotensin system (RAS). Through binding of the Ang II receptor subtype 1 (AT1R), Ang II controls saltwater and blood pressure homeostasis (1). Components of the RAS are also found within several tissues where locally produced Ang II acts as a cytokine, modulating cellular responses in paracrine and autocrine fashions (2). Tissue RAS also contributes to homeostatic responses. However, deregulated and chronic production of tissue Ang II may be an important mechanism linking uncontrolled activation of local RAS to cardiovascular diseases (3, 4). Regardless of the synthesis pathway, numerous studies have clearly established the effects of Ang II on vascular remodeling events such as atherosclerosis and hypertrophy, which are mediated in part by the growth and inflammatory properties of the octapeptide on vascular smooth muscle cells (VSMC) (5–8). As such, we previously demonstrated that the hypertrophic property of Ang II in VSMC is largely dependent on the MEK-ERK signaling cascade and the incapacity of the octapeptide to down-regulate the cell cycle inhibitor p27Kip1 (7, 8). Another important signaling pathway that is often involved in chronic inflammatory diseases is the NF-κB pathway (see Refs. 9–13 for reviews). Indeed, aberrant NF-κB regulation has been described in human atherosclerotic plaques (14, 15) and a recent mouse model of atherosclerosis in which NF-κB activation was blunted specifically in endothelial cells by genetic means highlighted the key role of the latter as a crucial promoter of atherosclerosis development (16). This observation possibly highlights that many processes affecting the formation of atherosclerotic plaques converge to the activation of NF-κB transcription factors (15).
The NF-κB family is represented by five members: p50, p65(RelA), c-Rel, p52, and RelB. In resting cells, they exist as homo- or heterodimers that are sequestered in the cytoplasm in an inactive form through their association with one of several inhibitory molecules, namely IκB-α, -β, -ϵ, p105, and p100. In the classical pathway, the first phase of NF-κB activation mainly consists of the regulated degradation of IκBα and is triggered by prototypical activators such as tumor necrosis factor (TNF)-α, lipopolysaccharide, IL-1β, and phorbol 12-myristate 13-acetate. These stimuli induce the phosphorylation of IκBα at Ser32 and Ser36 in the N-terminal signal responsive domain by the canonical IκB kinase (IKK) complex, which is composed of two catalytic subunits called IKKα and -β, and one regulatory subunit called IKKγ. Phosphorylated IκBα is subsequently polyubiquitinated and targeted to the 26 S proteasome complex, resulting in the release and nuclear accumulation of NF-κB, which can now stimulate target gene transcription (17). The IKK complex can also modulate the activity of the NF-κB pathway by directly targeting p65 (RelA) and c-Rel subunits (17). Notably, we and others have shown that the signaling cascade coupling the engagement of the AT1R to an inflammatory response involving NF-κB transcription factors required activation of the IKKβ subunit (5, 18). Due to the importance of the IKK/NF-κB module in several pathologies, the intricacy of IKK complex activation and modulation is currently under intensive investigation (19–22).
Like many protein kinases, both IKKα and IKKβ contain activation loops that need to be phosphorylated to become activated (23). Replacement of these phosphoacceptor sites within the T-loop in IKKβ (Ser177/Ser181) with alanines (IKKβ(AA)) prevents kinase activation, and thus, when overexpressed, acts in a dominant-negative fashion and prevents IKK complex and NF-κB activation (23). Importantly, both serines are phosphorylated in vivo in response to pro-inflammatory stimuli (23). IKKα also becomes phosphorylated at the corresponding serines (Ser176/Ser180) during cell stimulation. However, in most cell types, IKKα phosphorylation and activation is less critical than IKKβ (24, 25). In addition to phosphorylation, Lys63-linked polyubiquitination is another essential covalent modification required for IKK complex activation by membrane-bound receptors (19). Lys63-linked polyubiquitination reactions are proposed to create docking platforms for the recruitment and the activation of the IKK complex (through Lys63-linked IKKγ polyubiquitination) as well as for the activation of two mitogen-activated protein (MAP)-kinase-kinase-kinase (MAPKKK) family members, namely MEKK3 and TAK1 (19). These two protein kinases act as IKK-activating kinases (that induce IKKβ phosphorylation in the T-loop) (17, 19).
Genetic data about the molecular links between GPCR proximal events and activation of the IKK complex now demonstrate that a complex formed by CARMA3, Bcl10, and MALT1, better known as the CBM signalosome, constitute the missing link between G protein-coupled receptors (GPCRs) and IKK complex activation (20, 26–28). In this complex, the ubiquitin E3 ligase MALT1 could act as the effector protein in AT1R signaling by inducing Lys63-linked IKKγ polyubiquitination and IKK complex activation (26). On the other hand, in T cell receptor signaling, the E3 ubiquitin ligase TRAF6 and TAK1 act downstream of Bcl10 and MALT1 in mediating IKK complex activation (29). Thus, the identification of the E3 ubiquitin ligase and the IKK-activating kinase involved in coupling the AT1R to the activation of the IKK complex is still unknown. The present study was undertaken to characterize the signaling cascades controlling the phosphorylation and activation of the IKK complex in primary VSMC exposed to Ang II.
EXPERIMENTAL PROCEDURES
Reagent, Antibodies, and Plasmids
Angiotensin II was purchased from Sigma; platelet-derived growth factor (PDGF)-BB and TNF-α were purchased from BioSource (Camarillo, CA). The MEK1/2 inhibitors UO126 and PD184352, the PKCα/β inhibitor Gö6976, and the intracellular calcium-specific chelator BAPTA-AM were purchased from Calbiochem (Gibbstown, NJ). The selective RSK inhibitor BI-D1870 was kindly donated by Dr. Philippe Roux (Université de Montréal). p100 polyclonal antibody was a gift from Dr. John Hiscott (McGill University). The commercial antibodies were purchased from the following suppliers: anti-IKKα/β (SC-7607) and anti-TAK1 (SC-948) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-TRAF6 (IMG-5654-2) from Imgenex (San Diago, CA); anti-β-actin clone AC-74 (A5316) from Sigma; phospho-TAK1 (Thr184/Thr187) antibody (number 4531), anti-TAK1 (number 4505), phospho-IKKα/β (Ser180/Ser181) antibody (number 2681), phospho-ERK1/2 (Thr202–Tyr204) antibody (number 9101), anti-ERK1/2 (number 9102), anti-PKCα (number 2056), anti-RSK1/2/3 (number 9347), and anti-NIK (number 7211) from Cell Signaling Technology (Beverly, MA). Glutathione S-transferase (GST)-IκBα (amino acids 1–54) has been described (18). The GST-IKKβ T-loop (amino acids 125–208) recombinant protein was produced by subcloning PCR-amplified fragments in pGEX-KG. The resulting construct was transformed in Escherichia coli and following isopropyl β-d-thiogalactopyranoside (IPTG) induction (1 mm for 3 h at 37 °C), purified over a glutathione-agarose column (Amersham Biosciences). The expression plasmid pTrack-FLAG-IKKβ (K44A), the pRL-TK, and the luciferase NF-κB reporter, pLuc-(PRDII)2, were provided by Dr. John Hiscott (McGill University, Montreal, QC). The pCDNA3.1-HA-AT1R plasmid was kindly donated by Dr. Stéphane Laporte (McGill University, Montreal, QC). The pCMVT-TAK1 K63W plasmid was provided by Dr. Jun Ninomiya-Tsuji (North Carolina State University, Raleigh, NC). The expression construct pCMV5-MEKK3 WT was obtained from Gary L. Johnson and purchased through Addgene (Cambridge, MA) (plasmid 12186). From this vector, the pCMV5-MEKK3 K391M plasmid was produced by site-directed mutagenesis.
Cell Types
Low passage primary rat aortic VSMCs were obtained from Dr. Darren Richard (Hôtel-Dieu de Québec, QC, Canada) and grown in high glucose DMEM supplemented with 10% fetal bovine serum (FBS). Cultures were maintained at 37 °C in a humidified atmosphere of 95% air and 5% CO. All experiments were conducted on cells at passage levels 9–16. Quiescent VSMCs were obtained by incubation of 95% confluent cell cultures in serum-free high glucose DMEM, Ham's F-12 (1:1) supplemented with 15 mm Hepes (pH 7.4), 0.1% low endotoxin bovine serum albumin (Sigma), and 5 μg/ml of transferrin (Sigma) for 48 h. For experiments with pharmacological inhibitors, the cells were treated with vehicle alone or with the indicated concentrations of inhibitors for 30 min before addition of Ang II. QbI-HEK 293A cells, purchased from Q-Biogene (Carisbad, CA), were cultured in high glucose DMEM supplemented with 10% FBS.
Reporter Gene Assays
QbI-HEK 293A in 24 wells (100 000 cells/well) were transfected by calcium phosphate coprecipitation method with 50 ng of pRLTK reporter (Renilla luciferase for internal control), 100 ng of pCDNA3.1-HA-AT1R, 200 ng of NF-κB reporter (pLUC-PRDII), and an increasing amount of the dominant-negative forms of pTrack-FLAG-IKKβ K44A, pCMVT-TAK1 K63W, or pCMV5-MEKK3 K391M. Cells were harvested at 72 h posttransfection, lysed in passive lysis buffer (Promega, Madison, WI), and assayed for dual-luciferase activity with use of 10 μl of lysate according to the manufacturer's instructions. All firefly luciferase values were normalized to Renilla luciferase to control for transfection efficiency.
RT Real Time-PCR Analysis
Total RNA was isolated using TRIzol (Invitrogen), DNase-treated (Sigma), and reverse transcribed (1 μg) with the SuperScript VILO cDNA Synthesis Kit (Invitrogen, Burlington, ON) according to the manufacturer's instructions. Real time PCRs were subsequently performed using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) with the following forward and reverse primers: rat interleukin (IL6), 5′-TCCTACCCCAACTTCCAATGCTC-3′, 5′-TTGGATGGTCTTGGTCCTTAGCC-3′; rat 18S, 5′-GGGAGGTAGTGACGAAAAATAACAAT-3′, 5′-TTGCCCTCCAATGGATCCT-3′; human IL8, 5′-CTCTCTTGGCAGCCTTCCTGATT-3′, 5′-AACTTCTCCACAACCCTCTGCAC-3′; human β-actin, 5′-AACTCCATCATGAAGTGTGACG-3′, 5′-GATCCACATCTGCTGGAAGG-3′. qPCRs were performed using the following schedule: denaturation was performed for 5 min at 95 °C with 15 s between each cycle; annealing was performed for 20 s at 55 °C for 18S, at 60 °C for β-actin, and at 65 °C for IL6 and IL8; primer extension was done for 25 s at 72 °C.
RNA Interference
VSMCs were transfected with 20 nm siRNA duplexes using Dharmafect 3 reagent. The ON-TARGET plus siRNA targeting PKCα (siRNA1, 5′-GGAGAGAGAUGACGUACGA-3′; siRNA2, 5′-GGAAUGAGUCCUUCACGUU-3′), TAK1 (siRNA1, 5′-GGUGAUAACACGCCGGAAA-3′; siRNA2, 5′-AUACCAAUGGCUCGGAUAA-3′; siRNA3, 5′-CAUAUCAGGCAACGGGCAA-3′; siRNA4, 5′-CUGAAAUAGAAGCGAGGAU-3′), TRAF6 (siRNA1, 5′-GAGAACAGAUGCCUAAUCA-3′; siRNA2, 5′-GGACAAAGUUGCCGAGAUG-3′; siRNA3, 5′-GUGUUUGGCUGUCACGAAA-3′; siRNA4, 5′-AGAGCAUGCAUGUGAGCGA-3′), RSK1 (5′-UCGAGAUUCUUCUGCGGUA-3′), RSK2 (5′-GGAAUAAGCUGUAUCGCAA-3′), RSK3 (5′-CAGAUUAGGUGCCGGACCA-3′), and IKKβ (5′-AAACCAGCAUCCAGAUUGA-3′) were purchased from Dharmacon (Lafayette, CO).
Immunoblot Analysis
After the different treatments, cells were washed twice with ice-cold phosphate-buffered saline (PBS), and whole cell extracts were prepared using Triton X-100 lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 50 mm sodium fluoride, 5 mm EDTA, 40 mm β-glycerophosphate, 1 mm sodium orthovanadate, 10−4 m phenylmethylsulfonyl fluoride, 10−6 m leupeptin, 10−6 m pepstatin A, 1% Triton X-100, 10% glycerol) for 30 min at 4 °C. Lysates were clarified by centrifugation at 13,000 × g for 10 min, and equal amounts of lysate proteins (30–60 μg) were subjected to electrophoresis on 7.5 or 12% acrylamide gels. Proteins were electrophoretically transferred to Hybond-C nitrocellulose membranes (Amersham Biosciences) in 25 mm Tris, 192 mm glycine, and 20% methanol. Immunoblot analysis for each antibody was carried out according to the manufacturer's instructions. In some Western blot experiments, cellular extracts were equally divided (μg) and used in parallel.
In Vitro Kinase Assays
The phosphotransferase activity of the IKK complex and TAK1 was measured as described previously (18). Briefly, 500 μg of whole cell extracts were incubated for 4 h at 4 °C with specific antibody to IKKα/β (SC-7607) or TAK1 (SC-948) preabsorbed to protein A-Sepharose beads. The immune complexes were washed twice with lysis buffer and twice with kinase buffer (20 mm Hepes, pH 7.4, 25 mm NaCl, 1 mm EGTA, 20 mm MgCl2, 1 mm dithiothreitol (DTT), 5 mm p-nitrophenyl phosphate, 20 mm β-glycerophosphate, 1 mm sodium orthovanadate, 10−4 m phenylmethylsulfonyl fluoride). IKK complex and TAK1 activities were assayed by resuspending the beads in 40 μl of kinase buffer containing 2 μg of GST-IκBα (amino acids 1–54) for IKK complex or GST-IKKβ T-loop (amino acids 125–208) for TAK1, 20 μm ATP, and 20 μCi of [γ-32P]ATP. The reactions were incubated at 30 °C for 60 min and stopped by the addition of 5× Laemmli's sample buffer. The samples were analyzed by SDS-gel electrophoresis. Following Coomassie staining, the gels were dried and exposed to a gel documentation device (Typhoon scanner 9410, Amersham Biosciences) for imaging and quantification.
Electrophoretic Mobility Shift Assays (EMSA)
After the different treatments, VSMCs were washed with ice-cold PBS and resuspended in 200 μl of buffer A (10 mm Hepes, pH 7.4, 10 mm KCl, 0.1 mm EDTA, 1 mm sodium fluoride, 20 mm β-glycerophosphate, 1 mm sodium orthovanadate, 10−4 m phenylmethylsulfonyl fluoride, 10−6 m leupeptin, and 10−6 m pepstatin A). The cells were incubated on ice for 15 min. Nonidet P-40 (10%) was added to a final concentration of 0.5%; the cells were vortexed vigorously and centrifuged for 30 s at 4 °C. The nuclear pellets were washed in 100 μl of buffer A and centrifuged for 30 s at 4 °C. The pellets were then resuspended in 50 μl of 20 mm Hepes, pH 7.4, 0.4 m NaCl, 1 mm EDTA, 1 mm sodium orthovanadate, 10−4 m phenylmethylsulfonyl fluoride, 10−6 m leupeptin, and 10−6 m pepstatin A and incubated on ice for 20 min. Supernatants were collected after a centrifugation of 10 min at 4 °C. 10 μg of nuclear extracts were subjected to EMSA with the NF-κB oligonucleotide: 5′-ACCAAGAGGGATTTCACCTAAATC-3′ and the NF-κB 5X binding buffer (25 mm Tris-HCl, pH 7.5, 5 mm EDTA, 125 μg/ml of poly(dI:dC), 5 mm DTT, 1 mg/ml of BSA, 30% glycerol). In each reaction, 500,000 cpm of [γ-32P]ATP-labeled probe was used, and bandshifts were resolved on 5% polyacrylamide gels in 0.5× TBE running buffer.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 5.0 for Mac (GraphPad Software, San Diego, CA). Comparison of two groups was carried out using a two-tailed unpaired t test, and comparison of more than two groups was carried out with one-way analysis of variance and a Bonferroni post-test. Statistical significance was accepted at a p value below 0.05. The number of independent experiments is denoted by n.
RESULTS
Essential Role of IKKβ in the Proinflammatory Actions of Ang II
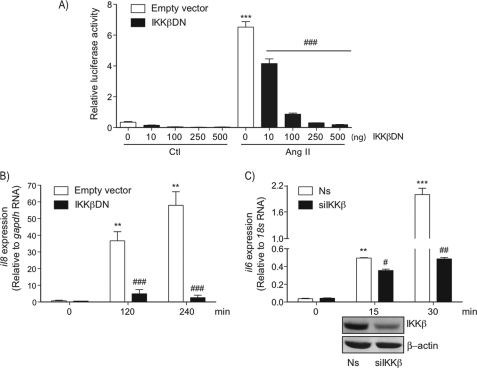
We and others have recently demonstrated AT1R-mediated activation of the IKK complex in primary VSMC exposed to Ang II (5, 18). Other studies have rather suggested a role of the noncanonical pathway, mediated by the MAPKKK family member NF-κB-inducing kinase (NIK) and IKKα, in the proinflammatory effect of Ang II (30, 31). To ascertain the role of the IKKβ subunit in the proinflammatory effect of the octapeptide, we first verified the Ang II response in a heterologous cell system overexpressing the AT1R and a dominant-negative version of IKKβ (IKKβ DN). Expression of the AT1R in 293 cells allows Ang II to strongly enhance NF-κB promoter activity, which was significantly inhibited by co-transfecting increasing amounts of IKKβ DN (Fig. 1A). IKKβ subunit was also essential for Ang II-induced il8 transcription in 293 cells (Fig. 1B) as well as rapid il6 transcription in VSMC, where the IKKβ subunit level was reduced by means of siRNA treatment (Fig. 1C). Together these data confirmed the essential role of the IKKβ subunit in the proinflammatory action of Ang II.
FIGURE 1.
Role of IKKβ in the proinflammatory actions of Ang II. A, QbI-HEK 293A cells were transiently transfected with the NF-κB reporter plasmid PRDII-Luc together with plasmids encoding for the AT1R and the indicated amount of IKKβ DN or an empty vector. Cells were serum starved for 24 h and then exposed to Ang II (100 nm) for 18 h before cell lysates were prepared for luciferase assays. Data are mean ± S.E. (n = 3). B, QbI-HEK 293A cells were co-transfected with plasmids encoding AT1R and IKKβ DN or an empty vector. At 24 h post-transfection, cells were serum starved for 24 h and then exposed to Ang II (100 nm) for the indicated times. RNA was extracted and submitted to a RT-PCR analysis using primers for il8 and gapdh as an internal RNA control. Data are mean ± S.D. (n = 3). C, VSMCs were transfected with a nonsilencing (Ns) RNA duplex or a RNA duplex that specifically targets IKKβ. At 48 h, post-transfection cells were serum starved for 24 h and then exposed to Ang II (100 nm) for the indicated times. RNA was extracted and submitted to a RT-PCR using primers for il6 and 18S as internal RNA controls. The lower panel shows the efficiency of IKKβ silencing using Western blot analysis. Data are mean ± S.D. (n = 3). *, significantly above conditions without Ang II treatment; #, significantly below the Ang II response; a single symbol indicates p < 0.05, two symbols indicate p < 0.01, and three symbols indicate p < 0.001.
In cultured primary B cells and mouse embryonic fibroblasts, the expression level of NIK is undetectable because it is constitutively degraded by the proteasome (32). Activation of the noncanonical NF-κB pathway by a select group of TNF receptors such as CD40 and the lymphotoxin-β receptor is rather a long process, which first needs stabilization of NIK through degradation of the E3 ligase TRAF3 (32). Once accumulated to a certain threshold, this MAPKKK then induces T-loop phosphorylation of IKKα, an effect that ultimately leads to the processing of p100 to p52 (19). In accordance with a lack of a significant effect of Ang II in inducing IKKα phosphorylation following minutes of stimulation (see supplemental Fig. S1A and Fig. 2A), the expression level of TRAF3 was only marginally affected by Ang II treatment for up 1 h of stimulation, which correlated with the incapacity to detect significant expression of endogenous NIK and the processing of p100 (see supplemental Fig. S1, B and C). Therefore, phosphorylation of IKKβ is likely the signaling mechanism by which the AT1R (and other GPCR, see below) is coupled to the rapid induction of the NF-κB pathway.
Early Signaling Events Implicated in T-loop Phosphorylation and Activation of IKKβ
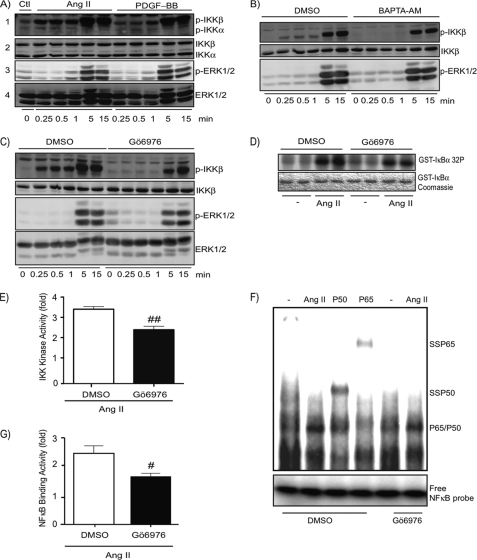
The use of an anti-phospho-IKKα/β (Ser180/Ser181) antibody in Western blot analysis revealed that Ang II induced a rapid (as early as 15 s) and sustained T-loop phosphorylation of IKKβ (Fig. 2A). The phosphosignal of IKKβ further increases after 5 and 15 min of stimulation. PDGF-BB also induced T-loop phosphorylation of IKKβ but only at times where protein kinases ERK1 and ERK2 were phosphorylated.
FIGURE 2.
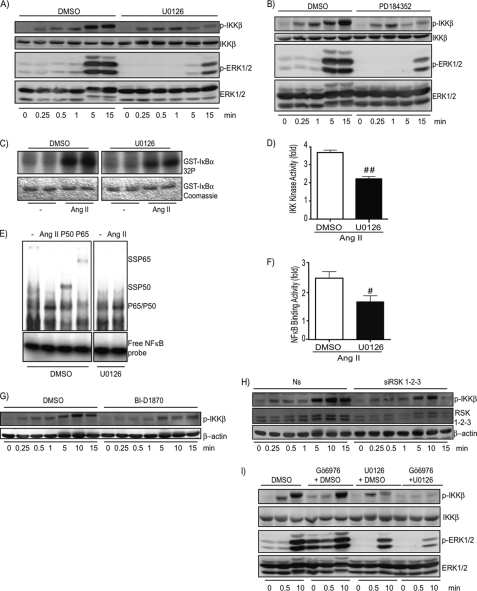
Early signaling events implicated in T-loop phosphorylation and activation of IKKβ in response to Ang II. A, quiescent VSMCs were treated with Ang II (100 nm) or PDGF-BB (50 ng/ml) for the indicated times. Cell extracts were prepared and subjected to immunoblotting analysis using the indicated antibodies. Data are representative of at least 4 different experiments. Cellular extracts were used either on 7.5 (panels 2 and 4) or 10% (panels 1 and 3) polyacrylamide gels. B and C, quiescent VSMCs were pre-treated with BAPTA-AM (15 μm), Gö6976 (10 μm), or DMSO (0.1%) for 30 min before Ang II (100 nm) treatment and used as described above. One of four independent experiments with similar results is shown. D, quiescent VSMCs were pre-treated with Gö6976 (10 μm) or DMSO (0.1%) for 30 min before Ang II (100 nm) exposure for 15 min. Cell lysates were prepared and analyzed for IKK activity using an in vitro kinase assay. One of three independent experiments with similar results is shown. E, densitometric analysis of IKK phosphotransferase activity presented in D. Data are mean ± S.E. of the three pooled experiments. ##, significantly below the DMSO response; p < 0.01. F, quiescent VSMCs were pre-treated with Gö6976 (10 μm) or DMSO (0.1%) for 30 min before Ang II (100 nm) exposure for 15 min. Nuclear extracts were prepared and subjected to EMSA using NF-κB-specific oligonucleotides as a probe. P50 and P65 represent the use of antibodies to supershift (SS) the inducible DNA binding complex composed of p65 and p50 subunits. One of three independent experiments with similar results is shown. G, densitometric analysis of NF-κB binding activity presented in F. Data are mean ± S.E. of the three pooled experiments. #, significantly below the DMSO response; p < 0.05.
We decided to use this assay to decipher the signaling cascades responsible for phosphorylation and activation of IKKβ. Ang II treatment of VSMC resulted in a very rapid phosphorylation of IKKβ, an effect likely mediated by the generation of second messengers such as calcium and diacylglycerol. Thus, we next verified the role of diacylglycerol/calcium-dependent PKCs (known as conventional PKCs) in IKKβ and NF-κB activation. Pretreatment of VSMC with the calcium chelator BAPTA-AM and the conventional PKC inhibitor Gö6976 abrogated the early signaling events leading to IKKβ phosphorylation (Fig. 2, B and C). The same results were observed for other agonists (thrombin and bradykinin) that bind GPCRs coupled to the Gαq-phospholipase Cβ pathway (data not shown). Importantly, the decrease in IKKβ phosphorylation caused by these treatments correlated with a significant reduction in the ability of Ang II to increase the phosphotransferase activity of IKKβ as well as the DNA binding activity of NF-κB dimers composed of p65 and p50 (see Fig. 2, D–G). Together, these results demonstrate the involvement of a PKC-dependent pathway controlling early phosphorylation of IKKβ, which is a modification required for full activation of the IKK complex pathway following AT1R engagement.
A Role of TRAF6-TAK1 Pathway in T-loop Phosphorylation of IKKβ by Ang II
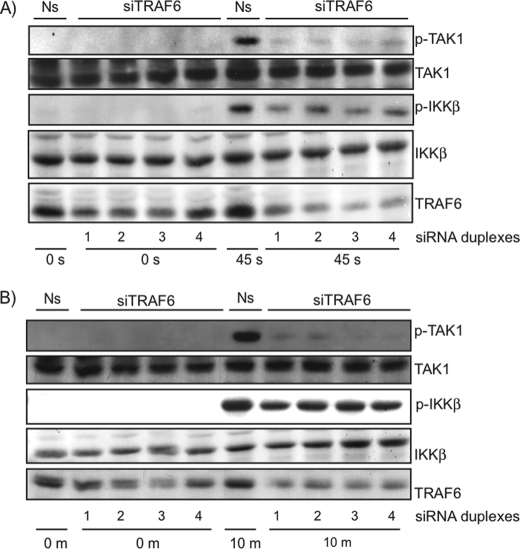
Direct phosphorylation of IKKβ by the atypical family member PKCζ has been demonstrated (33). To our knowledge, direct phosphorylation of the IKK complex by conventional PKCs has never been described. Rather, PKC isoforms play important roles in cytokine and antigen signaling, leading to IKK complex activation through phosphorylation of TRAF2 (34) and scaffolding proteins CARMA1 and CARMA3 (35). Importantly, CARMA3 was recently demonstrated to play important roles in GPCR signaling to the NF-κB pathway (20, 26). Phosphorylated CARMA3 is thought to recruit Bcl10 and MALT1 to form the CBM signalosome. This complex then activates the IKK complex through an ubiquitination-dependent pathway involving the E3 ubiquitin ligases MALT1 or TRAF6 (29, 36). Because it is still unknown which E3 ubiquitin ligases are used by the AT1R to activate the IKK complex, we next verified the role of TRAF6 in Ang II-induced IKKβ T-loop phosphorylation. Lowering the expression level of TRAF6 with four different siRNA duplexes resulted in a dramatic reduction of phosphorylated IKKβ in the early time point of stimulation with Ang II (Fig. 3A). It also affected IKKβ phosphorylation after 10 min of stimulation but to a lesser extent (Fig. 3B). These data demonstrate the importance of the early signaling events converging to the activation of TRAF6, which is required for phosphorylation of IKKβ. They also suggest the role of another intracellular pathway independent of TRAF6 in later time points.
FIGURE 3.
Role of TRAF6 in T-loop phosphorylation of IKKβ and TAK1 in VSMC. A and B, VSMC were transfected with a nonsilencing (Ns) RNA duplex or 4 different RNA duplexes that specifically target TRAF6. At 48 h post-transfection, cells were serum starved for 24 h and then exposed to Ang II (100 nm) for the indicated times. Cell extracts were prepared and subjected to immunoblotting analysis using the indicated antibodies. One of four independent experiments with similar results is shown.
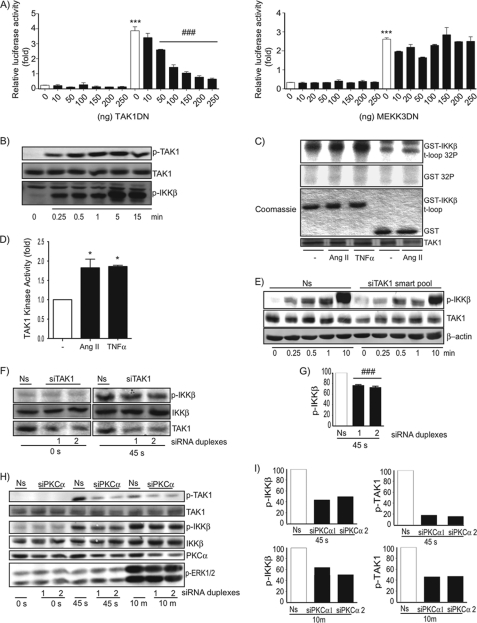
Receptor-dependent recruitments and oligomerization of TRAF6 is proposed to increase the latter's polyubiquitinating activity, which results in the recruitment and activation of two MAPKKK family members, TAK1 and MEKK3 (17, 19). Thus, we next verified the roles of these two proteins kinases in AT1R signaling to the IKK complex. Increasing amounts of DNA plasmid encoding for a dominant-negative version of TAK1, but not MEKK3, significantly inhibited Ang II-induced NF-κB promoter activation in 293 cells expressing the AT1R (Fig. 4A). We therefore addressed whether stimulation of the AT1R was coupled to the activation of TAK1 in VSMC. Phosphoblot analysis demonstrated that Ang II-induced phosphorylation of TAK1 and IKKβ followed an identical kinetic pattern (Fig. 4B). Importantly, the Ang II-dependent TAK1 phosphosignal observed in Western blot analysis was totally dependent on the presence of TRAF6 (Fig. 3). Moreover, it correlated with an increase in TAK1 phosphotransferase activity toward a recombinant GST-IKKβ peptide containing the activating T-loop sequence (Fig. 4, C and D). Importantly, as observed for TRAF6, the efficacy of silencing the expression level of TAK1 with a mixture of four different siRNA duplexes (smartpool) (Fig. 4E) or distinct siRNA duplexes (Fig. 4, F and G) correlated with a significant diminution in the early phosphorylation of IKKβ, an effect that also affected the full IKKβ phosphorylation observed after 10 min of stimulation (Fig. 4E). Thus, a TRAF6-TAK1 signaling cascade is involved in Ang II-induced IKKβ phosphorylation and activation.
FIGURE 4.
Implication of a PKCα-TRAF6-TAK1 signaling cascade in Ang II-induced T-loop phosphorylation and activation of IKKβ in VSMCs. A, QbI-HEK 293A cells were transiently transfected with the NF-κB reporter plasmid PRDII-Luc together with plasmids encoding for AT1R and the indicated amount of TAK1 DN (left panel), MEKK3 DN (right panel), or an empty vector. Cells were serum starved for 24 h and then exposed to Ang II (1 μm) for 18 h before cell lysates were prepared for luciferase assays. Data are mean ± S.E. (n = 3). *, significantly above conditions without Ang II treatment; #, significantly below the Ang II response; p < 0.001. B, quiescent VSMCs were treated with Ang II (100 nm) for the indicated times. Cell extracts were prepared and subjected to immunoblotting analysis using the indicated antibodies. One of three independent experiments with similar results is shown. C, quiescent VSMCs were treated with Ang II (100 nm) or TNFα (20 ng/ml) for 15 min. Cell lysates were prepared and analyzed for TAK1 activity using an in vitro kinase assay. One of two independent experiments with similar results is shown. D, densitometric analysis of TAK1 phosphotransferase activity presented in C. Data are mean ± S.D. of the two pooled experiments. *, significantly above conditions without Ang II treatment; p < 0.05. E and F, VSMCs were transfected with a nonsilencing (Ns) RNA duplex or a mixture of silencing RNA duplexes (E) or two specific silencing RNA duplexes (F) that target TAK1. At 48 h post-transfection, cells were serum starved for 24 h and then exposed to Ang II (100 nm) for the indicated times. Cell extracts were prepared and subjected to immunoblotting analysis using the indicated antibodies. One of three independent experiments with similar results is shown. G, densitometric analysis of the data presented in F. Data are mean ± S.E. of the three pooled experiments. ###, significantly below the Ns; p < 0.001. H, VSMCs were transfected with a nonsilencing RNA duplex or different silencing RNA duplexes that specifically target PKCα. At 48 h post-transfection, cells were serum starved for 24 h and then exposed to Ang II (100 nm) for the indicated times. Cell extracts were prepared and subjected to immunoblotting analysis using indicated antibodies. One of three independent experiments with similar results is shown. I, densitometric analysis of the data presented in H.
The mechanistic details of CBM signalosome formation and the specific PKC isoforms that link the AT1R to CBM remain to be identified (26). Because of the effect of the conventional PKC inhibitor on IKKβ phosphorylation (Fig. 2), we then decided to verify the role of the PKCα isoform in the early TRAF6-TAK1 signaling event. Reducing the expression level of PKCα with two different siRNA duplexes significantly diminished Ang II-induced TAK1 phosphorylation in primary VSMC (Fig. 4, H and I). Importantly, this approach also affected the phosphorylation of IKKβ, indicating that PKCα acts upstream of TAK1, likely at the level of CARMA3 (35).
A MEK1/2-ERK-RSK Pathway Is Involved in Late Signaling Events Leading to Phosphorylation and Activation of IKKβ by Ang II
We observed that delayed phosphorylation of IKKβ occurred when ERK1/2 were activated (starting at 5 min of stimulation with Ang II; Fig. 2, A–C). To verify whether the MEK1/2-ERK1/2 pathway could be involved in IKKβ T-loop phosphorylation and activation, two unrelated allosteric MEK1/2 inhibitors, UO126 and PD184352, were used to efficiently block the phosphorylation of ERK1/2 in response to Ang II (Fig. 5, A and B). Interestingly, only the late phase of Ang II-induced IKKβ phosphorylation (5–15 min of stimulation) was affected by these treatments. The same results were observed with the use of thrombin as another GPCR ligand coupled to the Gαq pathway (data not shown). The use of UO126 also affected the capacity of Ang II to fully activate the phosphotransferase activity of the IKK complex and the DNA binding activity of NF-κB (Fig. 5, C–F). Because RSK is a well defined target of ERK1/2 protein kinases, we next addressed its role in the late phase of IKKβ phosphorylation. As observed for the MEK1/2 inhibitors, the use of BI-D1870, a novel RSK1–4 inhibitor (37), significantly affected the late phase of IKKβ phosphorylation (Fig. 5G). This novel compound inhibitor also had a weak effect on the early phase possibly due to the fact that it was shown to partially inhibit PKCα in vitro (37). However, the use of siRNA against RSK1, -2, and -3 clearly indicated a role of RSKs isoforms in the late T-loop phosphorylation of IKKβ by Ang II (Fig. 5H). Importantly, both the early phase and late phase of Ang II-induced IKKβ T-loop phosphorylation were completely blunted when the conventional PKC and MEK1/2 inhibitors were used simultaneously (Fig. 5I).
FIGURE 5.
A MEK-ERK-RSK pathway is implicated in late signaling events leading to phosphorylation and activation of IKKβ by Ang II in VSMCs. A and B, quiescent VSMCs were pre-treated with U0126 (10 μm), PD184352 (2 μm), or DMSO (0.1%) for 30 min before Ang II (100 nm) treatment. Cell extracts were prepared and subjected to immunoblotting analysis using the indicated antibodies. One of three independent experiments with similar results is shown. C, quiescent VSMCs were pretreated with U0126 (10 μm) or DMSO (0.1%) for 30 min before Ang II (100 nm) exposure for 15 min. Cell lysates were prepared and analyzed for IKK activity using an in vitro kinase assay. One of three independent experiments with similar results is shown. D, densitometric analysis of the IKK phosphotransferase activity presented in C. Data are mean ± S.E. the three pooled experiments. ##, significantly below the DMSO response; p < 0.01. E, quiescent VSMCs were pre-treated with U0126 (10 μm) or DMSO (0.1%) for 30 min before Ang II (100 nm) exposure for 15 min. Nuclear extracts were prepared and subjected to EMSA using NF-κB-specific oligonucleotide as a probe. P50 and P65 represent the use of antibodies to supershift (SS) the inducible DNA binding complex composed of p65 and p50 subunits. One of three independent experiments with similar results is shown. F, densitometric analysis of NF-κB binding activity presented in E. Data are mean ± S.E. the three pooled experiments. #, significantly below the DMSO response; p < 0.05. G, quiescent VSMCs were pre-treated with BI-D1870 (10 μm) or DMSO (0.1%) for 30 min before Ang II (100 nm) treatment. Cell extracts were prepared and subjected to immunoblotting analysis using the indicated antibodies. One of three independent experiments with similar results is shown. H, VSMCs were transfected with a nonsilencing (Ns) RNA duplex or different silencing RNA duplexes that specifically target RSK1, -2, and -3 isoforms. At 48 h post-transfection, cells were serum starved for 24 h and then exposed to Ang II (100 nm) for the indicated times. Cell extracts were prepared and subjected to immunoblotting analysis using the indicated antibodies. One of two independent experiments with similar results is shown. I, quiescent VSMCs were pre-treated with Gö6976 (10 μm) and/or with U0126 (10 μm) and/or with DMSO (0.1%) for 30 min before Ang II (100 nm) treatment. Cell extracts were prepared and subjected to immunoblotting analysis using the indicated antibodies. One of three independent experiments with similar results is shown.
Dual Pathways Control the Inflammatory Response of VSMC Exposed to Ang II
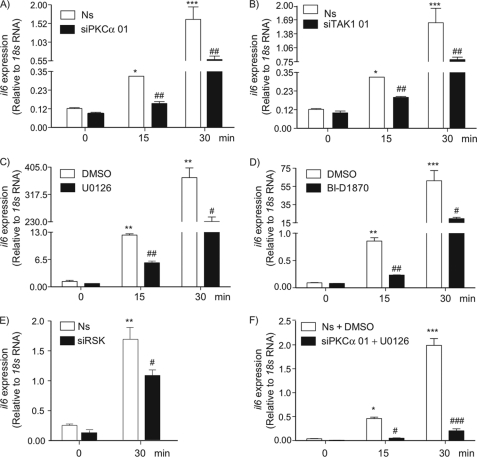
Our data demonstrate that the AT1R is coupled to two independent intracellular pathways leading to T-loop phosphorylation of IKKβ and required for full activation of the IKK complex and the DNA binding activity of the NF-κB dimers. The contributions of inflammatory cytokines, including IL-6, to the pathogenesis of atherosclerosis have been well documented (38–41). Thus, to further substantiate the role of these pathways in the proinflammatory actions of Ang II, we next conducted RT-qPCR analysis to measure il6 mRNA expression in VSMC exposed to pharmacological inhibitors and siRNA duplexes targeting TAK1, PKCα, and RSK isoforms. Reducing the expression level of PKCα and TAK1 significantly diminished the induction of il6 mRNA in response to Ang II (Fig. 6, A and B). This effect was also observed in cells exposed to MEK1/2 and RSK inhibitors as well as siRNA duplexes targeting RSK isoforms (Fig. 6, C–E). Importantly, the proinflammatory effect of the octapeptide was totally blunted by co-treatment with a siRNA duplex targeting PKCα and the UO126 MEK1/2 inhibitor (Fig. 6F).
FIGURE 6.
Involvement of PKCα, TAK1, and the MEK-ERK-RSK pathway in proinflammatory actions of Ang II in VSMCs. A and B, VSMCs were transfected with a nonsilencing (Ns) RNA duplex or different silencing RNA duplexes that specifically target PKCα or TAK1. At 48 h post-transfection, cells were serum starved for 24 h and then exposed to Ang II (100 nm) treatment for the indicated times. C, quiescent VSMCs were pre-treated with U0126 (10 μm) or DMSO (0.1%) for 30 min before Ang II (100 nm) treatment for the indicated times. D, quiescent VSMCs were pre-treated with BI-D1870 (10 μm) or DMSO (0.1%) for 30 min before Ang II (100 nm) treatment for the indicated times. E, VSMCs were transfected with a nonsilencing (Ns) RNA duplex or different silencing RNA duplexes that specifically target RSK1, -2, and -3 isoforms. At 48 h post-transfection, cells were serum starved for 24 h and then exposed to Ang II (100 nm) treatment for the indicated times. F, VSMCs were transfected with a nonsilencing RNA duplex or RNA duplex that specifically targets PKCα. At 48 h post-transfection, cells were serum starved for 24 h and then exposed to U0126 (10 μm) or DMSO (0.1%) for 30 min before Ang II (100 nm) treatment for the indicated times. RNA was extracted and submitted to a RT-PCR using primers for il6 and 18S as an internal control. Data are mean ± S.D. (n = 3). *, significantly above conditions without Ang II treatment; #, significantly below the Ang II response; a single symbol indicates p < 0.05, two symbols indicate p < 0.01, and three symbols indicate p < 0.001.
DISCUSSION
When providing protection against infection, it is generally thought that a controlled inflammatory response is beneficial for the host. However, fundamental research has compiled evidence that inflammation can become detrimental if deregulated. The best example of this deregulation is observed in septic shock, which occurs after a non-controlled acute inflammatory response to infection. Much less is known, however, about the causes and molecular mechanisms of chronic inflammation, which is now recognized as an underlying factor in the development and progression of many chronic diseases such as cancer, type II diabetes, autoimmune disorders, and cardiovascular diseases. Therefore, the delineation of the molecular mechanisms and, particularly, the signaling pathways involved in the regulation of genes encoding cytokines and chemokines is of high priority for identifying new potential therapeutic strategies to combat these diseases. Through the induction of a repertoire of NF-κB-regulated genes such as IL-6, MCP-1, IL-8, RANTES, VCAM-1, and ICAM-1, it is now recognized that Ang II participates in these key events of the inflammatory response leading to the development of atherosclerosis (42). Whereas the pathways leading to NF-κB activation following treatment with prototypical activators such as TNF-α, lipopolysaccharide, IL-1β, and antigen receptors is well characterized (17), the molecular understanding of the signaling pathways that are involved in the coupling of GPCR, such as AT1R to NF-κB, is still unclear. Previous reports have suggested that the proinflammatory activity of Ang II relied on the activation of the noncanonical NF-κB pathway (30, 31). However, this pathway is unlikely to be responsible for the early NF-κB signature that takes place in VSMC exposed to Ang II because the expression level of NIK is almost undetectable in quiescent VSMC exposed to Ang II for up to 1 h. On the other hand, numerous studies have demonstrated the role of conventional PKCs in Ang II-induced NF-κB activation (43–45). Studies on antigen receptor signaling have also highlighted the role of PKC in this process. Following T and B cell receptor engagement, the novel isoform PKCθ (in T cells) and the conventional isoform PKCβ (in B cells) phosphorylate the scaffold protein CARMA1, which in turn recruits Bcl10 and MALT1 to form the CBM complex. This leads to the activation of MALT1 and TRAF6 ubiquitin E3 ligases and to IKK complex activation (46, 47). As opposed to CARMA1, whose expression is largely restricted to lymphocytes, CARMA3 was recently demonstrated to be highly expressed in Ang II-sensitive tissues and involved in NF-κB activation by Ang II (26). However, it was still unknown how this complex regulates the activation of the IKK complex following Ang II stimulation. Through a series of biochemical, molecular, and genetic approaches, our work identifies two novel effectors in Ang II signaling: the E3 ubiquitin ligase TRAF6 and the serine-threonine protein kinase TAK1. Moreover, we demonstrate a novel role of the MEK1/2-ERK-RSK pathway in coupling the AT1R to the activation of the IKK complex. We propose that an early PKCα-CBM-TRAF6-TAK1 pathway is responsible for the second messenger-dependent phosphorylation and activation of IKKβ. This early phosphorylation of IKKβ by TAK1 is then followed by a second wave of IKKβ phosphorylation that requires the MEK1/2-ERK1/2-RSK pathway (see Fig. 7 for a detailed mechanism). Several observations support this proposition. First, pharmacological inhibition of conventional PKCs and intracellular calcium chelation totally blunt the early T-loop phosphorylation of IKKβ and affected full activation of the IKK complex (as observed by the reduced phosphosignal, the reduced phosphotransferase activity, and the reduced DNA binding activity (Fig. 2)). Second, our data demonstrate, for the first time, the phosphorylation and activation of TAK1 by Ang II, which closely follows the kinetic pattern of IKKβ phosphorylation (Fig. 4, B and C). Third, silencing the expression of PKCα affected both T-loop phosphorylation of TAK1 and IKKβ (Fig. 4H), demonstrating that PKCα acts upstream of these protein kinases in AT1R signaling. Moreover, silencing the expression of PKCα also affected il6 mRNA induction (Fig. 6A). Fourth, silencing the expression of TRAF6 and TAK1 also diminished: 1) the onset of IKKβ phosphorylation (Figs. 3A and 4, E–G); 2) the sustained phosphorylation of IKKβ observed at 10 min of stimulation (Figs. 3B and 4E); and 3) il6 mRNA induction by Ang II (Fig. 6B). Fifth, delayed phosphorylation of IKKβ requires the MEK1/2-ERK1/2-RSK pathway and is responsible for full activation of the IKK complex and NF-κB activation (Figs. 5, and 6, C–E). Finally, targeting both the PCKα-TRAF6-TAK1 and the MEK1/2-ERK1/2-RSK pathways totally blunts the phosphorylation of IKKβ (Fig. 5I) and the proinflammatory actions of Ang II (Fig. 6F). Another GPCR ligand, lysophosphatidic acid (LPA), was recently shown to also use a CARMA3-TRAF6 pathway to activate the IKK complex (20). However, in opposition to our results, the authors propose a model in which TRAF6 mediates LPA-induced NF-κB activation through an IKKβ phosphorylation-independent mechanism. Thus, in LPA signaling, TRAF6 would be involved in the polyubiquitination of IKKγ-associated proteins without controlling the activation of a putative IKK-activating kinase such as TAK1. In addition, MEKK3, instead of TAK1, acts as the IKK-activating kinase in their proposed model (48). These discrepancies between LPA and Ang II signaling to the IKK complex are currently unknown but could be related to the use of different cellular models and technologies (mouse embryonic fibroblasts versus primary VSMC, and transient siRNA knockdown versus gene disruption).
FIGURE 7.
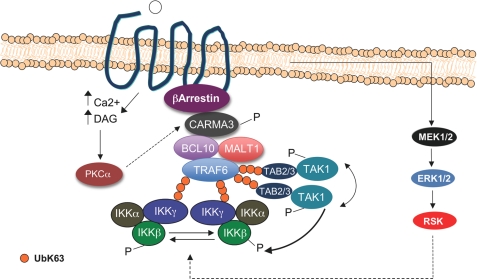
Proposed model for Ang II-mediated IKK activation in VSMC. Following binding to AT1R, PKCα induces the formation of the CBM-TRAF6 complex, which is likely recruited to the AT1R via β-arrestin2 (54). Once recruited to the AT1R, TRAF6 becomes activated, autoubiquitinates, and possibly induces Lys63-linked polyubiquitination of several downstream effectors such as IKKγ and TAK1 adapter proteins TAB2 and TAB3. Lys63-linked polyubiquitination is thought to provide targeted proteins with an ability to engage in new protein interactions with molecules containing ubiquitin binding domains. Because IKKγ and TAB2/3 contain ubiquitin binding domains, Lys63-linked polyubiquitination therefore provides the molecular means to orchestrate the interaction between protein complexes allowing TAK1 and the IKK complex to become activated through transphosphorylation. Moreover, once activated, TAK1 phosphorylates IKKβ to further activate the IKK complex. In addition to the role played by the PKC-CBM-TRAF6 complex, our study also reveals that the optimal activation of the IKK complex is triggered by the MEK1/2-ERK-RSK pathway. DAG, diacylglycerol.
Autoubiquitination of TRAF6 at Lys124 is required for its activity toward TAK1 and IKK (19). Activated TRAF6 is also proposed to induce Lys63-linked polyubiquitination of several downstream effectors such as IKKγ and the TAK1 adapter proteins TAB2 and TAB3 allowing transphosphorylation of the IKK complex and TAK1, respectively (see Fig. 7 for a detailed mechanism). In addition to the polyubiquitination of downstream effectors, it was recently demonstrated in vitro that TAK1 and the IKK complex become activated by free Lys63-linked polyubiquitin chains produced by TRAF6 (21). This new concept of free Lys63-linked polyubiquitin chains acting as second messenger molecules could be as important as diacylglycerol for conventional and novel PKC activation. More work is needed to verify whether the AT1R, and GPCR activation in general, induce the production of free Lys63-linked polyubiquitin chains.
Our data also demonstrate that MEK1/2-ERK1/2 modules serve as activators of the IKK complex by stimulating RSK. Through the phosphorylation of the NF-κB p65 subunit, the RSK family has already been proposed to act as effectors leading to activation of the NF-κB pathway in Ang II-stimulated cells (6). On the other hand, DNA damaging agents use a RSK pathway to increase the phosphotransferase activity of the IKK complex (49). However, the molecular details of IKK activation following activation of ERK1/2-RSK remain elusive because RSK does not directly phosphorylate IKKβ (49). In this scenario, it is possible that ERK-mediated phosphorylation of RSK promotes increased interaction of RSK with the IKK complex. Once in this microenvironment, RSK could target IKKγ, enhancing IKKβ phosphotransferase activity. Work is in progress to verify this hypothesis.
In conclusion, we propose that a portion of AT1R signaling to the IKK complex used similar intracellular pathways as T cell and B cell receptors, i.e. PKC-CMB-TRAF6-TAK1-IKK complex. The extent to which the present mechanism can account for the known proinflammatory actions of Ang II in the cardiovascular system is unknown at the moment. Whereas the therapeutic potential for PKC in atherosclerosis has recently been discussed (50), it is worth mentioning that TRAF6 is also now considered an important effector of vascular remodeling-associated diseases such as atherosclerosis and restenosis (51–53). The demonstration that the ERK-RSK pathway is also required for full IKK complex and NF-κB transcription factor activation by Ang II was unexpected but could have important implications for future effective treatment. Indeed, inhibition of the MEK1/2 pathway could diminish both the growth (8) and proinflammatory actions of Ang II (this study), two cellular processes involved in tissue remodeling events.
Supplementary Material
This work was supported in part by Canadian Institutes of Health Research Grant MOP-53282 and the Heart and Stroke Foundation of Canada (to M. J. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- Ang II
- angiotensin II
- AT1R
- Ang II AT1 receptor
- RAS
- renin angiotensin system
- VSMC
- vascular smooth muscle cells
- IKK
- IκB kinase
- RSK
- ribosomal S6 kinase
- TAK1
- tumor growth factor β activating kinase-1
- TRAF6
- TNF receptor-associated factor-6
- PLC
- phospholipase C
- NIK
- NF-κB inducing kinase
- LPA
- lysophosphatidic acid
- GPCR
- G protein-coupled-receptors
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- DMSO
- dimethyl sulfoxide
- DN
- dominant-negative.
REFERENCES
- 1.de Gasparo M., Catt K. J., Inagami T., Wright J. W., Unger T. (2000) Pharmacol. Rev. 52, 415–472 [PubMed] [Google Scholar]
- 2.Bader M. (2010) Annu. Rev. Pharmacol. Toxicol. 50, 439–465 [DOI] [PubMed] [Google Scholar]
- 3.Paul M., Poyan Mehr A., Kreutz R. (2006) Physiol. Rev. 86, 747–803 [DOI] [PubMed] [Google Scholar]
- 4.Lee M. A., Böhm M., Paul M., Ganten D. (1993) Circulation 87, IV7–13 [PubMed] [Google Scholar]
- 5.Zhang L., Cheng J., Ma Y., Thomas W., Zhang J., Du J. (2005) Circ. Res. 97, 975–982 [DOI] [PubMed] [Google Scholar]
- 6.Zhang L., Ma Y., Zhang J., Cheng J., Du J. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1148–1153 [DOI] [PubMed] [Google Scholar]
- 7.Servant M. J., Coulombe P., Turgeon B., Meloche S. (2000) J. Cell Biol. 148, 543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Servant M. J., Giasson E., Meloche S. (1996) J. Biol. Chem. 271, 16047–16052 [DOI] [PubMed] [Google Scholar]
- 9.Karin M. (2005) Proc. Am. Thorac. Soc. 2, 386–390; Discussion 394–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karin M., Greten F. R. (2005) Nat. Rev. Immunol. 5, 749–759 [DOI] [PubMed] [Google Scholar]
- 11.Barnes P. J., Karin M. (1997) N. Engl. J. Med. 336, 1066–1071 [DOI] [PubMed] [Google Scholar]
- 12.Monaco C., Paleolog E. (2004) Cardiovasc. Res. 61, 671–682 [DOI] [PubMed] [Google Scholar]
- 13.Karin M., Lawrence T., Nizet V. (2006) Cell 124, 823–835 [DOI] [PubMed] [Google Scholar]
- 14.Monaco C., Andreakos E., Kiriakidis S., Mauri C., Bicknell C., Foxwell B., Cheshire N., Paleolog E., Feldmann M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 5634–5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Winther M. P., Kanters E., Kraal G., Hofker M. H. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 904–914 [DOI] [PubMed] [Google Scholar]
- 16.Gareus R., Kotsaki E., Xanthoulea S., van der Made I., Gijbels M. J., Kardakaris R., Polykratis A., Kollias G., de Winther M. P., Pasparakis M. (2008) Cell Metab. 8, 372–383 [DOI] [PubMed] [Google Scholar]
- 17.Vallabhapurapu S., Karin M. (2009) Annu. Rev. Immunol. 27, 693–733 [DOI] [PubMed] [Google Scholar]
- 18.Douillette A., Bibeau-Poirier A., Gravel S. P., Clément J. F., Chénard V., Moreau P., Servant M. J. (2006) J. Biol. Chem. 281, 13275–13284 [DOI] [PubMed] [Google Scholar]
- 19.Skaug B., Jiang X., Chen Z. J. (2009) Annu. Rev. Biochem. 78, 769–796 [DOI] [PubMed] [Google Scholar]
- 20.Grabiner B. C., Blonska M., Lin P. C., You Y., Wang D., Sun J., Darnay B. G., Dong C., Lin X. (2007) Genes Dev. 21, 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Z. P., Sun L., Chen X., Pineda G., Jiang X., Adhikari A., Zeng W., Chen Z. J. (2009) Nature 461, 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu M., Skaug B., Zeng W., Chen Z. J. (2009) Mol. Cell 36, 302–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delhase M., Hayakawa M., Chen Y., Karin M. (1999) Science 284, 309–313 [DOI] [PubMed] [Google Scholar]
- 24.Hu Y., Baud V., Delhase M., Zhang P., Deerinck T., Ellisman M., Johnson R., Karin M. (1999) Science 284, 316–320 [DOI] [PubMed] [Google Scholar]
- 25.Li Z. W., Chu W., Hu Y., Delhase M., Deerinck T., Ellisman M., Johnson R., Karin M. (1999) J. Exp. Med. 189, 1839–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAllister-Lucas L. M., Ruland J., Siu K., Jin X., Gu S., Kim D. S., Kuffa P., Kohrt D., Mak T. W., Nuñez G., Lucas P. C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 139–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D., You Y., Lin P. C., Xue L., Morris S. W., Zeng H., Wen R., Lin X. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klemm S., Zimmermann S., Peschel C., Mak T. W., Ruland J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun L., Deng L., Ea C. K., Xia Z. P., Chen Z. J. (2004) Mol. Cell 14, 289–301 [DOI] [PubMed] [Google Scholar]
- 30.Choudhary S., Lu M., Cui R., Brasier A. R. (2007) Mol. Endocrinol. 21, 2203–2217 [DOI] [PubMed] [Google Scholar]
- 31.Wu L., Iwai M., Li Z., Li J. M., Mogi M., Horiuchi M. (2006) J. Hypertens. 24, 123–130 [DOI] [PubMed] [Google Scholar]
- 32.Zarnegar B. J., Wang Y., Mahoney D. J., Dempsey P. W., Cheung H. H., He J., Shiba T., Yang X., Yeh W. C., Mak T. W., Korneluk R. G., Cheng G. (2008) Nat. Immunol. 9, 1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lallena M. J., Diaz-Meco M. T., Bren G., Payá C. V., Moscat J. (1999) Mol. Cell. Biol. 19, 2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S., Wang L., Dorf M. E. (2009) Mol. Cell 33, 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wegener E., Krappmann D. (2007) Sci. STKE 2007, pe21. [DOI] [PubMed] [Google Scholar]
- 36.McAllister-Lucas L. M., Lucas P. C. (2008) Nat. Immunol. 9, 231–233 [DOI] [PubMed] [Google Scholar]
- 37.Sapkota G. P., Cummings L., Newell F. S., Armstrong C., Bain J., Frodin M., Grauert M., Hoffmann M., Schnapp G., Steegmaier M., Cohen P., Alessi D. R. (2007) Biochem. J. 401, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seino Y., Ikeda U., Ikeda M., Yamamoto K., Misawa Y., Hasegawa T., Kano S., Shimada K. (1994) Cytokine 6, 87–91 [DOI] [PubMed] [Google Scholar]
- 39.Charo I. F., Taubman M. B. (2004) Circ. Res. 95, 858–866 [DOI] [PubMed] [Google Scholar]
- 40.Brasier A. R. (2010) Cardiovasc. Res. 86, 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Y., Runge M. S., Brasier A. R. (1999) Circ. Res. 84, 695–703 [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y., Ruiz-Ortega M., Lorenzo O., Ruperez M., Esteban V., Egido J. (2003) Int. J. Biochem. Cell Biol. 35, 881–900 [DOI] [PubMed] [Google Scholar]
- 43.Kalra D., Sivasubramanian N., Mann D. L. (2002) Circulation 105, 2198–2205 [DOI] [PubMed] [Google Scholar]
- 44.Jamaluddin M., Meng T., Sun J., Boldogh I., Han Y., Brasier A. R. (2000) Mol. Endocrinol. 14, 99–113 [DOI] [PubMed] [Google Scholar]
- 45.Brasier A. R., Jamaluddin M., Han Y., Patterson C., Runge M. S. (2000) Mol. Cell. Biochem. 212, 155–169 [PubMed] [Google Scholar]
- 46.Shinohara H., Kurosaki T. (2009) Immunol. Rev. 232, 300–318 [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto R., Wang D., Blonska M., Li H., Kobayashi M., Pappu B., Chen Y., Wang D., Lin X. (2005) Immunity 23, 575–585 [DOI] [PubMed] [Google Scholar]
- 48.Sun W., Li H., Yu Y., Fan Y., Grabiner B. C., Mao R., Ge N., Zhang H., Fu S., Lin X., Yang J. (2009) Cell. Signal. 21, 1488–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panta G. R., Kaur S., Cavin L. G., Cortés M. L., Mercurio F., Lothstein L., Sweatman T. W., Israel M., Arsura M. (2004) Mol. Cell. Biol. 24, 1823–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Churchill E., Budas G., Vallentin A., Koyanagi T., Mochly-Rosen D. (2008) Annu. Rev. Pharmacol. Toxicol. 48, 569–599 [DOI] [PubMed] [Google Scholar]
- 51.Lutgens E., Lievens D., Beckers L., Wijnands E., Soehnlein O., Zernecke A., Seijkens T., Engel D., Cleutjens J., Keller A. M., Naik S. H., Boon L., Oufella H. A., Mallat Z., Ahonen C. L., Noelle R. J., de Winther M. P., Daemen M. J., Biessen E. A., Weber C. (2010) J. Exp. Med. 207, 391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zirlik A., Bavendiek U., Libby P., MacFarlane L., Gerdes N., Jagielska J., Ernst S., Aikawa M., Nakano H., Tsitsikov E., Schönbeck U. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1101–1107 [DOI] [PubMed] [Google Scholar]
- 53.Miyahara T., Koyama H., Miyata T., Shigematsu H., Inoue J., Takato T., Nagawa H. (2004) Cardiovasc. Res. 64, 154–164 [DOI] [PubMed] [Google Scholar]
- 54.Sun J., Lin X. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 17085–17090 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.