Abstract
The inhibitory Smads (I-Smads), i.e. Smad6 and Smad7, are negative regulators of transforming growth factor-β (TGF-β) family signaling. I-Smads inhibit TGF-β family signaling principally through physical interaction with type I receptors (activin receptor-like kinases), so as to compete with receptor-regulated Smads (R-Smads) for activation. However, how I-Smads interact with type I receptors is not well understood. In the present study, we found that Smad7 has two modes of interaction with type I receptors. One is through a three-finger-like structure in the MH2 domain, consisting of residues 331–361, 379–387, and the L3 loop. The other is through a basic groove in the MH2 domain (Mochizuki, T., Miyazaki, H., Hara, T., Furuya, T., Imamura, T., Watabe, T., and Miyazono, K. (2004) J. Biol. Chem. 279, 31568–31574). We also found that Smad6 principally utilizes a basic groove in the MH2 domain for interaction with type I receptors. Smad7 thus has an additional mode of interaction with TGF-β family type I receptors not possessed by Smad6, which may play roles in mediating the inhibitory effects unique to Smad7.
Keywords: Bone Morphogenetic Protein (BMP), Receptor Regulation, Receptor Serine/Threonine Kinase, SMAD Transcription Factor, Transforming Growth Factor-β (TGF-β)
Introduction
Cytokines of the transforming growth factor-β (TGF-β) family, including TGF-βs, activins, and bone morphogenetic proteins (BMPs),3 are multifunctional proteins that regulate a wide variety of cellular processes, including growth, differentiation, apoptosis, motility, adhesion, and the production of extracellular matrix, from embryogenesis to adult tissue homeostasis. Misregulation of TGF-β family signaling leads to various developmental disorders and diseases, including fibrosis, autoimmune diseases, and multiple types of cancer. TGF-β family signaling is transduced through type I and type II receptors, both of which have serine/threonine kinase domains in their intracellular regions. Upon binding of ligands, two type II receptors and two type I receptors form heterotetrameric receptor complexes, in which constitutively active type II receptor kinases activate type I receptor kinases. The activated type I receptors then phosphorylate a subset of Smad proteins termed receptor-regulated Smads (R-Smads) at the C-terminal serine residues. Phosphorylated R-Smads then associate with Co-Smad (Smad4) to form activated Smad complexes, translocate into the nucleus, and regulate the transcription of various target genes in cooperation with transcription factors as well as co-activators/co-repressors (1–5).
In mammals, seven type I receptors (termed activin receptor-like kinases 1 through 7; ALK-1–7) have been identified. They can be further divided structurally into three subfamilies: the ALK-3/6 subfamily, ALK-1/2 subfamily, and ALK4/5/7 subfamily (3). The ALK-3/6 subfamily and ALK-1/2 subfamily of type I receptors transmit BMP-type signaling through phosphorylation of Smad1, 5, and 8 (6). On the other hand, the ALK-4/5/7 subfamily transmits TGF-β/activin-type signaling through phosphorylation of Smad2 and 3. The molecular basis of the specific interaction between type I receptors and R-Smads has been elucidated in detail (7–11). The L45 loop of type I receptors and the L3 loop of R-Smads are determinants of the specificity of interaction between type I receptors and R-Smads.
In addition to R-Smads and Co-Smads, another class of Smad proteins, the inhibitory Smads (I-Smads), play crucial roles in negative regulation of TGF-β family signaling (12). I-Smads compete with R-Smads for activation (13–16) and recruit Smurfs and other HECT-type ubiquitin ligases (17–21) as well as the GADD34-PP1c complex (22) to signaling type I receptors, resulting in their down-regulation through ubiquitylation or dephosphorylation. I-Smads thus inhibit TGF-β family signaling principally through physical interaction with type I receptors, although alternative mechanisms have also been reported (12). We recently examined the specificity of I-Smads for each type I receptor (23–25). Smad6 efficiently inhibits signaling from the ALK-3/6 subgroup but only minimally inhibits that from the other subgroups of type I receptors, a finding attributed to the differential affinity of Smad6 for each type of type I receptor (24). In contrast, Smad7 stably interacts with all subfamilies of activated type I receptors and ubiquitously inhibits TGF-β family signaling (23).
It has been suggested that Smad7 interacts with type I receptors of TGF-β and BMP in different fashions (23, 26). The MH2 domains of I-Smads are responsible for inhibition of type I receptors. The N domain of Smad7 physically interacts with the Smad7 MH2 domain and enhances its inhibition of ALK-5, but not inhibition of ALK-6 (23). In addition, it has been shown that mutations of basic residues in the L3 loop (Lys-401 and Arg-409) of the Smad7 MH2 domain attenuate its association with both ALK-5 and ALK-6, whereas mutations in the α-helix1 (Lys-312 and Lys316) attenuate its association with ALK-5, but not with ALK-6 (26). These findings indicate that the mode of interaction with I-Smads and sensitivity to I-Smads vary among the subgroups of type I receptors. However, the molecular basis of the interaction between I-Smads and ALKs has not been clearly elucidated.
In the present study, utilizing the differential effects of Smad6 and Smad7 on ALK-2, we identified the important regions of Smad7 for interaction with type I receptors. The mode of interaction between type I receptors and I-Smads differed from that between R-Smads and type I receptors. We also found that Smad7 has two surfaces for interaction with type I receptors, whereas Smad6 has one. Usage of these two surfaces of Smad7 may affect its function as a negative regulator.
EXPERIMENTAL PROCEDURES
Cell Culture
C2C12, COS7, 293T, and HepG2 cells were obtained from American Type Culture Collection. C2C12 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 20% fetal bovine serum (FBS; Cell Culture Technologies) and antibiotics (50 units/ml streptomycin and 50 units/ml penicillin G; Invitrogen). COS7, 293T, and HepG2 cells were cultured in DMEM supplemented with 10% FBS and antibiotics. For HepG2 cells, nonessential amino acids (Invitrogen) were also supplemented. Cells were grown in a 5% CO2-humidified atmosphere at 37 °C.
Plasmid Constructions
The construction of constitutively active mutants of type I receptors (ALK-2(QD), ALK-3(QD), and ALK-5(TD)), deletion mutants and chimeras of Smad6 and Smad7 (Smad6N, Smad6C, Smad7N, Smad7C, Smad6/7, and Smad7/6), a point mutant of Smad7 (K316E), wild-type BMPR-II, Smad6, Smad7, Smurf1, and ubiquitin were described previously (13, 23, 26, 27). Other chimeras of Smad6 and Smad7 were generated by a PCR-based approach. All of the PCR products were sequenced before use.
Transfection, Cell Lysis, Immunoprecipitation, and Immunoblotting
Cells were transfected using FuGENE6 transfection reagent (Roche Diagnostics) according to the manufacturer's recommendations. Preparation of cell lysates, immunoprecipitation, and immunoblotting were performed as described previously (24). The antibodies used were as follows: anti-FLAG antibody (M2; Sigma), anti-HA antibody (3F10; Roche Diagnostics), anti-Myc antibody (9E10; Pharmingen), anti-phospho-Smad1/5 antibody (Cell Signaling), and anti-α-tubulin antibody (DM1A; Sigma).
Luciferase Reporter Assay
Luciferase reporter assay was performed as described previously (24). A BMP-responsive reporter construct, BRE-Luc (28), an activin-responsive reporter construct, AR3-Lux, and a TGF-β-responsive reporter construct, (CAGA)12-MLP-Luc (14, 29), were used. When AR3-Lux activity was measured, Fox H3 was co-tranfected (25). Equal expression of each mutant was confirmed by immunoblotting.
Homology Modeling
A structural model of Smad7 was constructed based on the three-dimensional structure of Smad2 (Protein Data Bank code 1DEV) using Biopackage (Molsoft).
RESULTS
MH2 Domains Are Responsible for the Differential Inhibitory Effects of I-Smads on Signaling from ALK-2
Smad7 efficiently inhibits BMP signaling from both ALK-2 and ALK-3. In contrast, Smad6 efficiently inhibits BMP signaling from ALK-3 but only weakly inhibits that from ALK-2 (24). By utilizing these differential effects of Smad6 and Smad7 on ALK-2 signaling, we investigated the molecular basis of the Smad7-ALK-2 interaction.
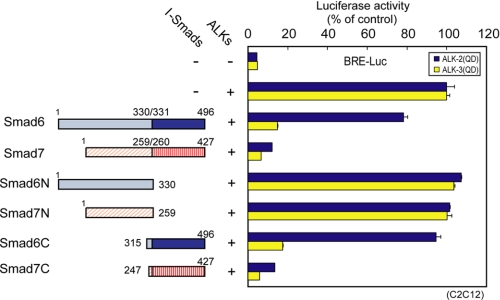
We first examined which parts of I-Smads are responsible for the differential inhibition of signaling from ALK-2, with the use of truncated mutants of Smad6 and Smad7. Smad6 and Smad7 were divided into N-terminal and C-terminal fragments (1–330 and 315–496 for Smad6, 1–259 and 247–427 for Smad7, denoted Smad6N, Smad6C, Smad7N, and Smad7C), and their inhibitory effects on signaling from ALK-2 and ALK-3 were determined using luciferase reporter assay (Fig. 1). The isolated N domains of I-Smads (Smad6N and Smad7N) failed to repress signaling from both ALK-2 and ALK-3. Smad7C inhibited ALK-2 signaling as effectively as wild-type Smad7, whereas Smad6C had less inhibitory effect on ALK-2 signaling, similar to the full-length Smad6. Consistent with these results, Smad7C interacted with ALK-2 more stably than Smad6C, whereas the N domains of both I-Smads failed to interact with ALK-2 and ALK-3 (supplemental Fig. S1A).
FIGURE 1.
MH2 domains are responsible for the differential inhibitory effects of I-Smads on signaling from ALK-2. Smad6 and Smad7 were divided into N-terminal (residues 1–330 and 1–259, respectively) and C-terminal (residues 315–496 and 247–427, respectively) moieties (schematically shown left), and inhibitory effects on ALK-2 and ALK-3 signaling were determined by luciferase reporter assay using BRE-Luc in C2C12 cells (right). Expression of each construct was confirmed by immunoblotting. Results are given as percentages of control. Error bars represent S.D.
The MH2 domains of I-Smads were thus found to be responsible for the differential inhibitory effects on signaling from ALK-2. In contrast, it is known that the N domain of Smad7 is required for inhibition by Smad7 of signaling from ALK-5 (23).
L3 Loops of I-Smads Do Not Determine the Specificity of Interaction with Type I Receptors
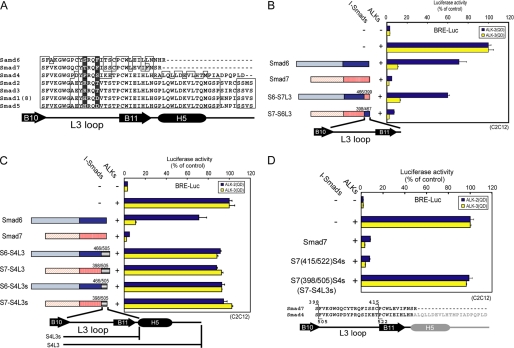
The L3 loops in the vicinity of the C terminus of R-Smads are crucial for the specific interaction of R-Smads with type I receptors (9). Amino acid sequences of the L3 loop are invariant among TGF-β/activin-specific R-Smads (Smad2 and 3) and among BMP-specific R-Smads (Smad1, 5, and 8), respectively (Fig. 2A). Swapping of the L3 loops of Smad1 and 2 results in switching of specificity of their C-terminal phosphorylation by type I receptors (9). We therefore examined whether the L3 loops of I-Smads are responsible for the specificity of interaction with type I receptors. We constructed mutants of Smad6 and 7 in which the regions spanning the L3 loop to the C terminus had been swapped (Fig. 2, A and B, left) and determined their inhibitory effects on ALK-2 and -3 signaling. We found that swapping of the region did not affect inhibition of ALK-2 and ALK-3 signaling (Fig. 2B, right). Binding experiments between I-Smad mutants with ALK-2 and ALK-3 also gave consistent results (supplemental Fig. S1B). We therefore concluded that the L3 loops of I-Smads do not determine the specificity of interaction with type I receptors.
FIGURE 2.
Role of the L3 loop of I-Smads in the interaction with type I receptors. A, alignment of amino acid sequences of the L3 loops in human Smad proteins is shown. Determinants of the specificity of interaction with type I receptors (8) are shadowed. B–D, inhibitory effects of I-Smad chimeric proteins were determined by luciferase reporter assay using BRE-Luc in C2C12 cells (right). Structures of the chimeric proteins are schematically shown at left (B and C) or bottom (D). Expression of each construct was confirmed by immunoblotting. Results are given as percentages of control. Error bars represent S.D. B, regions spanning the L3 loop to the C terminus of Smad6 (residues 467–496) and Smad7 (residues 399–427) were swapped. C, regions spanning the L3 loop to the C terminus of Smad6 (residues 467–496) and Smad7 (residues 399–427) were replaced by the corresponding region of Smad4 (residues 505–552 for S6-S4L3 and S7-S4L3) or the region in which the helix-5 (H5) and the C terminus of Smad4 were truncated (residues 505–531 for S6-S4L3s and S7-S4L3s). D, C-terminal region of Smad7 was progressively swapped with the corresponding region of Smad4.
L3 Loops of I-Smads Are Involved in Interaction with Type I Receptors
Next, to investigate whether the L3 loops of I-Smads are required for their inhibition of BMP signaling, we constructed chimeras of Smad6 or Smad7 with Smad4 because Smad4 is unable to interact with type I receptors. S6-S4L3 and S7-S4L3 are mutants based on Smad6 and 7, respectively, in which the regions spanning the L3 loop to the C terminus are replaced by the corresponding region of Smad4 (residues 505–552 for Smad4). S6-S4L3s and S7-S4L3s are short versions of S6-S4L3 and S7-S4L3, in which helix-5 (H5) and the C terminus of Smad4 (residues 505–531) are truncated (Fig. 2C, left). S6-S4L3, S7-S4L3, S6-S4L3s, and S7-S4L3s all lacked interaction with ALK-2/3 (supplemental Fig. S1B) and failed to suppress signaling from these type I receptors (Fig. 2C, right). Replacement of the L3 loop of S7-S4L3s by that of Smad7 resulted in recovery of inhibition (Fig. 2D) as well as interaction with ALK-2 and ALK-3 (supplemental Fig. S1B). These findings indicate that the L3 loops of I-Smads are required for their inhibitory effects on ALK-2 and ALK-3 through physical interaction. The L3 loops of I-Smads are thus required for but not determinative of the differential effects of Smad6 and Smad7 on ALK-2 signaling, suggesting that other region(s) in I-Smads play crucial roles in determining the specificity of interaction with BMP type I receptors.
Identification of the Regions in Smad7 Responsible for Specific Inhibition of BMP Signaling from ALK-2
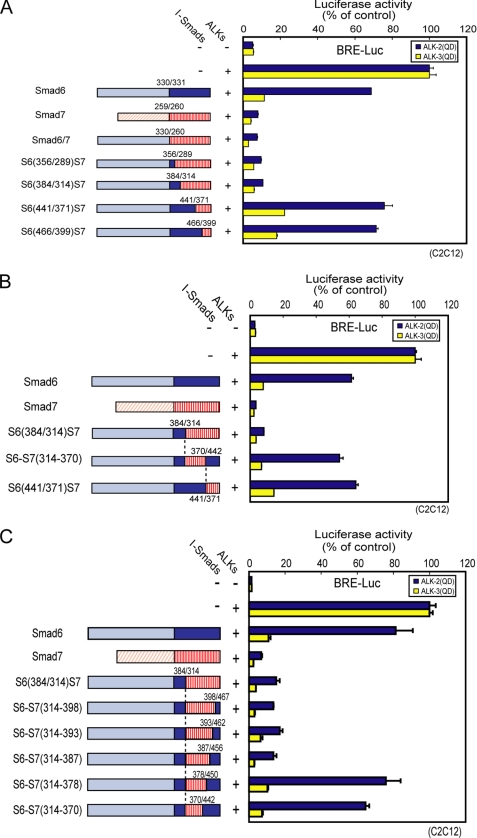
To identify which region(s) of Smad7 are determinants of the inhibitory effect on ALK-2 signaling, we constructed several Smad6/Smad7 chimeras and examined their inhibitory effects on BMP signaling from ALK-2 and -3 (Fig. 3). The chimeric protein Smad6/7 has the N domain from Smad6 (1–330) and the MH2 domain from Smad7 (260–427). The regions in the MH2 domain derived from Smad7 were then gradually replaced by the corresponding regions of Smad6 (Fig. 3A, left). Smad6/7, S6(356/289)S7, and S6(384/314)S7 strongly inhibited signaling from both ALK-2 and ALK-3. On the other hand, S6(441/371)S7 and S6(466/399)S7 efficiently inhibited signaling from ALK-3 but only weakly inhibited that from ALK-2 (Fig. 3A). These findings indicate that residues 314–370 of Smad7 play an important role in efficient inhibition of ALK-2 signaling. We next constructed a Smad6 mutant that contained residues 314–370 of Smad7 in place of the corresponding region of Smad6 (denoted S6-S7(314–370), Fig. 3B, left). S6-S7(314–370), however, failed to inhibit ALK-2 signaling (Fig. 3B, right). The inhibitory effects on ALK-2 thus also require region(s) downstream of residue 371 of Smad7.
FIGURE 3.
Mapping of the regions in Smad7 required for inhibition of ALK-2. Inhibitory effects of Smad6/Smad7 chimeric proteins were determined by luciferase reporter assay using BRE-Luc in C2C12 cells (right). Structures of the chimeric proteins are schematically shown at left. Expression of each construct was confirmed by immunoblotting. Results are given as percentages of control. Error bars represent S.D. A, MH2 domain of Smad6 was gradually substituted by the corresponding regions of Smad7. B, residues 314–370 of Smad7 were introduced into Smad6 in place of the corresponding region of Smad6. C, Smad7 sequences of S6(384/314)S7 were gradually substituted by the corresponding regions of Smad6 from the C terminus.
We next attempted to specify the regions crucial for inhibition of ALK-2 signaling. First, we constructed four more Smad6/Smad7 chimeras that were intermediates between S6(384/314)S7 and S6(441/371)S7 (supplemental Fig. S2, left). The inhibitory effects on ALK-2 signaling became less potent with gradual substitution of residues 331–361 of Smad7 with corresponding residues of Smad6 (supplemental Fig. S2, right). Thus, the structure of the entire region comprising residues 331–361 of Smad7 appears to be important for the inhibition of ALK-2.
We also gradually replaced Smad7 sequences of S6(384/314)S7 with corresponding regions of Smad6 from the C terminus (Fig. 3C, left). Replacement of residues 379–387 of Smad7 with the corresponding region of Smad6 altered the inhibitory effect on BMP signaling from Smad7-type to Smad6-type (Fig. 3C, right). These findings indicate that residues 379–387 of Smad7 are involved in the inhibition of ALK-2. We further examined physical interaction of all of the chimeric mutants used in Fig. 3, A–C, with ALK-2 and ALK-3, which was well consistent with their inhibitory effects (supplemental Fig. S1, C and D).
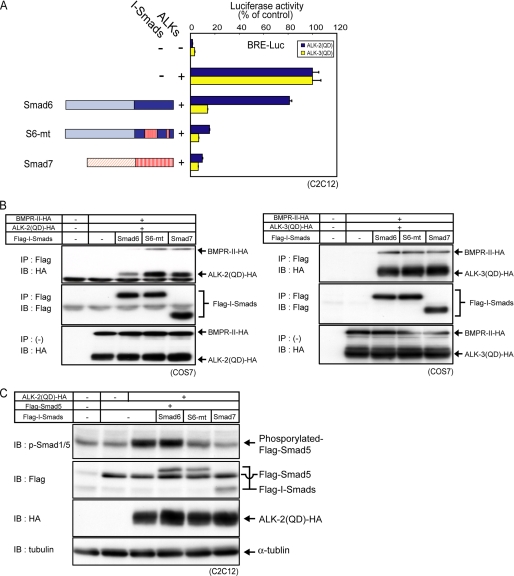
Finally, we constructed a Smad6 mutant that contained residues 331–361 and 379–387 of Smad7 (denoted S6-mt) and confirmed that these regions were sufficient for inhibition of signaling from ALK-2, as determined by luciferase reporter assay, physical interaction with ALK-2, and R-Smad phosphorylation (Fig. 4, A–C).
FIGURE 4.
A Smad6 mutant containing residues 331–361/379–387 of Smad7 (S6-mt) inhibits ALK-2 signaling. A, inhibitory effects of I-Smads and the mutant on ALK-2 and ALK-3 signaling were determined by luciferase reporter assay using BRE-Luc in C2C12 cells. Expression of each construct was confirmed by immunoblotting. Results are given as percentages of control. Error bars represent S.D. B, physical interaction of I-Smads and the mutant with ALK-2 and ALK-3 is shown. COS7 cells were transfected with the indicated plasmids. I-Smads were immunoprecipitated (IP), and co-precipitated ALK-2, or ALK-3 was visualized by immunoblotting (IB; top panel). Precipitated I-Smads (middle panel) and expression of receptors (bottom panel) are also shown. C, effects of I-Smads and the mutant on phosphorylation of Smad5 in transfected C2C12 cells stimulated with constitutively active ALK-2 are shown. Phosphorylation of exogenous Smad5 was visualized by immunoblotting using anti-phospho-Smad1/5.
Smad7 Interacts with TGF-β Family Type I Receptors through Two Distinct Regions
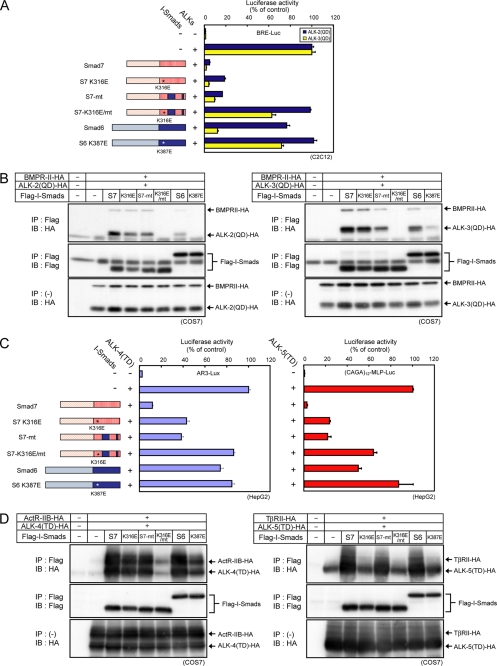
We next constructed a Smad7 mutant, in which residues involved in the interaction with ALK-2 were replaced by corresponding Smad6 residues (denoted S7-mt) (Fig. 5A, left). Contrary to our expectation, S7-mt inhibited ALK-2 signaling as well as wild-type Smad7 and interacted with ALK-2 (Fig. 5, A and B), indicating that there are other site(s) involved in recognition of BMP type I receptors by Smad7. It has been reported that a basic groove composed of four basic residues (Lys-312, Lys-316, Lys-401, and Arg-409) in the MH2 domain of Smad7 plays critical roles in the interaction of Smad7 with ALK-5 (26). We therefore examined whether the basic groove is also involved in the interaction with ALK-2 and ALK-3. Although mutating one of the four basic residues, Lys-316, of wild-type Smad7 to a glutamic acid did not affect its inhibitory activity, the same mutation in S7-mt (denoted S7-K316E/mt) resulted in attenuation of the inhibitory activity and the interaction with ALK-2/3 (Fig. 5, A and B). These findings indicate that Smad7 interacts with BMP type I receptors through the basic groove, independent of the region identified above. In contrast, introduction of the corresponding mutation into Smad6 (K387E) resulted in loss of inhibition by Smad6 of signaling by ALK-3 (Fig. 5A) and interaction with ALK-3 (Fig. 5B).
FIGURE 5.
Smad7 utilizes two distinct regions for inhibition of TGF-β family type I receptors. The regions in Smad7 responsible for efficient inhibition of ALK-2 signaling (residues 331–361 and 379–387) were replaced by the corresponding regions of Smad6 (S7-mt), and a lysine in the basic groove (Lys-316 for Smad7 and S7-mt, or Lys-387 for Smad6) was converted to glutamic acid (schematically shown at left). A, inhibitory effects of I-Smad mutants on ALK-2 and ALK-3 signaling were determined by luciferase reporter assay using BRE-Luc in C2C12 cells. Expression of each construct was confirmed by immunoblotting. B, physical interaction of I-Smads with ALK-2 and ALK-3 is shown. COS7 cells were transfected with the indicated plasmids. I-Smads were immunoprecipitated (IP), and co-precipitated each type I receptor was visualized by immunoblotting (IB; top panel). Precipitated I-Smads (middle panel) and expression of type I receptors (bottom panel) are also shown. C, inhibitory effects of mutant I-Smads on ALK-4 and ALK-5 signaling were determined by luciferase reporter assay using AR3-Lux or (CAGA)12-MLP-Luc respectively, in HepG2 cells. Expression of each construct was confirmed by immunoblotting. Results are given as percentages of control. Error bars represent S.D. D, physical interaction of I-Smads with ALK-4 and ALK-5 is shown. COS7 cells were transfected with the indicated plasmids. I-Smads were immunoprecipitated, and co-precipitated each type I receptor was visualized by immunoblotting (top panel). Precipitated I-Smads (middle panel) and expression of type I receptors (bottom panel) are also shown.
We next examined inhibitory effects of the same set of Smad7 mutants on signaling from ALK-4 and ALK-5 (Fig. 5C) and their interaction with these receptors (Fig. 5D). We obtained essentially the same results for ALK-4. However, we obtained inconsistent results for ALK-5. S7-K316E failed to interact with ALK-5, indicating that Smad7 interacts with ALK-5 principally thorough the basic groove as previously reported (26). Unexpectedly, S7-K316E still retained an inhibitory effect on signaling from ALK-5, which may be due to regulation of ALK-5 signaling by Smad7 at other levels (30). We also examined effects of Smad6 and its mutant Smad6-K387E on signaling from ALK-4 and ALK-5 and their interaction with these receptors. Smad6 interacted with ALK-4 and ALK-5 through the basic groove (Fig. 5D) but inhibited signaling only marginally (ALK-4) or moderately (ALK-5) (Fig. 5C). The underlying mechanism for these inert interactions remains to be elucidated.
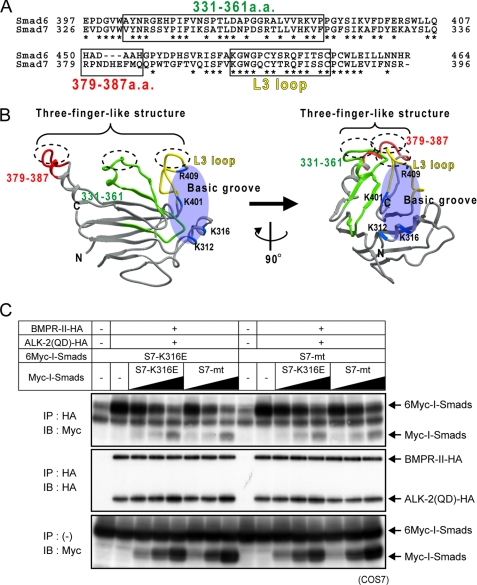
Mapping of the Important Regions for Interaction with ALKs on a Three-dimensional Structural Model of Smad7
As described above, we have shown that multiple regions including the L3 loop and residues 331–361 and 379–387 of Smad7 are responsible for interaction with ALKs. We aligned the amino acid sequences of Smad6 and Smad7 containing these regions (Fig. 6A). The amino acid sequences in residues 331–361 of Smad7 are conserved in Smad6 to the same extent as in other regions (53.3%). Residues 379–387 of Smad7 are not conserved in Smad6. We next mapped the regions required for efficient interaction with ALKs on a structural model of the Smad7 MH2 domain, which was constructed by homology modeling based on the MH2 domain of Smad2 (Protein Data Bank Code 1DEV) (Fig. 6B). The L3 loop, residues 379–387, and certain amino acids among residues 331–361 are present on a common surface of the three-dimensional structure of Smad7, comprising a three-finger-like structure, whereas the basic groove is located on a different side of the molecule. These findings suggest that, when Smad7 interacts with BMP type I receptors, two distinct modes of interaction exist, one through the three-finger-like structure and the other through the basic groove.
FIGURE 6.
Two distinct surfaces on Smad7 in interaction with type I receptors. A, alignment of amino acid sequences of Smad6 and Smad7. Residues involved in ALK-2 interaction are boxed, and identical residues in Smad6 and 7 are shown with asterisks. B, three-dimensional structural model of the MH2 domain of Smad7 based on the three-dimensional structure of Smad2 (PDB code 1DEV). Residues 331–361 are shown in green; residues 379–387, red; the L3 loop (residues 401–414), yellow; and the basic residues in the basic groove (Lys-312, Lys-316, Lys-401, and Arg-409), blue. C, competition of I-Smad mutants for interaction with ALK-2. COS7 cells were transfected with HA-tagged ALK-2, BMPRII, and Myc- or 6Myc-tagged I-Smad mutants. Receptors were immunoprecipitated (IP), and co-precipitated I-Smad mutants were visualized by immunoblotting (IB; top panel). Precipitated receptors (middle panel) and expression of I-Smad mutants (bottom panel) are also shown.
Two Molecular Surfaces Have Similar Binding Affinities for Type I Receptors
We next compared the relative affinity of the two binding surfaces on Smad7 for ALK-2. As shown in Fig. 6C, increasing amount of S7-K316E and S7-mt competed with each other in binding to ALK-2, indicating that the two molecular surfaces have similar binding affinities for ALK-2.
Smurf1 ubiquitin ligase induces ubiquitin-dependent degradation of TGF-β family type I receptors in cooperation with I-Smads (18, 19). We therefore examined the cooperativity of Smad7 and its mutants (S7-K316E and S7-mt) with Smurf1. However, we found that S7-K316E and S7-mt equally enhanced Smurf1-dependent ALK-2 ubiquitylation (data not shown).
DISCUSSION
Ligands of the TGF-β family signal through type I receptor-mediated activation of R-Smads. Interaction between type I receptors and R-Smads is coordinated by robust molecular recognition. I-Smads are members of the Smad protein family which act as antagonists of Smad signaling principally through physical interaction with type I receptors to interrupt activation of R-Smads (13–16). However, the mode of interaction between I-Smads and type I receptors has remained unclear. In the present study, we found that Smad7 inhibits TGF-β family type I receptors through two distinct modes of interaction.
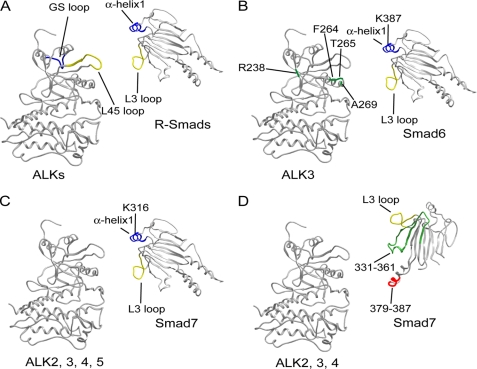
Recognition of R-Smads by activated type I receptors appears to involve two matching components (Fig. 7A) (11). The interaction between the L3 loop of R-Smads and the L45 loop of the type I receptor determines substrate specificity (7–10). The interaction between the basic groove and the phosphorylated GS loop appears to ensure recognition of R-Smads by activated receptors (11). The basic groove comprises two residues in the L3 loop and two residues in α-helix1 (26).
FIGURE 7.
Models of I-Smad-receptor interaction. A, model of R-Smad-receptor interaction. The L3 loop of R-Smad and the L45 loop of receptor are shown in yellow, and the α-helix1 of R-Smad and GS loop is in blue. B, model of Smad6-ALK-3 interaction. The L3 loop of Smad6 is shown in yellow; α-helix1, blue; and the four residues of ALK-3 responsible for interaction with Smad6 (Arg-238, Phe-264, Thr-265, and Ala-269) (23), green. C and D, two modes of Smad7-receptor interaction. Residues 331–361 are shown in green; residues 379–387, red; the L3 loop (residues 401–414), yellow; and the α-helix1, blue. All three-dimensional receptor structures in A–D are based on ALK-5 (PDB code 1B6C); R-Smad in A is based on Smad2 (PDB code 1DEV); I-Smads in B–D are as constructed in Fig. 6B.
In the interaction of I-Smads with type I receptors, the L3 loops were found to be required for but not determinative of the specificity of interaction with these receptors. Smad6 and Smad7 also have a basic groove consisting of the L3 loop and the α-helix1. Mutation of a basic residue within the α-helix1 of Smad6, but not Smad7, decreased the magnitude of inhibition of ALK-3 signaling and association with ALK-3. These findings indicate that Smad6 principally utilizes the basic groove in its functional interaction with ALK-3. In ALK-3, the region along the nucleotide-binding loop and the αC helix, which is located near the L45 loop but apart from the GS loop, is a determinant of interaction with Smad6 (24) (Fig. 7B). The mode of interaction of Smad6 with ALK-3 thus differs from that of R-Smads with type I receptors.
Smad7 utilizes two alternative surfaces for interaction with type I receptors (Fig. 7, C and D). One is the basic groove (Fig. 7C) and the other the three-finger-like structure (Fig. 7D), both of which include the L3 loop as an essential constituent. The three-finger-like structure includes residues 331–361 and 379–387 in addition to the L3 loop. Residues 379–387 of Smad7 form a loop-like structure on the side opposite the L3 loop on an identical surface (Fig. 6B). On sequence alignment of Smad6 and Smad7, no amino acid residue in the 379–387 region of Smad7 coincided with that of Smad6. Moreover, the amino acid sequences of this region of Smad6 are divergent, and only 38% amino acids are conserved even between human and mouse (supplemental Fig. S3); this explains why this region is not utilized in Smad6 for interaction with type I receptors. In the case of residues 331–361, inhibition of ALK-2 signaling became less potent as the length of the amino acid sequence of Smad7 substituted with that of Smad6 increased. Within residues 331–361, there is a protruding loop-like structure on the same surface as residues 379–387 and the L3 loop. Smad7 thus possibly grabs type I receptors via this three finger-like loop structure.
For inhibition of ALK-5 signaling, Smad7 may have an alternative mode of regulation of ALK-5 signaling, which does not require its interaction with ALK-5 (12, 30). S7-K316E failed to interact with ALK-5, but still inhibited signaling from ALK-5. Remarkably, the three-finger-like structure of Smad7 is also indispensable for this mode of ALK-5 inhibition because S7-K316E/mt lost its inhibitory effect on ALK-5.
At present, it is unclear why Smad7 utilizes two alternative surfaces in its interaction with type I receptors. The interaction of Smad7 with type I receptors through the three-finger-like structure appears to be involved in Smad7-specific modulation of type I receptor function, which remains to be elucidated. It is also unclear how utilization of two surfaces of Smad7 in interaction with type I receptors is controlled. One possibility is that some posttranslational modifications of Smad7 or type I receptors affect the mode of interaction between them.
In Drosophila, one I-Smad (Daughters against Decapentaplegic, Dad) and three type I receptors (Thickveins, Tkv; Saxophone, Sax; and Baboon, Babo) have been identified. Tkv and Sax are orthologues of ALK-3/6 and ALK-1/2, respectively, and transduce BMP-type signaling, whereas Babo is an orthologue of ALK-4/5/7 and transduces activin-type signaling (31). Dad strongly interacts with Tkv and Sax but not with Babo (25). The prototypic function of I-Smads thus appears to inhibit BMP type I receptors.
In mammals, novel types of I-Smads, i.e. Smad6 and Smad7, have emerged via gene duplication events. Although Dad does not recruit dSmurf to type I receptors (32), mammalian I-Smads have acquired the ability to cooperate with Smurf family ubiquitin ligases (20–24). In addition, the two I-Smads have distinct target specificity on type I receptors. Smad6 exhibits differential inhibitory effects on two subfamilies of BMP type I receptors. On the other hand, Smad7 has acquired the ability to inhibit ALK-4/5/7 in addition to ALK1/2 and ALK3/6, together with utilization of a distinct binding surface in its interaction with type I receptors. Control of TGF-β family type I receptor function by I-Smads via various mechanisms through multiple surfaces results in the generation of spatiotemporally fine tuned signaling activity.
Supplementary Material
Acknowledgments
We thank Keiko Yuki for skilled technical assistance and Masao Saitoh for technical advice.
This work was supported by KAKENHI Grants-in-aid for Scientific Research and the Global Center of Excellence Program from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- BMP
- bone morphogenetic protein
- ALK
- activin receptor-like kinase
- I-Smad
- inhibitory Smad
- R-Smad
- receptor-regulated Smad.
REFERENCES
- 1.Derynck R., Zhang Y. E. (2003) Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 2.Brown K. A., Pietenpol J. A., Moses H. L. (2007) J. Cell. Biochem. 101, 9–33 [DOI] [PubMed] [Google Scholar]
- 3.Schmierer B., Hill C. S. (2007) Nat. Rev. Mol. Cell Biol. 8, 970–982 [DOI] [PubMed] [Google Scholar]
- 4.Heldin C.-H., Landström M., Moustakas A. (2009) Curr. Opin. Cell Biol. 21, 166–176 [DOI] [PubMed] [Google Scholar]
- 5.Miyazawa K., Shinozaki M., Hara T., Furuya T., Miyazono K. (2002) Genes Cells 7, 1191–1204 [DOI] [PubMed] [Google Scholar]
- 6.Miyazono K., Kamiya Y., Morikawa M. (2010) J. Biochem. 147, 35–51 [DOI] [PubMed] [Google Scholar]
- 7.Feng X. H., Derynck R. (1997) EMBO J. 16, 3912–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y.-G., Hata A., Lo R. S., Wotton D., Shi Y., Pavletich N., Massagué J. (1998) Genes Dev. 12, 2144–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo S. R., Chen Y.-G., Shi Y., Pavletich N. P., Massagué J. (1998) EMBO J. 17, 996–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persson U., Izumi H., Souchelnytskyi S., Itoh S., Grimsby S., Engström U., Heldin C.-H., Funa K., ten Dijke P. (1998) FEBS Lett. 434, 83–87 [DOI] [PubMed] [Google Scholar]
- 11.Wu G., Chen Y.-G., Ozdamar B., Gyuricza C. A., Chong P. A., Wrana J. L., Massagué J., Shi Y. (2000) Science 287, 92–97 [DOI] [PubMed] [Google Scholar]
- 12.Nakao A. (2006) in Smad Signal Transduction (ten Dijke P., Heldin C.-H. eds) pp. 379–395, Springer, Dordrecht, The Netherlands [Google Scholar]
- 13.Imamura T., Takase M., Nishihara A., Oeda E., Hanai J., Kawabata M., Miyazono K. (1997) Nature 389, 622–626 [DOI] [PubMed] [Google Scholar]
- 14.Hayashi H., Abdollah S., Qiu Y., Cai J., Xu Y. Y., Grinnell B. W., Richardson M. A., Topper J. N., Gimbrone M. A., Jr., Wrana J. L., Falb D. (1997) Cell 89, 1165–1173 [DOI] [PubMed] [Google Scholar]
- 15.Nakao A., Afrakhte M., Morén A., Nakayama T., Christian J. L., Heuchel R., Itoh S., Kawabata M., Heldin N.-E., Heldin C.-H., ten Dijke P. (1997) Nature 389, 631–635 [DOI] [PubMed] [Google Scholar]
- 16.Souchelnytskyi S., Nakayama T., Nakao A., Morén A., Heldin C.-H., Christian J. L., ten Dijke P. (1998) J. Biol. Chem. 273, 25364–25370 [DOI] [PubMed] [Google Scholar]
- 17.Kavsak P., Rasmussen R. K., Causing C. G., Bonni S., Zhu H., Thomsen G. H., Wrana J. L. (2000) Mol. Cell 6, 1365–1375 [DOI] [PubMed] [Google Scholar]
- 18.Ebisawa T., Fukuchi M., Murakami G., Chiba T., Tanaka K., Imamura T., Miyazono K. (2001) J. Biol. Chem. 276, 12477–12480 [DOI] [PubMed] [Google Scholar]
- 19.Murakami G., Watabe T., Takaoka K., Miyazono K., Imamura T. (2003) Mol. Biol. Cell 14, 2809–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komuro A., Imamura T., Saitoh M., Yoshida Y., Yamori T., Miyazono K., Miyazawa K. (2004) Oncogene 23, 6914–6923 [DOI] [PubMed] [Google Scholar]
- 21.Kuratomi G., Komuro A., Goto K., Shinozaki M., Miyazawa K., Miyazono K., Imamura T. (2005) Biochem. J. 386, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi W., Sun C., He B., Xiong W., Shi X., Yao D., Cao X. (2004) J. Cell Biol. 164, 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanyu A., Ishidou Y., Ebisawa T., Shimanuki T., Imamura T., Miyazono K. (2001) J. Cell Biol. 155, 1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goto K., Kamiya Y., Imamura T., Miyazono K., Miyazawa K. (2007) J. Biol. Chem. 282, 20603–20611 [DOI] [PubMed] [Google Scholar]
- 25.Kamiya Y., Miyazono K., Miyazawa K. (2008) FEBS Lett. 582, 2496–2500 [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki T., Miyazaki H., Hara T., Furuya T., Imamura T., Watabe T., Miyazono K. (2004) J. Biol. Chem. 279, 31568–31574 [DOI] [PubMed] [Google Scholar]
- 27.Fujii M., Takeda K., Imamura T., Aoki H., Sampath TK., Enomoto S., Kawabata M., Kato M., Ichijo H., Miyazono K. (1999) Mol. Biol. Cell 10, 3801–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korchynskyi O., ten Dijke P. (2002) J. Biol. Chem. 277, 4883–4891 [DOI] [PubMed] [Google Scholar]
- 29.Dennler S., Itoh S., Vivien D., ten Dijke P., Huet S., Gauthier J.-M. (1998) EMBO J. 17, 3091–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S., Fei T., Zhang L., Zhang R., Chen F., Ning Y., Han Y., Feng X.-H., Meng A., Chen Y. G. (2007) Mol. Cell. Biol. 27, 4488–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raftery L. A., Korochkina S., Cao J. (2006) in Smad Signal Transduction (ten Dijke P., Heldin C.-H. eds) pp. 55–73, Springer, Dordrecht, The Netherlands [Google Scholar]
- 32.Liang Y.-Y., Lin X., Liang M., Brunicardi F. C., ten Dijke P., Chen Z., Choi K.-W., Feng X.-H. (2003) J. Biol. Chem. 278, 26307–26310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.