Abstract
Abnormal hyperphosphorylation of the microtubule-associated protein Tau is a hallmark of Alzheimer disease and related diseases called tauopathies. As yet, the exact mechanism by which this pathology causes neurodegeneration is not understood. The present study provides direct evidence that Tau abnormal hyperphosphorylation causes its aggregation, breakdown of the microtubule network, and cell death and identifies phosphorylation sites involved in neurotoxicity. We generated pseudophosphorylated Tau proteins by mutating Ser/Thr to Glu and, as controls, to Ala. These mutations involved one, two, or three pathological phosphorylation sites by site-directed mutagenesis using as backbones the wild type or FTDP-17 mutant R406W Tau. Pseudophosphorylated and corresponding control Tau proteins were expressed transiently in PC12 and CHO cells. We found that a single phosphorylation site alone had little influence on the biological activity of Tau, except Thr212, which, upon mutation to Glu in the R406W background, induced Tau aggregation in cells, suggesting phosphorylation at this site along with a modification on the C-terminal of the protein facilitates self-assembly of Tau. The expression of R406W Tau pseudophosphorylated at Thr212, Thr231, and Ser262 triggered caspase-3 activation in as much as 85% of the transfected cells, whereas the corresponding value for wild type pseudophosphorylated Tau was 30%. Cells transfected with pseudophosphorylated Tau became TUNEL-positive.
Keywords: MAPs, Microtubules, Neurodegeneration, Tau, Tubulin, Abnormal Hyperphosphorylation Of Tau, Microtubule-Associated Proteins, Neurofibrillary Degeneration, Pair Helical Filaments, Microtubule Assembly
Introduction
The microtubule-associated protein (MAP)3 Tau accumulates in abnormally hyperphosphorylated state forming intracellular filamentous deposits in several neurodegenerative diseases that cause dementia (1). These dementia disorders are collectively known as tauopathies. This family of diseases includes Alzheimer disease (AD), adults with Down syndrome, frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17), amyotrophic lateral sclerosis, cortical basal degeneration, dementia pugilistica, Pick disease, and tangle-only dementia. Despite their diverse phenotypic manifestations, brain dysfunction, and degeneration, these tauopathies are linked to the progressive accumulation of filamentous hyperphosphorylated Tau inclusions, and these inclusions, together with the absence of other disease-specific neuropathological abnormalities except amyloid β deposition in AD and Down syndrome, provide circumstantial evidence implicating abnormal Tau in disease onset and/or progression.
Tau is the major neuronal MAP. The other neuronal MAPs are MAP1A, MAP1B, and MAP2. In vitro, Tau promotes the assembly of tubulin into microtubules and stabilizes the microtubule structure (2). Microtubules support axoplasmic transport. The vast majority of axonal proteins are synthesized in the neuronal cell body and transported through the axon along the microtubule tracks. Axonal transport occurs throughout the life of a neuron and is essential to its growth and survival. Microtubules lie along the axis of the axon and provide the main cytoskeletal “tracks” for transport. In the neurons of patients with AD, the microtubule system is believed to be disrupted, and axonal transport is interrupted, preventing vesicles to reach the synapses, and slowly and steadily, the synapses degenerate associated with retrograde degeneration.
In the central nervous system, Tau is a family of six proteins derived from a single gene by alternative splicing of its pre-mRNA (3, 4). The human brain Tau isoforms range from 352 to 441 amino acids. They differ in whether they contain three or four tubulin-binding domains/repeats of 31 or 32 amino acids, each near the C terminus, and two, one, or no inserts of 29 amino acids, each in the N-terminal region of the molecule. The isoform expression and its degree of phosphorylation are regulated developmentally. All six isoforms have been reported to be present in an abnormally hyperphosphorylated state in neurofibrillary tangles of paired helical filaments admixed with straight filaments (1, 5–7). The Alzheimer hyperphosphorylated Tau contains ∼8 mol of phosphate/mol of protein compared with ∼3 mol of phosphate/mol of the normal protein (8) and results from phosphorylation at several new sites.
In AD, hyperphosphorylation of Tau appears to precede the appearance of the tangles (8, 9). Tau is phosphorylated at Ser and Thr by several protein kinases, in vitro and in vivo, including glycogen synthase kinase-3, Cdk5, protein kinase A, calcium/calmodulin-dependent protein kinase II, casein kinase 1, protein kinase C, MAPK, and cyclin-dependent kinase 2 (reviewed in Refs. 10 and 11). It has been shown that phosphorylation of Tau decreases its interaction with microtubules, and certain sites such as Thr212, Ser214, Thr231, Ser235, and Ser262 are the major sites in the inhibition of the binding of Tau to microtubules (12–17). It also has been shown that phosphorylation of Tau filaments by glycogen synthase kinase-3β induces formation of tangles (18). We demonstrated previously that in vitro hyperphosphorylation of Tau by brain kinases converts Tau into a molecule that is able both to self-assemble into tangles of filaments and to disrupt microtubules by binding normal Tau, MAP1A, MAP1B, and MAP2 (19–22). Tau can self-assemble when phosphorylated with a combination of isolated kinases (23), and the biological activity of Tau (microtubule-binding, disruption, and self-assembly) can be differentially regulated by different kinases (24). Studying the self-assembly properties of the first microtubule-binding domain of Tau, Zhou et al. (25) showed by turbidity, electron microscopy, circular dichroism (CD), and NMR spectroscopy that phosphorylation at Ser262 could accelerate Tau assembly into filaments.
The discovery of mutations in the Tau gene, which cosegregate with the disease in FTDP-17, provided unequivocal evidence that Tau abnormalities alone are enough to cause neurodegenerative disease (26–28). Three different types of Tau mutations have been described: missense, deletion, and intronic. The missense mutations have been reported to result in the substitution of one amino acid. The intronic 5′ to exon 10 mutations result in overexpression of four-repeat Tau (26, 28). The exact molecular mechanism of neurodegeneration by which the mutations cause the disease is not yet understood. Like individuals with AD, FTDP-17 patients with Tau mutations show accumulations of abnormally hyperphosphorylated Tau as neurofibrillary tangles. All of the mutations discovered in Tau are dominant, suggesting that the effect of Tau mutations results in a gain of toxic function (29).
In a previous study, we also have shown that the FTDP-17 point mutations in Tau make this protein more prone to be phosphorylated with brain kinases and that these mutated Tau proteins can self-assemble into filaments at a lower stoichiometry of phosphorylation than the wild type Tau (30). Normal Tau self-assembles when ∼10 mol of phosphate/mol of protein are incorporated, whereas the corresponding value for FTDP-17 mutated Tau is only ∼5 mol of phosphate/mol of protein.
All of these previous observations taken together suggested that hyperphosphorylation of Tau was a pivotal step in neurodegeneration and that the hyperphosphorylation probably induced a conformational change in the Tau molecule, which made it inhibitory and that the FTDP-17 mutant Tau acquired this gain of toxic function more readily (31).
The present study provides direct evidence that abnormal hyperphosphorylation causes a breakdown of microtubule network and aggregation of Tau and cell death and identifies some of the phosphorylation sites that are involved in converting normal Tau to a toxic protein. In this study, we transiently transfected PC12 and CHO cells; expressed Tau pseudophosphorylated by mutation of Ser or Thr into Glu individually and in different combinations; and examined the microtubule network, Tau aggregation, and cell death. We found that no single phosphorylation site induced a dramatic change except Thr212, phosphorylation of which induced aggregation of Tau. The combination of phosphorylation of Thr212, Thr231, and Ser262 in Tau in cells induced caspase activation and TUNEL-positive staining, making Tau a toxic molecule, and this toxicity was higher in R406W than the wild type Tau.
EXPERIMENTAL PROCEDURES
Materials
Sodium borohydride was purchased from ICN Pharmaceutials (Casta Mesa, CA). All other reagents used in this study were purchased from Sigma unless otherwise indicated. The following primary antibodies were used: mouse monoclonal antibody DM1A to α-tubulin (1:2000, Sigma); mouse monoclonal antibody Tau C3 to Asp421 Tau (32); rabbit polyclonal antibodies 134d (33), 111e (34), and 92e (35) to total Tau (1:5000); monoclonal antibody Tau-1 to Tau nonphosphorylated at Ser198/199/202 (1:50,000) (36); and polyclonal antibodies to p-Thr181, p-Ser214, p-Ser396, and p-Ser422 Tau (1:1000) (BIOSOURCE, Camarillo, CA). The secondary antibodies used were Alexa 488-conjugated goat anti-mouse antibody and Alexa 594-conjugated goat anti-rabbit antibody (1:1000) (Molecular Probes, Eugene, OR).
Site-directed Mutagenesis
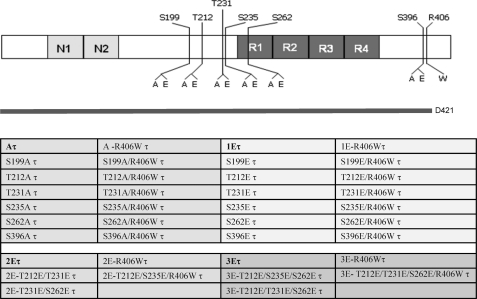
To mimic phosphorylation, Ser199, Thr212, Thr231, Ser235, Ser262, and Ser396 in Tau were mutated to Glu and, as a nonphosphorylation control, to Ala individually or in combination, and Ser422 to a stop codon to generate truncated Tau, Asp421 Tau (Fig. 1), by site-directed mutagenesis using a QuikChange II XL site-directed mutagenesis kit (Stratagene) for the single sites or a QuikChange multisite-directed mutagenesis kit (Stratagene) following the manufacturer's instructions. The incorporation of the mutations in the Tau was verified by sequencing of the plasmids.
FIGURE 1.
Tau constructs employed for transfection of cells. N1 and N2 represent the two amino-terminal inserts, and R1, R2, R3, and R4 represent the four microtubule-binding domain repeats. Indicated are the S/T sites, which were pseudophosphorylated in wild type or R406W mutated Tau (τ) to Glu (E) and as control to Ala (A) in the present study. Asp421 Tau is shown (D421).
Cell Culture and Transfections
CHO cells (obtained from ATCC, Manassas, VA) were grown in 25-cm2 flasks at 37 °C, and 5% CO2 in F12K medium (ATCC) supplemented with 10% fetal bovine serum, 100 international units/ml penicillin, 100 μg/ml streptomycin. Cells were plated in eight-well culture slides coated with poly-d-lysine (Becton Dickinson Labware, Bedford, MA) for 24 h prior to use and then transfected with expression plasmids using LipofectamineTM 2000 (Invitrogen) according to the manufacturer's instructions. Briefly, 2 μg of DNA was diluted in 50 μl of Opti-MEM I reduced serum medium and mixed with Lipofectamine 2000 for 20 min at room temperature. The mixture was overlaid on cells containing medium without serum and antibiotics. After 6 h, the cells were replenished with fresh, regular culture medium. Studies were carried out 24–48 h post-transfection and when the transfection efficiency was ∼25–35%.
Immunocytochemistry to Detect Microtubules and Tau, with or without Extraction of Cells
To double label Tau and microtubules, the cells were rinsed once with warm (37 °C) phosphate-buffered saline (PBS) and once with warm PEM (80 mm PIPES, 5 mm EGTA, 1 mm MgCl2, pH 6.8) and then were either fixed with 0.3% glutaraldehyde containing 0.5% Nonidet P-40 in PEM for 10 min at 37 °C or were extracted with warm 0.2% Triton X-100, 10 μm taxol in PEM for 1 min at 37 °C. Extracted cells were rinsed with warm PEM twice and fixed. After fixation, the cells were treated with 0.5% Triton X-100 in TBS for 10 min and rinsed with PBS. Then, the cells were treated with sodium borohydride (10 mg/ml in PBS) for 7 min and incubated with 0.1 m glycine in PBS for 20 min. After rinsing with PBS, the cells were treated with 4% normal horse serum containing 0.1% Tween 20 in PBS for 30 min and immunostained with primary antibodies, followed by incubation with Alexa 488-conjugated goat anti-mouse antibody (1:1000) and Alexa 594-conjugated goat anti-rabbit antibody (1:1000) (Invitrogen).
TUNEL, Tau, and Caspase Staining
For TUNEL and Tau staining, cells were fixed with 4% formaldehyde in PBS for 20 min. First, the apoptotic cells were labeled with FITC by a TUNEL apoptosis detection kit (Millipore) according to the manufacturer's instructions. Then, the total Tau was immunolabeled as described above. The primary antibody used was 134d, and the secondary antibody was Alexa 594-conjugated goat anti-rabbit antibody. For double labeled active caspase-3 and Tau, the primary antibodies used were rabbit polyclonal antibody to active caspase-3 (1:50; Abcam, Cambridge, MA) and mouse monoclonal antibody TAU-5 (1:100; Becton Dickinson Biosciences, Bedford, MA). Representative images were generated using a PCM 2000 Confocal Imaging System (Nikon, Melville, NY) or an Act-2U Digital Imaging System (Nikon). The National Institutes of Health Image J program, version 1.32j (http://rsb.info.nih.gov/ij/) was used to count double labeled cells.
Binding of Normal Tau to Pseudophosphorylated Tau
The binding of normal Tau to the pseudophosphorylated Tau was studied by a dot blot overlay assay (22, 37). Taking advantage of the heat stability of Tau, CHO cells transfected with different Tau constructs were extracted with 100 mm MES, pH 6.7, 1 mm EGTA, 150 mm NaCl, 1 mm β-mercaptoethanol and centrifuged at 100,000 × g for 60 min, and the supernatant was heated for 10 min in a boiling water bath. After removing the denatured proteins by centrifugation for 10 min at 100,000 × g, 50 ng of each pseudophosphorylated Tau was spotted on nitrocellulose, blocked with 5% nonfat dry milk in 100 mm Mes buffer, pH 6.7 for 1 h. After blocking, the membrane was overlaid with normal recombinant Tau (5 μg/ml) for 3 h. The membrane was then washed and fixed with 0.5% formaldehyde, and the aldehyde groups were neutralized with 9% glycine in 50 mm Tris buffer, pH 7.4, containing 150 mm NaCl (TBS). The Tau bound was detected with Tau-1 antibody (36); this antibody recognizes Tau when it is nonphosphorylated at Ser195, Ser198, Ser199, and/or Ser202 (38). Samples in which overlay with normal recombinant Tau was substituted with BSA were used to deduct any background binding. A third strip of the membrane was used to detect total Tau with a mixture of phosphoindependent polyclonal antibodies against Tau: 134d (33), 111e (34), and 92e (35) to normalize the data.
RESULTS
Pseudophosphorylation at Multiple Sites Is Required to Have a Strong Impact on the Binding of Tau to Microtubules
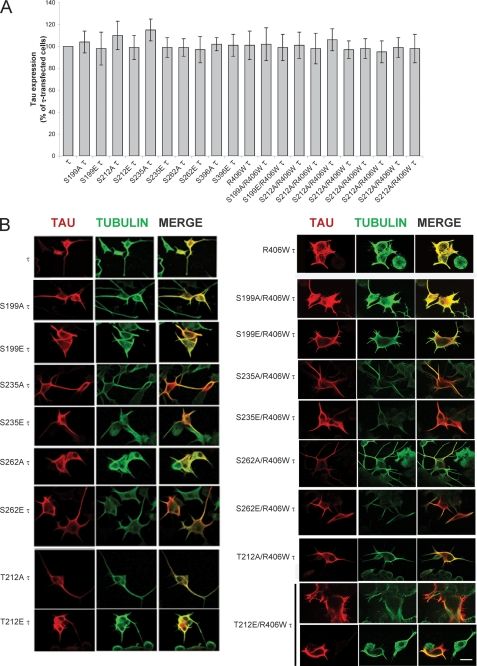
Previously, by in vitro studies, we found that the conformation of abnormally hyperphosphorylated Tau needed to sequester normal Tau might involve among other sites phosphorylation at Ser199, Thr212, Ser235, and Ser262 and, for self-assembly, further phosphorylation at Thr231 and Ser396 (30). To test the participation of these phosphorylation sites on Tau binding to microtubules and self-assembly in cells, we generated vectors containing Tau and R406W Tau that were mutated individually at Ser199, Thr212, Thr231, Ser235, Ser262, and Ser396 to Ala or to Glu to prevent or mimic phosphorylation at these sites (Fig. 1). cDNAs of Tau, R406W Tau, and all the mutants generated were employed to transiently transfect PC-12 cells or CHO cells. After 6 h of incubation with DNA, the medium was replaced with low serum medium containing 5% serum and 50 ng/ml of NGF to induce differentiation in the case of PC12 cells or with regular medium in the case of CHO cells. After 48 h, the cells were fixed and processed for immunocytochemistry. Cells with at least 25% transfection efficiency were employed for this study. The level of Tau expression was determined by quantitating the inmunofluorescence of Tau relative to the tubulin staining of microtubules for each of the constructs used (Fig. 2A). For each of the constructs, the levels of transfections were very similar. When Tau was expressed, its staining colocalized with microtubules, the processes were long, and microtubules appeared to form bundles in PC-12 cells (Fig. 2B). In general, mutations of Ser/Thr to Ala resembled the patterns observed with the protein nonmutated at these sites. When the Ser or Thr were mutated to Glu at Ser235 and Ser262, especially in R406W Tau background, the colocalization of Tau with microtubules was markedly reduced. Pseudophosphorylation at Thr212 in R406W Tau also showed only a partial colocalization with microtubules (Fig. 2B). These findings suggested that pseudophosphorylation of Tau at Thr212, Ser235, and Ser262 reduced its binding to microtubules.
FIGURE 2.
Expression of single-site pseudophosphorylated Tau (1E Tau and 1E R406W Tau) in PC12 cells. A, the levels of Tau (τ) expression in the cells was measured by quantifying the immunofluorescence of Tau relative to that of tubulin, using ImageJ software. The ratio of Tau to tubulin was considered 100% for cells transfected with wild type Tau. Error bars, SD. B, PC12 cells were transfected with pseudophosphorylated Tau for 24–48 h, and its expression was studied by immunocytochemistry as described. The cells were double labeled with 134d (Tau, red fluorescence) and DM1A (tubulin, green fluorescence). Merge is shown in yellow. Bar, 25 μm.
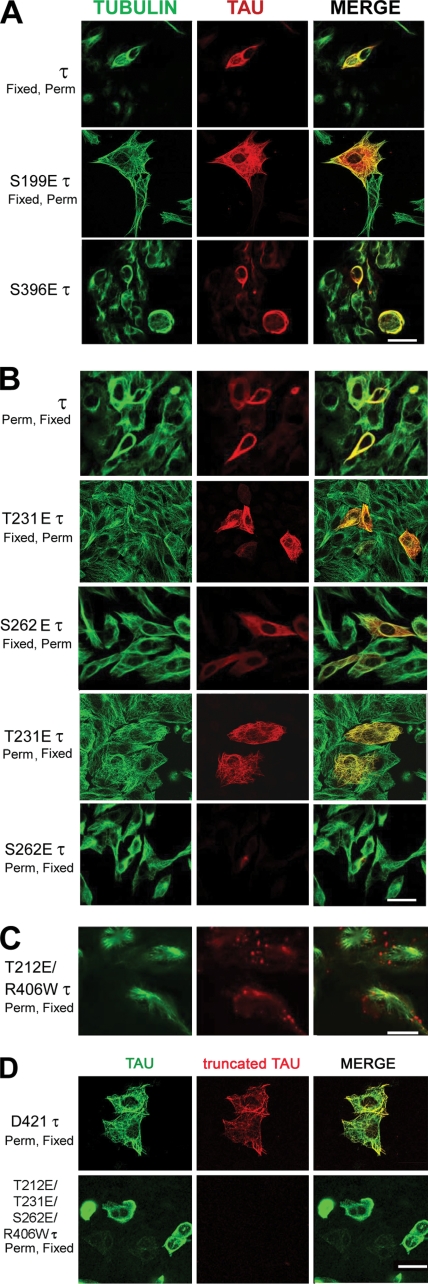
To better visualize the microtubule network and detect Tau-microtubule interaction, we used CHO cells for transfection (Fig. 3). Cells were fixed and permeabilized (Fig. 3A) or were permeabilized before fixing to detect Tau associated to microtubules (Fig. 3). When the cells were fixed before permeabilization, Tau staining was diffuse, but it also colocalized with microtubules. Nevertheless, when the cells were permeabilized first under microtubule-stabilizing conditions and then fixed, although the normal Tau immunostaining remained similar (Fig. 3 compare τ Fixed, Perm with τ Perm, Fixed) the pseudophosphorylated Tau bound to microtubules was very much decreased (Fig. 3B, compare S262E τ Fixed, Perm with S262E τ Perm, Fixed). Although all Tau proteins expressed remained associated to the microtubules the staining appeared weaker in the permeabilized cells when Tau was pseudophosphorylated, suggesting that the presence of the negative charge in a position that interferes with Tau binding to tubulin decreased Tau binding to microtubules; this effect was most marked in the case of S262E (Fig. 3B). This finding is in agreement with the previous report showing that Ser262 has a very strong effect on Tau-microtubule binding (14, 16, 39). Pseudophosphorylation at other sites, including Thr231, did not appear to have a major impact on microtubule binding (Fig. 3B). Truncated Tau, D421 Tau, bound to microtubules similarly to wild type Tau (Fig. 3D).
FIGURE 3.
Expression of single-site pseudophosphorylated Tau (1E Tau and 1E R406W Tau) and Asp421 truncated Tau (Asp421 Tau and Asp421/R406W Tau) in CHO cells. A, cells were transfected with pseudophosphorylated or truncated Tau, and its expression was studied by immunocytochemistry at 24–48 h as described. The cells were double labeled with 134d (Tau, red fluorescence) and DM1A (tubulin, green fluorescence). Merge is shown in yellow. B, cells transfected with S262E Tau. The cells were fixed before (Fixed) or after (Perm) permeabilization. S262E Tau bound weakly to microtubules. C, cells transfected with T212E/R406W Tau. After 48 h, the cells were permeabilized with 0.1% Nonidet P-40 before fixed and processed for immunocytochemistry and double labeled with 134d (Tau, red) and DM1A (tubulin, green). T212E/R406W Tau appeared to form aggregates devoid of tubulin, decorating the microtubules. Bar, 25 μm. D, cells transfected with Asp421 Tau and double labeled with 134d (Tau, green fluorescence) and Tau C3 (truncated Tau, red fluorescence). Bar, 16 μm.
Pseudophosphorylation of R406W Tau at Thr212 induced Tau aggregates that decorated the microtubule network in CHO cells, suggesting that phosphorylation at this position could be involved in Tau gaining the conformation that promotes Tau-Tau interaction (Fig. 3C). Of all the Tau constructs studied in cells, this Tau-staining pattern was seen only with T212E/R406W Tau. The difference observed with this pseudophosphorylated Tau does not appear to be due to different levels of expression of Tau. We did not observe any differences in transfection efficiency. These results indicate that R406W Tau probably aggregates when Thr212 is phosphorylated.
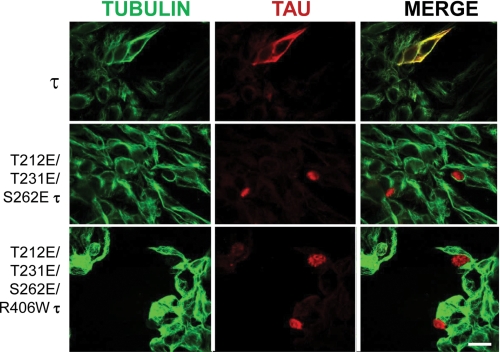
To test the effect of more than one phosphorylation sites on Tau, multiple site-directed mutagenesis was performed. We were able to generate Tau proteins with two and three pseudophosphorylation sites. Using transient transfection, we studied the effect of expressing these pseudophosphorylated Tau proteins in CHO cells as described above. T212E/T231E/S262E Tau and T212E/T231E/S262E/R406W Tau appeared to dissociate from microtubules and form aggregates in the cell nucleus (Fig. 4). These findings suggested that the presence of a negative charge at these three positions was able to reduce Tau binding to the microtubules much more than at only one phosphorylated site studied. Tau binds to microtubules with a Kd of ∼100 nm (40). Hyperphosphorylation of Tau at Thr212, Thr231, and Ser262 is known to inhibit this binding and results in its accumulation in the cytoplasm. Tau has a consensus nuclear localization sequence KKXK (amino acid residues (140–143) (41) through which it might be translocated by one or more importins to the nucleus. The pseudophosphorylation of Tau at Ser212, Thr231, and Ser262 might make it a better substrate for interaction with an importin than pseudophosphorylation at Ser212 alone and, hence, the differences in their aggregations in nucleus versus cytoplasm. In the nucleus, the hyperphosphorylated Tau, like histones, might bind to the DNA through its highly basic domains. Previously, we have shown that Tau can interact with DNA (42). Nuclear localization of hyperphosphorylated Tau also was reported previously (43, 44).
FIGURE 4.
CHO cells transfected with either Tau or T212E/T231E/S262E Tau or T212E/T231E/S262E/R406W Tau. After 48 h, the cells were permeabilized with 0.1% Nonidet P-40 before fixing and then processed for immunocytochemistry and double labeled with 134d (Tau) and DM1A (tubulin). Merge is shown in yellow. 3E Tau appeared to localize in the nucleus, and tubulin staining was markedly reduced in the transfected cells. Bar, 25 μm.
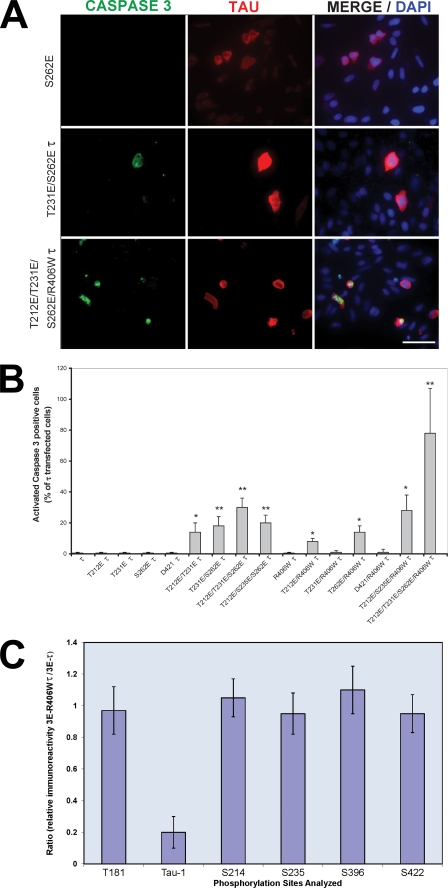
Pseudophosphorylated Tau Induces Caspase Activation and TUNEL-positive Cells
To investigate the sites of pseudophosphorylation that made Tau toxic, CHO cells transfected individually with the different Tau constructs were double stained for Tau and activated caspase-3 and counterstained with DAPI. The cells were fixed and stained as described above, and both Tau and caspase-positive cells were counted. A total of at least 300 cells were counted per transfection. Expression of 1E Tau was unable to induce caspase-3 activation (Fig. 5). T212E/T231E/S262E Tau and T212E/T231E/S262E/R406W Tau induced proapoptotic behavior. In the case of T212E/T231E/S262E/R406W Tau, the effect was much higher than that of T212E/T231E/S262E Tau. Thus, the marked increase in the induction of caspase-3 activation in T212E/T231E/S262E/R406W Tau-transfected cells could be due to the mutation R406W and/or the higher level of endogenous phosphorylation of this Tau than T212E/T231E/S262E Tau. We investigated this possibility by quantitative immuno-dot blots using antibodies specific to different Tau phosphoepitopes (Thr181, Ser198/199/202, Ser214, Ser235, Ser396, and Ser422; Fig. 5C). No significant differences were found in the sites investigated between 3E Tau and 3E R406W Tau, with the exception of Tau-1 (reactive when Ser198/199/202 are not phosphorylated). Tau-1 immunoreactivity for 3E R406W Tau was 20% of that of 3E-Tau, suggesting that the FTDP-17-mutated Tau was considerably more phosphorylated at this site than 3E Tau. Taken together, these results clearly indicate that phosphorylation at Thr212, Thr231, and Ser262 induce a toxic property to Tau, but this property can be enhanced either by the conformational change induced by R406W mutation and/or by the subsequent phosphorylation at Ser198/199/202, the Tau-1 site.
FIGURE 5.
Caspase activation in Tau-transfected CHO cells. A, cells were transfected with pseudophosphorylated wild type and R406W-mutated Tau proteins. After 48 h, the cells were double labeled with 134d (Tau) and activated caspase-3. Cells were counterstained with DAPI to visualize the nuclei. Bar, 50 μm. B, quantification of cells double stained for Tau and activated caspase-3. Approximately 300 cells were counted in each transfection. T212E/T231E/S262E/R406W Tau induced the highest caspase activation in the transfected cells. Error bars, SD. *, p < 0.05; **, p < 0.01; Error bars, SD. *, p < 0.05; **, p < 0.01. C, cells were transfected with T212E/T231E/S262E Tau or T212E/T231E/S262E/R406W Tau. (After 48 h, the cells were harvested, and phosphorylation of Tau at different sites (Thr181, Ser199/202 (Tau-1), Ser214, Ser235, Ser396, and Ser422) was determined by quantitative immuno-dot blots using antibodies specific to total and different phospho-Tau proteins.) The results are shown as the ratio of the immunoreactivity for T212E/T231E/S262E/R406W Tau (3E R406W Tau) to T212E/T231E/S262E Tau (3E Tau). No significant differences (p < 0.5) were found in the sites investigated between 3E Tau and 3E R406W Tau, with the exception of the Tau-1 site (reactive when Ser198/199–202 are not phosphorylated); Error bars, SD.
Truncation of Tau at Asp421 following its abnormal hyperphosphorylation has been reported previously (45). Activated caspase-3 can cleave Tau at Asp421. Truncated Tau can be detected with the monoclonal antibody Tau C3. We generated wild type and R406W Tau truncated at position 421 by mutating Ser422 to a STOP codon. The expression of truncated Tau in CHO cells showed that truncated Tau bound to microtubules and did not activate caspase-3 (Figs. 3D and 5B). Expression of truncated Tau in cells expressing 3E R406W Tau was undetectable.
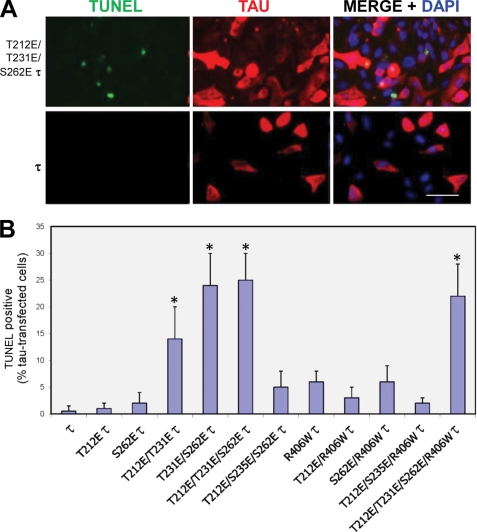
TUNEL staining enables investigation of DNA fragmentation during apoptosis. We investigated TUNEL staining in CHO cells transiently transfected with E Tau constructs. We quantitated the percentage of TUNEL-positive cells that were transfected with Tau. Cells transfected with single pseudophosphorylated Tau forms were TUNEL-negative, or the values were too low (<5% of the 30% transfected cells; i.e. from 100 cells counted, <2 cells were TUNEL-positive) to draw any conclusions. Cells transfected with T212E/T231E Tau, T231E/S262E Tau, T212E/T231E/S262E Tau, and T212E/T231E/S262E/R406W Tau had the highest percentage of Tau expressing TUNEL-positive cells. In the case of 3E R406W Tau, whereas ∼80% of the transfected cells were caspase-3-activated, only 23% were TUNEL-positive (Fig. 6). This finding is in agreement with the finding of Spires-Jones et al. (46) who showed in a reversible transgenic mouse model of tauopathy that after hours of imaging, neurons with neurofibrillary tangles were caspase-3-positive but TUNEL-negative. More recently, in the brain stem of AD cases, Wei et al. (47) showed that hyperphosphorylated Tau correlated with caspase activation, and only a smaller proportion of these neurons were TUNEL-positive.
FIGURE 6.
TUNEL staining in Tau-transfected CHO cells. A, CHO cells were transiently transfected with Tau and R406W Tau, and these Tau proteins pseudophosphorylated at various sites. After 48 h, the cells were double labeled with 134d (Tau) and TUNEL. Cells were counterstained with DAPI to visualize the nuclei. B, quantitation of cells double stained for Tau and TUNEL. The Tau construct used in each case is indicated. Approximately 300 cells were counted per Tau construct. T212E/T231E Tau, T231E/S262E Tau, T212E/T231E/S262E Tau, and T212E/T231E/S262E/R406W Tau induced apoptosis in the transfected cells. Bar, 50 μm. Error bars, SD. *, p < 0.05.
Pseudophosphorylated Tau Binds Normal Tau
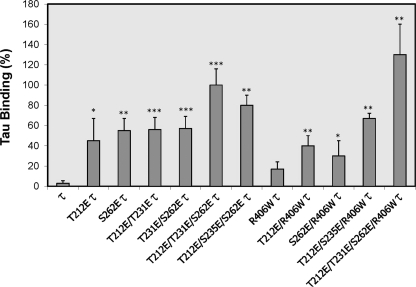
Previously, we had proposed that abnormally hyperphosphorylated Tau could induce neurodegeneration through a mechanism that involves microtubule disruption through the sequestration of normal MAPs (20, 21). According to this hypothesis, pseudophosphorylated Tau should bind normal Tau. To test Tau-E-Tau binding, we used overlay assay. Different Tau constructs including Tau, R406W Tau, 1E Tau, 2E Tau, 3E Tau and 1E, 2E, and 3E R406W Tau were transfected individually in CHO cells. After 48 h of transfection, the cells were harvested and lysed, and pseudophosphorylated Tau was isolated by heat treatment of the cell lysates as described under “Experimental Procedures.” The amount of Tau was detected by quantitative immuno-dot blot assay using wild type Tau as a standard.
To study the sequestration of normal Tau by E Tau proteins by overlay assay, 40 ng of E Tau was dotted on a nitrocellulose membrane and overlaid with 2 μg/ml normal Tau. E Tau was able to bind normal Tau (Fig. 7). Like AD abnormally hyperphosphorylated Tau (19, 21), E Tau sequestered normal Tau, and this Tau-Tau binding was the highest in the case of T212E/T231E/S262E/R406W Tau.
FIGURE 7.
Pseudophosphorylated Tau binds normal Tau. CHO cells were transfected with different pseudophosphorylated Tau constructs, and cell extracts enriched in Tau were obtained as described under “Experimental Procedures.” Binding of normal Tau to pseudophosphorylated Tau was quantified as described under “Experimental Procedures.” The binding of Tau to T212E/T231E/S262E Tau was taken as 100%. Pseudophosphorylated Tau bound to normal Tau.
DISCUSSION
Neurofibrillary tangles of abnormally hyperphosphorylated Tau are a hallmark of AD and related tauopathies. There is no disease described, in which Tau accumulates and is not hyperphosphorylated. Nevertheless, it has not been established whether hyperphosphorylation of Tau is a cause or a consequence of the process of neurodegeneration. Here, we show by employing pseudophosphorylation that: 1) AD-type abnormal hyperphosphorylation of Tau causes neurodegeneration and, 2) that Thr212, Thr231, and Ser262 are among the phosphorylation sites that make Tau cytotoxic. Furthermore, the FTDP-17 mutation R406W exacerbates this hyperphosphorylation-induced toxicity of Tau. Phosphorylation at these three critical sites promotes its binding to normal Tau and confers Tau an apoptotic behavior.
Previous reports on the biological activity and self-assembly of abnormally hyperphosphorylated Tau are consistent with the present study. Abnormally hyperphosphorylated Tau from AD brains (AD P-Tau) sequesters normal Tau and self-assembles into tangles of paired helical/straight filaments (19–22). The longest isoforms of the human four-repeat and the three-repeat Tau proteins, when expressed in yeast, were found to acquire pathological phosphoepitopes, assume a pathological conformation, and form aggregates (48). We also have shown that hyperphosphorylation of Tau by brain kinases inhibits its microtubule assembly promoting activity and induces its self-assembly into tangles of paired helical/straight filaments (49). Recently, Rankin et al. (18) demonstrated that phosphorylation of Tau by glycogen synthase kinase-3β is sufficient to induce the clustering of arachidonic acid-induced filaments into structures similar to the neurofibrillary tangle-like aggregates of Tau filaments found in AD brain.
In FTDP-17 with Tau mutations, this protein is found to be polymerized in an abnormally hyperphosphorylated state in the absence of plaques. We postulate that a common molecular mechanism, i.e. the abnormal hyperphosphorylation of Tau, leads to neurofibrillary degeneration in these clinically distinct diseases and that Tau phosphorylation can be modulated by its conformation. Our previous studies have shown that four of the FTDP-17 Tau mutations, R406W, V337M, G272V, and P301L, result in Tau proteins that are, in vitro, more favorable substrates for phosphorylation by brain protein kinases than the wild type Tau, and Tau 4L (2 N-inserts, 4R) more than Tau 3L (2 N-inserts, 3R) (30). We had found that the mutated Tau proteins polymerized into filaments in vitro when 4–6 mol of phosphate/mol of Tau were incorporated, whereas wild type Tau required ∼10 mol of phosphate/mol of protein to self-assemble. Mutated and wild type Tau proteins were able to sequester normal Tau upon incorporation of ∼4 mol of phosphate/mol of protein, which was achieved as early as 30 min of in vitro phosphorylation by brain kinases in the case of mutant Tau proteins. These findings taken together suggest that the mutations in Tau might cause neurodegeneration through rendering a molecule that is a more favorable substrate for hyperphosphorylation. This could mean that Tau phosphorylation is a key regulator of cellular processes, one that is carefully modulated not to over- or under-respond. Nevertheless, the particular sites that are involved in these abnormal functions were not known.
We and others (49, 50) had shown that the microtubule-binding domain of Tau was the domain involved in Tau-Tau interaction. We also postulated that the positive charge of the Tau molecule, concentrated in patches in the flanking region of Tau, could be responsible for the inhibition of its aggregation, because the presence of the two N-terminal inserts of Tau, which are highly negative, induced self-assembly of Tau (49). Upon phosphorylation, Tau also acquires the ability to bind normal Tau. These results suggest that at least two different conformational states of Tau are induced by phosphorylation: one in which the hyperphosphorylated Tau is able to bind normal Tau and one in which it is able to self-assemble into filaments. In agreement with our model, oxidation of Tau, by the addition of carbonyls to Lys also neutralizes the charge, and Tau filaments are formed under oxidative stress conditions (51). Eliminating the positive fragment of Tau by truncation also fits well into this model.
Based on our previous studies, we have selected, in the present study, sites to be modified by site-directed mutagenesis. When we modified one single site at a time, we found that the effects were not dramatic. In general, all the mono-pseudophosphorylated Tau proteins were able to bind to microtubules, although pseudophosphorylated Tau at Ser262 or Ser235 appeared to have a weaker binding because the immunoreactivity was very much reduced when the cells were permeabilized and then fixed and immunostained. Tau pseudophosphorylated at Thr212, though, was able to bind to microtubules; it formed aggregates on the microtubules, suggesting that the negative charge on Thr212, one of the most basic residues on Tau, is involved in the self-assembly of Tau. Consistent with the present study, Vandebroek et al. (48) found that Tau sites of monoclonal antibody AT100 (Thr212 and Ser214) were critical for the self-assembly of Tau when they transfected yeast with human Tau and induced inactivation of Pho85, an orthologue of Cdk5.
The Tau phosphosites Thr212, Thr231, and Ser262 are present early in AD pathology (30, 52). Thr212 can be phosphorylated by Dyrk1A (dual-specificity tyrosine (Y) phosphorylation-regulated kinase 1A), coded in chromosome 21, and most individuals with trisomy 21 (Down syndrome) show early onset of AD. The amyloid precursor protein also is coded in chromosome 21; therefore, with the extra copy of chromosome 21, Down syndrome patients have three copies of Dyrk1A and amyloid precursor protein. Ryoo et al. (53) generated a transgenic mouse model overexpressing Dyrk1A. These mice have higher Tau phosphorylation at Thr212, and this hyperphosphorylated Tau did not promote microtubule assembly (54). Thr212 is in a very basic domain of Tau, so the impact of phosphorylation on this site is very strong (30). With respect to Thr231 and Ser262, there is evidence that these sites are very important in Tau binding to microtubules and that the combination of these sites dramatically decreases Tau microtubule-promoting activity of Tau (15, 55).
In the present report, we have used the pseudophosphorylation to study the effect of a particular site on Tau binding to microtubules, self-assembly, and cell toxicity. In a previous study, Fath et al. (56) pseudophosphorylated Tau at five Ser/Thr residues in the N-terminal region flanking the microtubule-binding domain (Ser198, Ser199, Ser202, Thr231, and Ser235) and five Ser/Thr residues in the C-terminal end (Ser396, Ser404, Ser409, Ser413, and Ser422); and observed no self-assembly but partial toxicity in PC-12 cells. More recently, this group generated a transgenic mouse line using this pseudophosphorylated Tau (57), and these mice did not show any significant neurodegeneration, probably because they did not include Tau pseudohosphorylation at Ser262, which is most implicated in inhibition of its interaction with microtubules (see Refs. 14–16 and 39). With regards to Ser262, Lijima et al. (58) using transgenic Drosophila expressing both human Abeta42 and Tau, showed that Tau phosphorylation at Ser262 plays a critical role in Abeta42-induced Tau toxicity because the toxicity of Tau was prevented by using a transgenic fly expressing a nonphosphorylatable form at position Ser262 instead of normal Tau. Another in vitro study used pseudophosphorylated Tau to study the influence of different Tau phosphorylation sites on assembly of Tau with polyanions (59) and found that Thr212 was an important site in terms of stabilizing the polymer. Leschik et al. (60) used pseudophosphorylated Tau at Ser198, Ser199, Ser202, Thr231, Ser235, Ser396, Ser404, Ser409, Ser413, and Ser422 to show that E-Tau is cytotoxic in primary neuronal culture, is the limiting factor in amyloid β-induced cell death, and is modulated by PS1 and amyloid β. In the present study, we have used wild type and FTDP-17 mutant Tau proteins, which we have shown previously to require a lower phosphorylation stoichiometry than the wild type Tau. The selection of sites was based on our previous study on kinetics of phosphorylation and binding of normal Tau and self-assembly (30). The present study suggests that pseudophosphorylated Tau, at Thr212, Thr231, and Ser262 (3E Tau) is a molecule that does not bind to microtubules, induces caspase activation, and shows as TUNEL-positive cells. Taken together, these results suggest that the combination of these three phosphorylation sites converts Tau into a toxic molecule.
In conclusion, the present study suggests a scenario where there is a phosphorylation/dephosphorylation imbalance, and key phosphorylation sites such as Thr212, Thr231, and Ser262 are phosphorylated and induce a conformational change in Tau. This abnormal hyperphosphorylation of Tau not only results in the loss of its normal function but also the gain of a toxic activity that causes disruption of the microtubule network and cell death. In light of the sites detected in the present study, we can speculate that phosphorylation of Tau by a combination of kinases, i.e. nonproline dependent kinases PKA or calcium/calmodulin-dependent protein kinase II and proline-dependent kinases glycogen synthase kinase-3β or Cdk5, could play a critical role in converting Tau from a functional to cytotoxic protein. This combination of kinases was shown previously to induce self-assembly and inhibition of microtubule assembly by Tau (23). It is possible that other combination of sites could also render toxicity to Tau, but sites reported in the present study are known to get phosphorylated early in AD neurodegeneration.
Acknowledgments
We are grateful to Janet Murphy for secretarial assistance. Dr. Ezzat El-Akkad helped prepare Fig. 1. Dr. Lester Binder provided the mouse monoclonal antibody Tau C3.
This work was supported, in whole or in part, by National Institutes of Health Grants AG019158 and AG028538 (to K. I.) and R15AG034524-01 (to A. D. A.). This work was also supported by the New York State Office of Mental Retardation and Developmental Disabilities; Alzheimer's Association (Chicago, IL) Grants IIRG-06-25836 (to K. I.) and IIRG-09-133206 (to A. D. A.); and Professional Staff Congress-City University of New York Research Award 62909-00 40 (to A. D. A.) and 80209-0416 (to A. D. A.).
- MAP
- microtubule-associated protein
- AD
- Alzheimer disease
- PIPES
- 1,4-piperazinediethanesulfonic acid
- Mes
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. (1975) Proc. Natl. Acad. Sci. U.S.A. 72, 1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. (1989) Neuron 3, 519–526 [DOI] [PubMed] [Google Scholar]
- 4.Himmler A., Drechsel D., Kirschner M. W., Martin D. W., Jr. (1989) Mol. Cell. Biol. 9, 1381–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goedert M., Spillantini M. G., Cairns N. J., Crowther R. A. (1992) Neuron 8, 159–168 [DOI] [PubMed] [Google Scholar]
- 6.Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. (1986a) J. Biol. Chem. 261, 6084–6089 [PubMed] [Google Scholar]
- 7.Iqbal K., Grundke-Iqbal I., Smith A. J., George L., Tung Y. C., Zaidi T. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 5646–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Köpke E., Tung Y. C., Shaikh S., Alonso A. C., Iqbal K., Grundke-Iqbal I. (1993) J. Biol. Chem. 268, 24374–24384 [PubMed] [Google Scholar]
- 9.Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., Seitelberger F., Grundke-Iqbal I., Iqbal K., Wisniewski H. M. (1989) Brain Res. 477, 90–99 [DOI] [PubMed] [Google Scholar]
- 10.Lee V. M., Goedert M., Trojanowski J. Q. (2001) Annu. Rev. Neurosci. 24, 1121–1159 [DOI] [PubMed] [Google Scholar]
- 11.Iqbal K., Liu F., Gong C. X., Alonso Adel C., Grundke-Iqbal I. (2009) Acta Neuropathol. 118, 53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins S. M., Johnson G. V. (1997) Brain Res. 767, 305–313 [DOI] [PubMed] [Google Scholar]
- 13.Brandt R., Lee G., Teplow D. B., Shalloway D., Abdel-Ghany M. (1994) J. Biol. Chem. 269, 11776–11782 [PubMed] [Google Scholar]
- 14.Drewes G., Trinczek B., Illenberger S., Biernat J., Schmitt-Ulms G., Meyer H. E., Mandelkow E. M., Mandelkow E. (1995) J. Biol. Chem. 270, 7679–7688 [DOI] [PubMed] [Google Scholar]
- 15.Sengupta A., Kabat J., Novak M., Wu Q., Grundke-Iqbal I., Iqbal K. (1998) Arch. Biochem. Biophys. 357, 299–309 [DOI] [PubMed] [Google Scholar]
- 16.Singh T. J., Wang J. Z., Novak M., Kontzekova E., Grundke-Iqbal I., Iqbal K. (1996) FEBS Lett. 387, 145–148 [DOI] [PubMed] [Google Scholar]
- 17.Liu F., Liang Z., Wegiel J., Hwang Y. W., Iqbal K., Grundke-Iqbal I., Ramakrishna N., Gong C. X. (2008) Faseb J. 22, 3224–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rankin C. A., Sun Q., Gamblin T. C. (2007) Mol. Neurodegener. 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alonso A., Zaidi T., Novak M., Grundke-Iqbal I., Iqbal K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6923–6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso A. D., Grundke-Iqbal I., Barra H. S., Iqbal K. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso A. C., Grundke-Iqbal I., Iqbal K. (1996) Nat. Med. 2, 783–787 [DOI] [PubMed] [Google Scholar]
- 22.Alonso A. C., Zaidi T., Grundke-Iqbal I., Iqbal K. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 5562–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J. Z., Grundke-Iqbal I., Iqbal K. (2007) Eur. J. Neurosci. 25, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F., Li B., Tung E. J., Grundke-Iqbal I., Iqbal K., Gong C. X. (2007) Eur. J. Neurosci. 26, 3429–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L. X., Zeng Z. Y., Du J. T., Zhao Y. F., Li Y. M. (2006) Biochem. Biophys. Res. Commun. 348, 637–642 [DOI] [PubMed] [Google Scholar]
- 26.Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R. C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J. M., Nowotny P., Che L. K., Norton J., Morris J. C., Reed L. A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P. R., Hayward N., Kwok J. B., Schofield P. R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B. A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. (1998) Nature 393, 702–705 [DOI] [PubMed] [Google Scholar]
- 27.Poorkaj P., Bird T. D., Wijsman E., Nemens E., Garruto R. M., Anderson L., Andreadis A., Wiederholt W. C., Raskind M., Schellenberg G. D. (1998) Ann. Neurol. 43, 815–825 [DOI] [PubMed] [Google Scholar]
- 28.Spillantini M. G., Murrell J. R., Goedert M., Farlow M. R., Klug A., Ghetti B. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 7737–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowther R. A., Goedert M. (2000) J. Struct. Biol. 130, 271–279 [DOI] [PubMed] [Google Scholar]
- 30.Alonso, Adel C., Mederlyova A., Novak M., Grundke-Iqbal I., Iqbal K. (2004) J. Biol. Chem. 279, 34873–34881 [DOI] [PubMed] [Google Scholar]
- 31.Alonso A. C., Li B., Grundke-Iqbal I., Iqbal K. (2008) Curr. Alzheimer Res. 5, 375–384 [DOI] [PubMed] [Google Scholar]
- 32.Gamblin T. C., Chen F., Zambrano A., Abraha A., Lagalwar S., Guillozet A. L., Lu M., Fu Y., Garcia-Sierra F., LaPointe N., Miller R., Berry R. W., Binder L. I., Cryns V. L. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10032–10037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatebayashi Y., Iqbal K., Grundke-Iqbal I. (1999) J. Neurosci. 19, 5245–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alonso, Adel C., Grundke-Iqbal I., Iqbal K. (1995) Brain Res. Mol. Brain Res. 31, 194–200 [DOI] [PubMed] [Google Scholar]
- 35.Grundke-Iqbal I., Vorbrodt A. W., Iqbal K., Tung Y. C., Wang G. P., Wisniewski H. M. (1988) Brain Res. 464, 43–52 [DOI] [PubMed] [Google Scholar]
- 36.Binder L. I., Frankfurter A., Rebhun L. I. (1985) J. Cell Biol. 101, 1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kremer L., Domínguez J. E., Avila J. (1988) Anal. Biochem. 175, 91–95 [DOI] [PubMed] [Google Scholar]
- 38.Szendrei G. I., Lee V. M., Otvos L., Jr. (1993) J. Neurosci. Res. 34, 243–249 [DOI] [PubMed] [Google Scholar]
- 39.Fischer D., Mukrasch M. D., Biernat J., Bibow S., Blackledge M., Griesinger C., Mandelkow E., Zweckstetter M. (2009) Biochemistry 48, 10047–10055 [DOI] [PubMed] [Google Scholar]
- 40.Chelsky D., Ralph R., Jonak G. (1989) Mol. Cell. Biol. 9, 2487–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goode B. L., Denis P. E., Panda D., Radeke M. J., Miller H. P., Wilson L., Feinstein S. C. (1997) Mol. Biol. Cell 8, 353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua Q., He R. Q., Haque N., Qu M. H., del Carmen, Alonso A., Grundke-Iqbal I., Iqbal K. (2003) Cell Mol. Life Sci. 60, 413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loomis P. A., Howard T. H., Castleberry R. P., Binder L. I. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 8422–8426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brady R. M., Zinkowski R. P., Binder L. I. (1995) Neurobiol. Aging 16, 479–486 [DOI] [PubMed] [Google Scholar]
- 45.Delobel P., Lavenir I., Fraser G., Ingram E., Holzer M., Ghetti B., Spillantini M. G., Crowther R. A., Goedert M. (2008) Am. J. Pathol. 172, 123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spires-Jones T. L., de Calignon A., Matsui T., Zehr C., Pitstick R., Wu H. Y., Osetek J. D., Jones P. B., Bacskai B. J., Feany M. B., Carlson G. A., Ashe K. H., Lewis J., Hyman B. T. (2008) J. Neurosci. 28, 862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wai M. S., Liang Y., Shi C., Cho E. Y., Kung H. F., Yew D. T. (2009) Biogerontology 10, 457–469 [DOI] [PubMed] [Google Scholar]
- 48.Vandebroek T., Vanhelmont T., Terwel D., Borghgraef P., Lemaire K., Snauwaert J., Wera S., Van Leuven F., Winderickx J. (2005) Biochemistry 44, 11466–11475 [DOI] [PubMed] [Google Scholar]
- 49.Alonso A. D., Zaidi T., Novak M., Barra H. S., Grundke-Iqbal I., Iqbal K. (2001) J. Biol. Chem. 276, 37967–37973 [DOI] [PubMed] [Google Scholar]
- 50.Pérez M., Valpuesta J. M., Medina M., Montejo de Garcini E., Avila J. (1996) J. Neurochem. 67, 1183–1190 [DOI] [PubMed] [Google Scholar]
- 51.Santa-María I., Smith M. A., Perry G., Hernández F., Avila J., Moreno F. J. (2005) Biochim. Biophys. Acta 1740, 472–480 [DOI] [PubMed] [Google Scholar]
- 52.Augustinack J. C., Schneider A., Mandelkow E. M., Hyman B. T. (2002) Acta Neuropathol. 103, 26–35 [DOI] [PubMed] [Google Scholar]
- 53.Ryoo S. R., Jeong H. K., Radnaabazar C., Yoo J. J., Cho H. J., Lee H. W., Kim I. S., Cheon Y. H., Ahn Y. S., Chung S. H., Song W. J. (2007) J. Biol. Chem. 282, 34850–34857 [DOI] [PubMed] [Google Scholar]
- 54.Ryoo S. R., Cho H. J., Lee H. W., Jeong H. K., Radnaabazar C., Kim Y. S., Kim M. J., Son M. Y., Seo H., Chung S. H., Song W. J. (2008) J. Neurochem. 104, 1333–1344 [DOI] [PubMed] [Google Scholar]
- 55.Sengupta A., Grundke-Iqbal I., Iqbal K. (2006) Neurochem. Res. 31, 1473–1480 [DOI] [PubMed] [Google Scholar]
- 56.Fath T., Eidenmüller J., Brandt R. (2002) J. Neurosci. 22, 9733–9741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hundelt M., Fath T., Selle K., Oesterwind K., Jordan J., Schultz C., Gotz J., von Engelhardt J., Monyer H., Lewejohann L., Sachser N., Bakota L., Brandt R. (2009) Neurobiol. Aging, in press [DOI] [PubMed] [Google Scholar]
- 58.Iijima K., Gatt A., Iijima-Ando K. (2010) Hum. Mol. Genet. 19, 2947–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Necula M., Kuret J. (2004) J. Biol. Chem. 279, 49694–49703 [DOI] [PubMed] [Google Scholar]
- 60.Leschik J., Welzel A., Weissmann C., Eckert A., Brandt R. (2007) J. Neurochem. 101, 1303–1315 [DOI] [PubMed] [Google Scholar]