Abstract
Myelinating Schwann cells (SCs) are highly plastic cells that are able to dedifferentiate and re-enter the cell cycle. However, the molecular signals controlling dedifferentiation are not completely understood. Because a connection between mitogenic signaling and myelin loss has been suggested, we investigated the role of cAMP, a strong inducer of the myelinating phenotype, and mitogenic factors activating receptor tyrosine kinases (RTKs) on SC dedifferentiation. We herein provide evidence indicating that cAMP was required to not only initiate but also maintain a state of differentiation because SCs rapidly dedifferentiated and became competent to resume proliferation upon the removal of cAMP stimulation. Surprisingly, isolated SCs could undergo multiple cycles of differentiation and dedifferentiation upon cAMP addition and removal, respectively, in the absence of mitogenic factors and without entering the cell cycle. Conversely, the activation of RTKs and the ERK cascade by a variety of growth factors, including neuregulin, was not sufficient to initiate dedifferentiation in the presence of cAMP. Importantly, a reduction of cAMP triggered dedifferentiation through a mechanism that required JNK, rather than ERK, activity and an induction of the expression of c-Jun, a transcriptional inhibitor of myelination. In summary, the reversible transition from an undifferentiated to a myelinating state was dependent on cAMP but independent of RTK signaling and cell cycle progression, further indicating that dedifferentiation and proliferation are uncoupled and differentially regulated events in SCs.
Keywords: Cell Differentiation, Cell Division, ERK, JNK, Jun Transcription Factor, Receptor Tyrosine Kinase, Krox-20, Schwann Cells, Myelination, Neuregulin
Introduction
The process of cellular dedifferentiation, in which a partially or terminally differentiated cell reverts to an earlier developmental stage to acquire self-renewal capability, usually occurs as part of a regenerative process. One of the most compelling examples of this type of cellular plasticity in vertebrate animals is shown during the regeneration of complex structures, such as limbs, tail, and spinal cord in urodele amphibians (1, 2). In contrast to other vertebrates, which show a much more restricted capacity for regeneration, urodeles respond to injury by forming a mesenchymal growth zone or blastema that largely derives from the dedifferentiation of various cell types, including skeletal muscle, SCs,2 and cartilage.
For reasons that are not yet understood, most adult mammalian cell types lack this high degree of plasticity (3). One exception are myelinating SCs of peripheral nerves, which are postmitotic cells that show the ability to dedifferentiate, re-enter the cell cycle, and re-differentiate during adulthood, all features that are prominently observed after nerve injury (4). Dedifferentiated SCs facilitate axonal growth into the injured area, and ultimately they promote nerve repair by ensheathing and remyelinating regenerated axons. Although the cellular mechanisms that underlie peripheral nerve regeneration are well understood, little is known about the molecular basis of adult mammalian SC plasticity. Recent evidence has suggested that intracellular pathways involved in SC dedifferentiation overlap at least partially with those involved in proliferation because the activation of the neuregulin receptor ErbB2 (5, 6), the ERK cascade (7), and the transcription factors c-Jun (8) and Notch (9) have been linked to the initiation of myelin loss. It is well established that ErbB2 activation is required for SC mitogenesis initiated by neuregulin, a component of the mitogenic signal of dorsal root ganglion axons (10), and there is evidence indicating that SCs require ERK activation, c-Jun, and Notch for cell cycle progression in developing nerves (9) and in response to neuregulin and adenosine (11–13). Although SCs of the distal nerve stumps lose their myelin and start proliferating shortly after injury, the question remains as to whether these processes are initiated by common or independent signaling events.
In this study, we used SCs growing in the absence of neurons as a simple in vitro system to investigate the connection between mitogenic signaling, cell cycle re-entry, and dedifferentiation. More specifically, we sought to investigate the role of cAMP, a key instructive signal for SC differentiation into a myelinating phenotype (14), and polypeptide growth factors activating receptor tyrosine kinase (RTK) signaling upon the reversal of the differentiated state. Overall, our data support the concept that dedifferentiation and cell cycle re-entry are differentially and independently controlled, and although they can occur one after another, they are not necessarily functionally coupled cellular events.
EXPERIMENTAL PROCEDURES
Materials
Non-selective cell-permeable cAMP analogs db-cAMP and CPT-cAMP were from Biolog (United States distributor, Axxora LLC, San Diego, CA). Recombinant heregulin-β1(177–244), a soluble peptide consisting of the EGF homology domain of β1-heregulin (hereafter referred to as “neuregulin”) was from Genentech (South San Francisco, CA). Recombinant purified platelet-derived growth factor-BB (PDGF-BB), insulin-like growth factor-1 (IGF-1), and fibroblast growth factor-2 (FGF-2) were from R&D Systems (Minneapolis, MN). Defined fetal bovine serum (FBS) was from HyClone (Logan, UT). Forskolin, cholera toxin, PMA, cycloheximide, actinomycin D, and antibodies against cyclic nucleotide phosphohydrolase (CNPase) were from Sigma. S100, ErbB2, and GFAP antibodies were from DAKO (Carpinteria, CA). MAG and PMP22 (peripheral myelin protein-22) antibodies were from Chemicon (Temecula, CA). Antibodies recognizing phosphorylated Akt, ERK, ErbB2, ErbB3, tyrosine, and protein kinase A (PKA) substrates were from Cell Signaling Technology (Beverly, MA). ErbB3 and c-Jun antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal antibodies against the PCNA and MCM2 (minichromosome maintenance protein 2) were from Abcam (Cambridge, MA) and Bethyl Laboratories (Montgomery TX), respectively. U0126 was from Promega Corp. (Madison, WI), and SP600125 (anthra[1,9-cd]pyrazol-6(2H)-one) was from Biomol (Plymouth Meeting, PA). All other protein kinase inhibitors were from Calbiochem. Bromodeoxyuridine (BrdU) antibody and DNase were from the BrdU detection kit (Amersham Biosciences). [3H]Thymidine (6.7 Ci/mmol) and SolvableTM were from PerkinElmer Life Sciences. Thy 1.1 and p75NGFR hybridoma cells were from the American Type Culture Collection (Manassas, VA). O1 and O4 hybridoma cells were provided by Dr. Schachner. Krox-20 antibodies were provided by Dr. Meijer. The expression vectors pCEV29-MEKEE and pCDNAIII-HA-ERK2 were provided by Dr. J. S. Gutkind.
Primary Cultures of Adult and Postnatal Schwann Cells
Rat SCs were prepared from adult sciatic nerves by a modification of a previously reported method (15). Nerve segments were explanted in non-coated dishes containing DMEM, 10% heat-inactivated FBS and sequentially transplanted to new dishes (2–3 times) to facilitate myelin removal and cell recovery after nerve tissue dissociation. Ten days after, nerve explants were dissociated with 0.25% dispase and 0.05% collagenase, and the cells were plated on a poly-l-lysine substrate (200 μg/ml). After 1 week in culture, contaminating fibroblasts were removed by a complement reaction using Thy 1.1 antibodies (30-min incubation followed by rabbit complement). The purified SCs were subsequently plated in poly-l-lysine substrate-coated 10-cm dishes and allowed to grow in expansion medium consisting of DMEM, 10% FBS supplemented with 2 μm forskolin, 20 μg/ml bovine pituitary extract (Biomedical Technologies, Inc., Stoughton, MA), and 10 nm neuregulin. All experiments were performed using SCs from passages 2–4 (2–8 population doublings) routinely cultured on poly-l-lysine substrate/laminin-coated dishes. Cultured adult SCs prepared following this procedure are competent to form myelin upon axonal contact both in vitro (15) and in vivo (16).
SCs from postnatal day 1 rat sciatic nerves were established by a modification of a standard method (17). Briefly, nerve tissue was dissociated by incubation with 0.1% collagenase and then with 0.25% trypsin. The resulting cell suspensions were plated and purified of contaminating fibroblasts by including 10 μm cytosine arabinoside in the culture medium (DMEM, 10% FBS) for 3 days. Subsequent steps were identical to those described for adult SCs. Purified adult and postnatal cultures consisted of >98% SCs based on immunostaining with anti-S100, a specific SC marker.
Transduction with Lentiviral Vectors and Transient Transfections
Established SC cultures were transduced at an early passage with a lentiviral vector encoding the green fluorescent protein (GFP) gene under the control of the cytomegalovirus promoter. The efficiency of GFP expression at 3 days after transduction was >99%. Importantly, we confirmed that the expression of GFP did not alter the dynamics of SC proliferation, differentiation, and dedifferentiation.
SCs, treated and non-treated with cAMP for 2 days, were transfected for 3 h in serum-free DMEM containing 0.3 μg of total plasmid DNA/condition together with the Lipofectamine Plus reagent (Invitrogen), according to the protocol suggested by the manufacturer. The percentage of transfected SCs was 20–30% based on GFP fluorescence from the expression vector pMAX-GFP (Amaxa Biosystems), which was routinely used as a positive control to estimate transfection efficiency and to make the total amount of transfected DNA equivalent in all conditions.
Cell Proliferation Assays
For experimentation, adult rat SCs were plated on poly-l-lysine substrate/laminin-coated 24-well dishes (50,000–70,000 cells/well) in DMEM containing 10% FBS. One day after plating, the medium was changed to HEPES-buffered DMEM containing 1% FBS (non-proliferating medium), and the cells were subjected to experimentation. SCs return to quiescence after removal of the mitogenic stimulus (i.e. neuregulin and forskolin) for 2 days, and the inclusion of 1% FBS in the culture medium, which was non-mitogenic for SCs, served to prevent the loss of cells by apoptosis as a consequence of serum and mitogen withdrawal (11). Importantly, adult SCs could be maintained for prolonged periods of time (at least 2 weeks) in non-proliferating medium if medium was frequently replaced (e.g. every 4 days) (18).
The incorporation of [3H]thymidine or the thymidine analog BrdU into nuclear DNA was assayed as a measure of S phase entry, as described previously (18). Briefly, cells were exposed to medium containing [3H]thymidine (0.25 μCi/well) or BrdU (1 μm) under the conditions described in the figure legends. The analogs were present throughout the incubation period. Cultures were assayed in triplicate samples in each experimental condition. Unless otherwise noted, mitogenic concentrations of growth factors were used for all stimulation experiments (10 nm neuregulin, 20 ng/ml PDGF-BB, 50 ng/ml IGF-1, 20 ng/ml FGF-2, and 10% FBS). PMA was used at 200 ng/ml. In some experiments, a mitogenic concentration of the adenylyl cyclase activator forskolin (2 μm) was used to enhance neuregulin- and serum-induced SC proliferation and thereby achieve a maximal mitogenic response (18). The incorporated tritium (liquid scintillation counting) or BrdU (immunofluorescence microscopy) was determined 2–3 days after the initial stimulation, as indicated. For BrdU incorporation, the cells were fixed sequentially with 4% paraformaldehyde and −20 °C methanol or alternatively with −20 °C methanol alone and blocked with 5% normal goat serum for 30 min. Cells were treated for 30 min with a 50% solution of DNase in the presence of anti-BrdU (1:300) and then incubated with Alexa594-conjugated secondary antibodies (Molecular Probes, Inc., Eugene, OR).
Antibodies recognizing MCM2 and PCNA, which label the nucleus of cells throughout the G1 and S phases of the cell cycle, were used as markers to identify proliferating cells (19, 20) by immunofluorescence microscopy or Western blot analysis. Only SCs exposed to growth factors or serum for at least 24 h labeled positive for nuclear MCM2 and PCNA expression, whereas SCs subjected to mitogen deprivation for 2–3 days were 98% negative for these markers.
Cell Differentiation and Dedifferentiation Assays
SCs were induced to acquire a differentiated phenotype by exposure for 3–4 days to membrane-permeable analogs of cAMP, as described previously (18). Unless otherwise noted, the non-selective cAMP analog CPT-cAMP was used at 250 μm for all stimulation experiments in non-proliferating medium. For dedifferentiation assays, cAMP-treated cells were subjected to additional treatments, as described for each experiment, and analyzed for the expression of markers for both myelinating and non-myelinating SCs as well as changes in cell morphology (live GFP expression in lentivirally infected SCs) over a time period of up to 3 days. To study the effect of RTK signaling on SC dedifferentiation, mitogenic concentrations of growth factors or vehicle (control) were added directly to the conditioned medium of cAMP-differentiated SCs, and the cells were analyzed 3 days after as indicated in the figure legends. To induce SC dedifferentiation, the medium of cAMP-differentiated SCs was replaced with fresh non-proliferating medium without cAMP-inducing agents. Three days after cAMP removal, the cells were analyzed as described above. In some experiments, a differentiating concentration of forskolin (20 μm) was used to prevent SC dedifferentiation (18). Importantly, the detection of cell proliferation and expression of phenotypic markers was done in parallel cultures subjected to identical experimental conditions. Cultures maintained from the outset in the absence of cAMP-inducing agents served as a control for undifferentiated cells.
Immunofluorescence Microscopy
Cultures were fixed for 10 min with 4% paraformaldehyde in PBS and then for 5 min with −20 °C methanol. Cultures were blocked in 5% normal goat serum in PBS; incubated overnight at 4 °C with the appropriate dilution of the primary antibody in 5% normal goat serum, PBS (1:200); and then rinsed three times with PBS prior to incubation with Alexa-conjugated (594 or 488) secondary antibodies (1:400, 1 h, room temperature). Labeling of O1 and O4 antigens was done by incubating living cells with hybridoma culture supernatant (20 min, room temperature) before fixation. Cells were mounted with Vectashield containing the nuclear dye DAPI (Vector Laboratories, Burlingame, CA) and analyzed by conventional fluorescence microscopy. Black and white images from immunofluorescence microscopy were artificially colorized, digitally processed, and arranged for presentation using Adobe Photoshop version 7.0 and Adobe Illustrator CS3. For cell quantification analysis, pictures from random fields were taken at low magnification (×10–20), and the number of cells labeled positive for the different markers was determined in reference to the total number of cells (DAPI staining). Cells were classified as positive or negative for the expression of the different markers in reference to non-treated controls and disregarding the variability shown by individual cells. At least 500 cells were analyzed per condition.
The phosphorylation of PKA substrates in methanol-fixed cells was assessed by using an antibody that recognizes PKA-specific phosphomotifs ((RR)X(S*/T*), where the asterisk indicates phosphorylation) in target proteins, as described previously (21).
Western Blots
Total cell lysates were prepared by resuspending the cells in a buffer consisting of 50 mm Tris, 150 mm NaCl, 1% SDS, and 0.5 mm DTT along with protease inhibitors (1 mm PMSF, 20 μg/ml aprotinin, and 20 μg/ml leupeptin) and phosphatase inhibitors (phosphatase mixtures I and II, Sigma). Protein lysates were combined with SDS sample buffer (400 mm Tris/HCl, pH 6.8, 10% SDS, 50% glycerol, 500 mm DTT, 2 μg/ml bromphenol blue), followed by 10 min of boiling. Equal protein samples were subjected to polyacrylamide gel electrophoresis under denaturing conditions (SDS-PAGE) and then transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA) by liquid transfer. Membranes were blocked with ECL blocking agent (Amersham Biosciences) in Tris-buffered saline containing 0.05% Tween 20 (TBS-T) and incubated overnight with a 1:1000 dilution of each primary antibody. The membranes were washed three times with TBS-T prior to incubation with horseradish peroxidase-conjugated secondary antibodies, 1:5000 (Promega). Immunoreactive protein bands were detected by enhanced chemiluminescence using ECL Advanced or ECL Plus, depending on signal intensity, according to the manufacturer's instructions (Amersham Biosciences). To determine the relative changes in the levels of expression of cell surface O1, SCs were labeled live by incubation with O1 antibodies and washed extensively before preparing the cell lysates as described above. O1 immunoglobulins were detected by enhanced chemiluminescence after incubating the membranes with anti-mouse horseradish peroxidase. Whenever appropriate, the unchanged expression of ErbB3, is shown as a control of equal cellular protein loading. The total levels of ERK and Akt expression served as a control for the treatments inducing ERK and Akt phosphorylation, respectively.
RESULTS
cAMP-differentiated SCs Did Not Dedifferentiate When Exposed to Growth Factors or Serum
Besides the growth-promoting effects of cAMP on growth factor-induced proliferation (11, 22, 23), cAMP promotes cell growth arrest and differentiation when SCs are exposed to prolonged stimulation with high doses of cAMP-stimulating agents (14, 18, 24). cAMP elevation induces the expression of markers typically associated with myelinating SCs (e.g. MAG and protein zero) and concomitantly decreases the expression of markers specific for the premyelinating or non-myelinating SC phenotype (e.g. p75NGFR and GFAP). It also induces a dramatic cell enlargement and the vacuolization of the cytoplasm (18). In this study, we used SCs differentiated with cAMP (referred to as “differentiated” SCs) as a simplified system to investigate the connection between mitogenic signaling and dedifferentiation. Throughout these studies, we concluded that SCs had undergone dedifferentiation if we observed 1) a decrease in the expression of myelin-related proteins and lipids (e.g. cell surface galactocerebroside (O1)), 2) an increase in the expression of premyelinating SC markers, 3) the reversal of cAMP-induced morphological transformation (i.e. the reacquisition of the characteristic spindle-shaped morphology of immature SCs), and 4) the release of cell growth arrest and/or the reinitiation of S phase entry. Non-cAMP-treated (non-differentiated) SCs, which exhibit an immature phenotype (i.e. they express low or undetectable levels of myelin-associated markers and readily undergo proliferation in response to mitogens (18)) served as controls.
In a previous study (18), we found that growth factors did not reduce the effect of cAMP on driving differentiation. Because available data suggested that key transducers of RTK signaling (e.g. ErbB and ERK) were involved in SC dedifferentiation, we first proceeded by testing whether neuregulin and other growth factors would promote the reversal of the differentiated characteristics once cells had fully differentiated. For this, SCs that had been differentiated with cAMP for 3 days were treated with growth factors, and the expression of phenotypic markers was evaluated over a time period of up to 3 days after growth factor administration. We observed that treatment of cAMP-differentiated SCs with a soluble neuregulin peptide that is sufficient to trigger the activation of the neuregulin co-receptor ErbB2-ErbB3 and SC proliferation (18) failed to revert the morphological transformation induced by cAMP or change the levels of expression of MAG and O1 (Figs. 1A and 6D) or p75NGFR and GFAP (Figs. 1B (bottom) and 6D), regardless of the concentration of neuregulin used (i.e. 1 or 100 nm). Similarly, stimulation of cAMP-differentiated SCs with 10% FBS or mitogenically active concentrations of purified PDGF, IGF, and FGF, which are all effective adult SC mitogens (18), failed to induce SC dedifferentiation in the presence of cAMP elevation (Fig. 1B and supplemental Fig. 1).
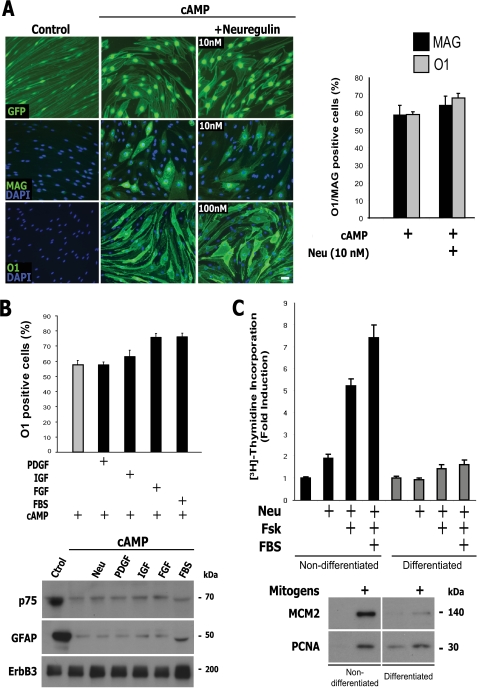
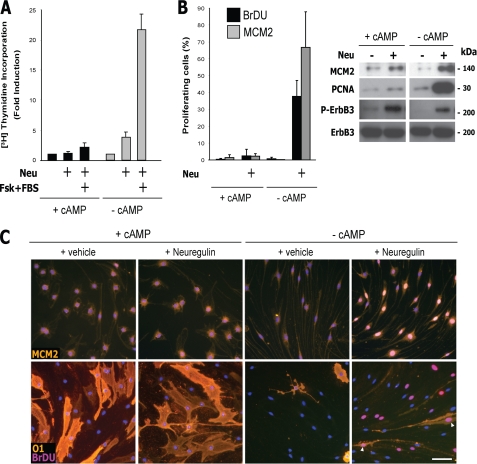
FIGURE 1.
Stimulation with RTK ligands or serum did not trigger SC dedifferentiation or proliferation in the presence of cAMP elevation. Effect of neuregulin, other polypeptide growth factors, and FBS on the expression of SC markers (A and B) and on cell proliferation (C) in cAMP-differentiated adult SCs. Mitogen- and serum-deprived SCs were left untreated (control) or treated with CPT-cAMP in medium non-supportive of proliferation. Three days after cAMP administration, the cells were stimulated with neuregulin (Neu), PDGF-BB, IGF-1, FGF-2, or 10% FBS for an additional 3-day period in medium containing cAMP (see “Experimental Procedures”). Cultures were photographed live (A, top left, GFP fluorescence of lentivirally infected SCs) or analyzed for the expression of the indicated markers by immunofluorescence microscopy (A, middle and bottom left, and B) and Western blot (B, bottom). Representative micrographs of SC cultures stained for MAG and O1 (A, left) and quantitative results (A (right) and B) are shown. In C, SCs were left untreated (Non-differentiated) or treated with CPT-cAMP for 3 days (Differentiated) before stimulation with neuregulin alone or in combination with forskolin (Fsk; 2 μm) and FBS (10%). The incorporation of [3H]thymidine (top) and the expression of the indicated markers (bottom) were determined 2 days after mitogenic stimulation. Mitogens consisted of neuregulin + forskolin + FBS. Neuregulin was used at 10 nm, unless otherwise indicated. In these and all subsequent graphs, bar heights are means of triplicate determinations; error bars represent S.D. Results are from one representative experiment of at least three independent experiments performed. Scale bar, 20 μm.
FIGURE 6.
Signals controlling SC dedifferentiation; effect of cAMP and growth factors on the regulation of Krox-20 and c-Jun expression. A, effect of inhibitors of transcription and translation on SC dedifferentiation. SCs were deprived of cAMP (−cAMP) either in the absence or presence of actinomycin D (10 μm) and cycloheximide (1 μm), and the expression of O1 was determined 3 days after. On the right, controls are shown to denote the inhibitory effect of actinomycin D and cycloheximide on the induction of differentiation by cAMP. B, effect of cAMP on the expression of Krox-20 and c-Jun. The temporal changes in the expression of Krox-20 and c-Jun were determined by Western blot in SCs initially stimulated with CPT-cAMP (top) and in cAMP-differentiated SCs that were either deprived of cAMP (−cAMP) or stimulated with fresh cAMP-containing medium (+cAMP) for the indicated time points (bottom). Note that the loss of Krox-20 precedes the loss of O1 expression, whereas the induction of c-Jun precedes the induction of GFAP expression after cAMP deprivation. C–E, effect of serum and growth factors on the expression of Krox-20 and c-Jun. SCs were left untreated (Non-differentiated; Nd) or treated with cAMP for 3 days (Differentiated) before growth factor stimulation, as indicated, and the expression of the indicated markers was analyzed after 3 days. In C, results are shown for adult (top) and postnatal (bottom) SCs. In E, PDGF, IGF, and FGF (P +I +F) were provided in combination. Scale bar, 50 μm.
Consistent with these observations, cAMP-differentiated SCs did not undergo S phase entry, as assessed by measuring the incorporation of [3H]thymidine (Fig. 1C, top), or transition into the G1 phase, as assessed by measuring the expression of the proliferation markers MCM2 and PCNA (Fig. 1C, bottom) when exposed to neuregulin alone or neuregulin in combination with 10% FBS and forskolin, the most potent mitogenic mixture known for SCs (15). This clearly contrasts with the high proliferative potential displayed by non-cAMP-treated SCs in response to this combination of mitogens (Fig. 1C) and further indicates that exposure to neuregulin alone or in combination with serum was not sufficient to overcome cAMP-induced differentiation or cell growth arrest.
The Activation of RTK Signaling in Differentiated SCs Was Not Sufficient to Promote Dedifferentiation
Growth factors activating RTKs exert biological effects through the stimulation of intracellular kinase cascades, including MEK-ERK and PI3K-Akt (25). In SCs, neuregulin-activated ERK and Akt signaling are both required for S phase progression (11, 12, 26). Because the possibility exists that differentiation would render SCs insensitive to mitogens, we next investigated whether differentiated SCs responded to growth factors by initiating signaling through ligand-activated RTKs. We therefore compared the activation of ERK and Akt in non-differentiated and differentiated SCs by detecting the activated forms of these kinases using phospho-specific antibodies. The results indicated that cAMP-differentiated SCs responded to neuregulin, FBS, and all of the above mentioned growth factors by increasing the phosphorylation of ERK and Akt (Fig. 2, B–D), confirming that SCs did not lose responsiveness to growth factors as a result of prolonged cAMP elevation and differentiation. In addition, the increased neuregulin-induced phosphorylation of ErbB2 and ErbB3 on key activating tyrosine residues (Fig. 2A) further indicated that cAMP-differentiated SCs expressed functional neuregulin co-receptors. The levels of total ErbB2 and ErbB3 proteins were not significantly affected by prolonged treatment with cAMP analogs (Fig. 2A).
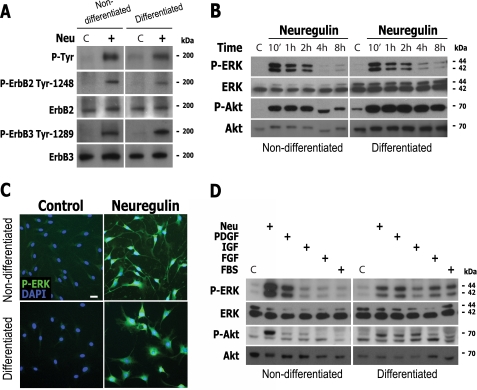
FIGURE 2.
RTK signaling was not sufficient to drive SC dedifferentiation in the presence of cAMP elevation. A–C, neuregulin-induced activation of ErbB2, ErbB3, ERK, and Akt in differentiated SCs. D, activation of ERK and Akt by neuregulin, other growth factors, and serum in differentiated SCs. Experimental conditions were identical to the ones described in the legend to Fig. 1 with the exception that stimulation with growth factors was done for 30 min (A, C, and D) or the times indicated in the figure (B). In all experiments, the response of non-differentiated SCs was compared with that of differentiated SCs, as indicated. The activation of ErbB2, ErbB3, ERK, and Akt was determined by using specific antibodies recognizing the phosphorylated forms of ErbB2 (Tyr-1248), ErbB3 (Tyr-1289), ERK1/2 (Tyr-204), and Akt (Ser-473), respectively. ErbB phosphorylation was also confirmed by using total anti-phosphotyrosine antibodies (P-Tyr). In B and D, the controls (C) were non-mitogen-treated cells. For the differentiated SC group, cAMP-stimulating agents were maintained throughout the course of the experiments. Scale bar, 10 μm.
Time course studies revealed that the activation of ERK and Akt in response to neuregulin reached maximum levels shortly after (10 min) stimulation but remained activated for the following 4–8 h, regardless of the state of cellular differentiation (Fig. 2B). As controls, representative micrographs of SC cultures immunolabeled with anti-phospho-ERK are shown to denote that ERK phosphorylation was enhanced in all of the cells within the population, including vacuolated SCs (Fig. 2C), confirming that highly differentiated SCs were sensitive to neuregulin stimulation. Overall, these results confirmed that the activation of RTK signaling in differentiated SCs was insufficient to drive dedifferentiation and cell cycle progression in the presence of cAMP.
cAMP-induced SC Differentiation and Morphological Transformation Were Reversible upon cAMP Removal
One important question was whether prolonged treatment with cAMP analogs led to reversible or non-reversible SC differentiation. Because SCs require repeated additions of cAMP-stimulating agents to maintain high levels of expression of myelin-specific markers (18), we examined whether simply removing the cAMP-stimulating agents from the culture medium would promote dedifferentiation. We observed that with the exception of the predifferentiation marker O4 (cell surface sulfatide; Fig. 3A), the expression of all the other myelin-associated markers tested was remarkably reduced 3 days after cAMP removal and that this temporally coincided with the recovery of an elongated bipolar morphology and the reacquisition of p75NGFR and GFAP expression (Fig. 3, A–C). However, the expression of S100 (Fig. 3A) and ErbB3 (Fig. 3C), which are SC-specific markers, was not apparently affected by cAMP addition and removal. A control for the effectiveness of cAMP treatment was demonstrated by the expression of intracellular phosphorylated PKA substrates (Fig. 3A, right).
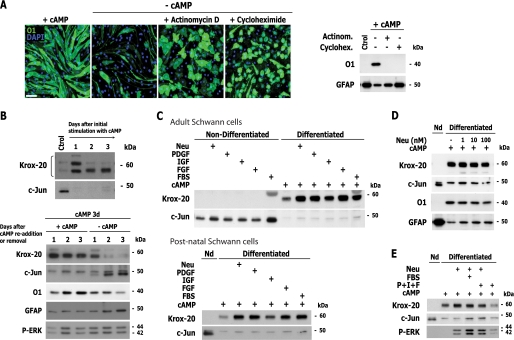
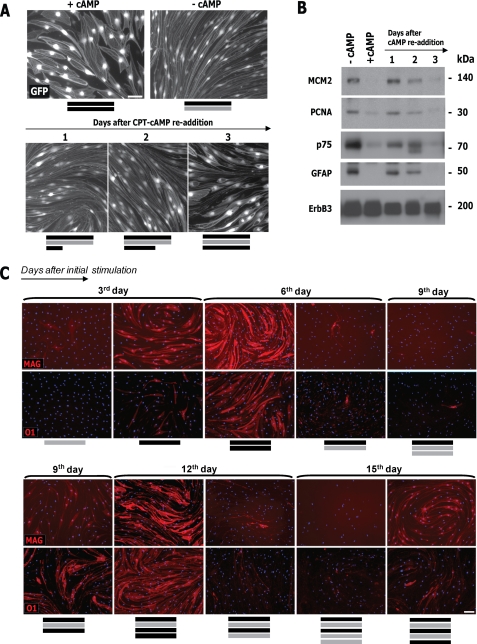
FIGURE 3.
Reversibility of cAMP-induced SC differentiation and dependence on cAMP. A, loss of myelin markers and induction of immature SC markers after cAMP deprivation. SCs were left untreated (Control) or treated with CPT-cAMP. On the third day poststimulation, culture medium was maintained (+cAMP) or replaced with medium without cAMP (−cAMP). Three days after, the expression of the indicated markers was analyzed by immunofluorescence microscopy. The expression of phosphorylated PKA substrates (P-PKAsub) is included to show the effectiveness of the cAMP treatment. B and C, temporal changes in SC morphology (B) and in the expression of SC-specific and proliferation markers (C) after cAMP deprivation. Conditions were as in A with the exception that SCs were lentivirally infected to express GFP, and medium containing vehicle (−cAMP; upper panels in B) or fresh CPT-cAMP (+cAMP; lower panels) was used for replacement. Cells were analyzed by immunofluorescence microscopy (B; GFP live fluorescence) and Western blot (C) at 1, 2, and 3 days after medium replacement. Note that differentiated SCs lose their reticulate appearance, indicative of the presence of intracellular vacuoles, and decrease their size shortly after cAMP removal, rendering cells that exhibit multiple processes (B, arrowhead). D, cAMP-stimulating agents prevent SC dedifferentiation. SCs were treated and analyzed as in A, with the exception that db-cAMP (1 mm), forskolin (20 μm), and cholera toxin (100 ng/ml) were included in the medium used for replacement. Black bars, presence of cAMP for 3 days; gray bars, absence of cAMP for 3 days. In B, the length of the bars is proportional to the incubation time period. DAPI was used to stain the cell nuclei (blue). Scale bars, 50 μm.
Live cell imaging of GFP-expressing SCs further revealed that the cells underwent a fast morphological transformation that coincided with the loss of the intracellular vacuoles within 24 h after the removal of the cAMP stimulus (Fig. 3B), indicating that the development of vacuoles was also a reversible feature of cAMP-induced differentiation (18). Importantly, different cell-permeable analogs of cAMP as well as agents that induce cAMP accumulation through different mechanisms, including forskolin and cholera toxin, effectively prevented SC dedifferentiation (Fig. 3D). This result suggests that a decrease in intracellular cAMP concentration but not the removal of an autocrine metabolite accumulated in the conditioned medium, was responsible for the maintenance of differentiation.
cAMP-induced Cell Growth Arrest Was Reversible upon cAMP Removal
We next analyzed whether SCs that had lost the expression of markers of differentiation recovered their ability to undergo proliferation. As shown in Fig. 3C, we observed that the levels of the G1-S markers MCM2 and PCNA did not increase upon the removal of the cAMP stimulus, indicating that SCs dedifferentiated without transitioning into the G1 phase in the absence of growth factors. We then measured the incorporation of [3H]thymidine and BrdU to assay the proliferative response of dedifferentiated SCs (i.e. cells deprived of cAMP-stimulating agents for 3 days) to growth factor stimulation. Results indicated that SCs deprived of cAMP, but not SCs maintained in cAMP-containing medium for an equivalent period of time, recovered their ability to transition into the S phase when exposed to growth factors (Fig. 4; shown only for neuregulin), which suggests that cAMP-induced cell cycle arrest was reversible upon the removal of the cAMP stimulus and subsequent exposure to individual mitogens. By labeling proliferative nuclei with BrdU, we confirmed that cAMP removal allowed SCs to dedifferentiate without undergoing S phase entry unless exposed to growth factors (Fig. 4, B and C). In addition, immunofluorescence detection of MCM2 and PCNA expression further confirmed that in the absence of growth factors, SCs dedifferentiated without transitioning into the G1 phase (Fig. 4, B and C). These results suggest that SC dedifferentiation was a prerequisite for S phase entry but that growth factors were required for the transition into the G1 and subsequent phases of the cell cycle.
FIGURE 4.
SCs become competent to resume proliferation after cAMP removal. Increase in DNA synthesis (A–C) and the expression of G1-S markers (B and C) in response to neuregulin in SCs deprived of cAMP-stimulating agents. SCs were grown for 3 days in the presence of CPT-cAMP and then for three additional days either in the absence (−cAMP) or presence (+cAMP) of CPT-cAMP before the addition of [3H]thymidine or BrdU together with the indicated mitogens. Cells were analyzed 3 days after mitogenic stimulation, as indicated in the figure. In B (right), the expression of phosphorylated ErbB3 on Tyr-1289 (P-ErbB3) is shown as a control for the effectiveness of neuregulin treatment. In C, the arrowheads point to representative BrdU-positive SCs expressing low but detectable levels of cell surface O1, indicative of dedifferentiation. DAPI staining is shown in blue. Scale bar, 50 μm.
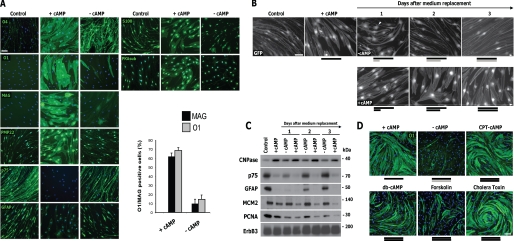
SCs Could Differentiate and Dedifferentiate Multiple Times without Entering the Cell Cycle
The results above suggested that SC differentiation and dedifferentiation were highly dependent on cAMP signals. Accordingly, SCs that had undergone one cycle of differentiation (cAMP administered for 3 days) followed by dedifferentiation (cAMP removed for 3 days) redifferentiated (e.g. reacquired the expression of myelin-associated markers) after receiving a second administration of cAMP analogs (Fig. 5). Live cell imaging of GFP-expressing SCs showed that a 3-day period of re-exposure to cAMP analogs was required for SCs to regain morphological differentiation (Fig. 5A). Time course studies confirmed that these morphological changes correlated with the induction of MAG and O1 expression (Fig. 5C) and the concomitant decrease in p75NGFR and GFAP expression (Fig. 5B). Strikingly, SCs underwent at least three cycles of differentiation in non-proliferating medium if cAMP-stimulating agents were alternatively added to or removed from the culture medium on a 3-day schedule (Fig. 5C). Because cAMP-stimulating agents were non-mitogenic when added in the absence of growth factors and serum (11), these results suggest that SCs redifferentiated without undergoing proliferation. In fact, the unchanged expression of proliferation markers confirmed the absence of cycling cells upon SC dedifferentiation and redifferentiation (Figs. 4, B and C, and 5B), further indicating that SCs alternate between differentiated and undifferentiated phenotypes without transitioning into the G1 phase unless exposed to growth factors. In summary, these results suggest that adult SCs are highly plastic in their response to cAMP and that the reduction of cAMP levels, but not signals emanating from activated RTKs, drives the withdrawal of the cells from the differentiated state.
FIGURE 5.
Cycles of SC differentiation and dedifferentiation under conditions non-supportive of proliferation. A and B, temporal changes in SC morphology (A) and in the expression of immature SC and proliferation markers (B) during SC redifferentiation. GFP-transduced SCs were subjected to alternating 3-day cycles of cAMP addition (+cAMP) and removal (−cAMP), as indicated by the black and gray bars, respectively. Cells were photographed live (A; GFP) or analyzed by Western blot for the markers indicated (B) at 1, 2, and 3 days after the readdition of CPT-cAMP. In B, note that prolonged cAMP treatment dramatically reduces the levels of MCM2 and PCNA expression. C, temporal changes in the expression of O1 and MAG in SCs undergoing multiple cycles of cAMP addition and removal. SCs (non-transduced) were deprived of mitogens and serum and subjected to alternating 3-day periods of cAMP presence (black bars) and absence (gray bars). At the times indicated, the cells were stained with MAG or O1 antibodies and analyzed by fluorescence microscopy. Of note, cells were maintained in non-proliferating medium throughout the time course of the experiment. Scale bars, 50 μm.
SC Dedifferentiation Was an Active Process That Required New Transcription and Translation
Evidence has accumulated that the onset of dedifferentiation requires the activation of a specific transcriptional program (27). To begin exploring whether SC dedifferentiation occurred through a passive or an active mechanism of action, SCs were deprived of cAMP analogs in the absence and presence of actinomycin D and cycloheximide, which are potent inhibitors of transcription and translation in eukaryotic cells, respectively. As shown in Fig. 6A (left), both of these inhibitors effectively prevented the loss of O1 expression and the recovery of a spindle-shaped morphology after cAMP removal, indicating that new RNA and protein biosynthesis was required for the phenotypic reversal of cAMP-induced differentiation. As controls, we show that actinomycin D and cycloheximide effectively prevented the induction of O1 expression and the reduction of GFAP expression after cAMP administration (Fig. 6A, right), suggesting that both differentiation and dedifferentiation require actively driven intracellular changes.
The Presence and Absence of cAMP Signals, but Not Growth Factor Signaling, Shifted the Balance between Early Transcriptional Promoters (Krox-20) and Inhibitors (c-Jun) of Myelination
An emerging concept in the SC field (28) is that the maintenance of the myelinated state is determined by a cross-antagonistic relationship between Krox-20, a master transcriptional regulator of myelination (29), and c-Jun, an inhibitor of myelination (8). Whereas Krox-20 is required for differentiation, c-Jun is required for dedifferentiation (8). Therefore, intracellular signaling cascades that decrease Krox-20 and/or increase c-Jun expression are expected to underlie the initiation of myelin loss and dedifferentiation (28). How Krox-20 and c-Jun are regulated by extracellular signals during dedifferentiation is not completely understood. To begin understanding the molecular mechanism that controls differentiation and dedifferentiation, we analyzed the changes in the expression of Krox-20 and c-Jun in SCs that were stimulated with and deprived of cAMP-stimulating agents, respectively. Results indicated that stimulation with cAMP was sufficient to rapidly (i.e. within 1 day) increase the expression of Krox-20 and concomitantly decrease the expression of c-Jun (Fig. 6B, top). Conversely, the levels of Krox-20 declined, and the levels of c-Jun increased 1–2 days after cAMP deprivation (Fig. 6B, bottom). Altogether, this indicates that persistent cAMP stimulation was required not only to initiate but also to maintain a high Krox-20/c-Jun ratio and, therefore, a differentiated state. However, we did not detect changes in the Krox-20/c-Jun ratio when differentiated SCs were treated with different growth factors, including neuregulin, or with 10% FBS in the presence of cAMP (Fig. 6C, right lanes), which is consistent with growth factors lacking an effect in driving dedifferentiation (Fig. 1). Importantly, these results were validated using cultures of both adult and postnatal SCs (Figs. 6C and 7 and supplemental Fig. 3). Growth factors also failed to change the expression of Krox-20 and c-Jun either when administered alone (Fig. 6C, left lanes) or concurrently with cAMP (Supplemental Fig. 2), which explains why these were not sufficient to promote differentiation or antagonize the differentiating effects of cAMP (18).
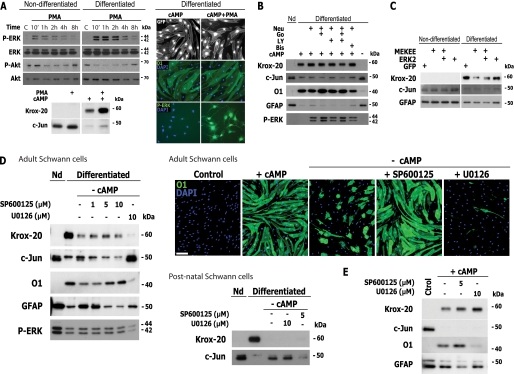
FIGURE 7.
Role of the JNK pathway, rather than MEK-ERK, on SC dedifferentiation. A, effect of PMA on SC dedifferentiation. Non-differentiated and cAMP-differentiated SCs were stimulated with PMA for the indicated times (A, top left panel), 30 min (A, bottom right panel), or 3 days (all other treatments), and the cells were analyzed by Western blot or immunofluorescence microscopy, as indicated. B, effect of Akt and PKC inhibition on neuregulin-stimulated ERK activation and on the expression of markers of differentiation. Differentiated SCs were stimulated with neuregulin either in the absence or presence of inhibitors of PKC (Gö6976 and bisindolylmaleimide; 1 μm) and PI3K-Akt (LY294002; 5 μm), and the expression of the indicated markers was evaluated 3 days after. C, effect of transfection of a constitutively activated MEK construct on dedifferentiation. Non-differentiated and differentiated SCs were transfected with MEKEE alone or in combination with ERK2, and the expression of the indicated markers was analyzed by Western blot 2 days after transfection. D, effect of JNK and MEK-ERK antagonists on SC dedifferentiation (D). SCs were deprived of cAMP (−cAMP) either in the absence or presence of the indicated concentrations of SP600125 or U0126, and the expression of the indicated markers was determined after 2 days (D). Results in adult SCs were validated using cultures of postnatal SCs, as indicated. The non-antagonistic effect of SP600125 and U0126 on the induction of SC differentiation by cAMP is shown as a control (E). Scale bars, 50 μm.
Evidence indicates that soluble neuregulin induces myelin loss without inducing axonal damage in vitro (30, 31). To further test whether neuregulin would counterbalance the effect of cAMP on maintaining the state of differentiation, we stimulated differentiated SCs with increasing concentrations of neuregulin peptide (Fig. 6D) and also administered neuregulin in combination with serum and other growth factors (Fig. 6E). The results indicated that concentrations of neuregulin ranging from 1 to 100 nm were not sufficient to reduce Krox-20 or enhance c-Jun expression (Fig. 6D), consistent with results shown in Fig. 1. Likewise, no apparent changes in the levels of expression of Krox-20 and c-Jun were observed when neuregulin was provided in combination with either 10% FBS or purified PDGF, IGF, and FGF (Fig. 6E), ruling out a possible synergistic contribution of different growth factors to the initiation of dedifferentiation.
Cumulatively, these results suggest that the presence and absence of cAMP signals initiate SC differentiation and its reversal, respectively, by shifting the balance between early transcriptional promoters (Krox-20) and inhibitors (c-Jun) of myelination and that this occurs independently of RTK signaling initiated by neuregulin or other growth factors.
The Activation of ERK by Growth Factors Was Not Sufficient to Drive Dedifferentiation
A previous study showed that persistent and selective ERK activation reduced the expression of Krox-20 and other myelin markers and induced demyelination of axons in co-cultures of SCs and dorsal root ganglion neurons (7). Therefore, we decided to explore other means to test whether ERK activation by growth factors was sufficient for dedifferentiation. Because growth factor-activated Akt, which displays promyelinating effects in SCs (26, 32), may counteract a possible dedifferentiating action of ERK, we investigated whether ERK activation would promote dedifferentiation in the absence of Akt signaling. We first chose to use the phorbol ester PMA, which by stimulating the activity of Raf, the upstream activating kinase for MEK, increases ERK signaling independently of Ras and RTK activation. PMA stimulated the phosphorylation of ERK in a manner similar to neuregulin but without increasing the phosphorylation of RTKs (not shown) or Akt (Fig. 7A, left). However, it failed to induce changes in the morphology of the cells (Fig. 7A, right) or the expression of markers of the myelinating and non-myelinating SC phenotype (Fig. 7A, right; shown only for O1). Although PMA induced strong ERK activation in differentiated SCs (Fig. 7A, right), it did not decrease Krox-20 or increase c-Jun expression (Fig. 7A, left), suggesting that ERK activation was not sufficient to drive dedifferentiation when activated alone or concurrently with Akt.
Because phorbol esters, as well as growth factors such as neuregulin, are also strong activators of PKC (Supplemental Fig. 3), we performed additional experiments to test whether ERK activation would induce dedifferentiation under conditions in which PKC and Akt activity were reduced. As a result, we stimulated ERK activation with neuregulin either in the absence or presence of pharmacological inhibitors of PKC (Gö6976 and bisindolylmaleimide) and PI3K-Akt (LY294002). Results indicated that neuregulin-induced ERK activation failed to change the ratio of Krox-20/c-Jun and the expression of other myelin and non-myelin markers under conditions in which both Akt and PKC activity were inhibited (Fig. 7B). This excludes the possibility that the activation of Akt, alone or together with PKC, would prevent neuregulin/ERK-mediated dedifferentiation.
We also transfected cAMP-treated SCs and undifferentiated SCs as a control with an expression vector encoding a constitutively active form of MEK1 (MEKEE), either alone or together with ERK2 (33). Consistent with published data (7), we observed that MEKEE overexpression decreased the expression of Krox-20 in differentiated SCs. Because the reduction of Krox-20 expression was not accompanied by an increase in the expression of c-Jun or other non-myelin genes (e.g. GFAP), we conclude that the selective activation of the ERK pathway may not be sufficient to drive all aspects of dedifferentiation.
The JNK-c-Jun Pathway, Rather than the MEK-ERK Pathway, Was Required for SC Dedifferentiation
Recent evidence has suggested that c-Jun is required for SC dedifferentiation. Conditional knock-out of c-Jun in SCs delays myelin sheath degradation after injury, whereas enforced expression of c-Jun inhibits myelination in vitro (8). Because we observed that c-Jun expression was increased upon a reduction in intracellular cAMP, we next studied whether there was a differential requirement of JNK versus MEK-ERK signaling upon the initiation of dedifferentiation because both of these mitogen-activated protein kinases (MAPKs) are known potential regulators of c-Jun expression and activity (34). We then used SP600125 and U0126 to interfere pharmacologically with the activity of JNK (35) and MEK (36), respectively, in SCs induced to dedifferentiate by cAMP deprivation. Results indicated that in adult and postnatal SCs, pretreatment with SP600125 effectively and dose-dependently prevented the loss of O1 expression, the reappearance of GFAP expression, and the recovery of a spindle-shaped morphology after the removal of the cAMP stimulus (Fig. 7D and supplemental Fig. 3). On the contrary, SCs that were deprived of cAMP in the presence of U0126 showed an enhanced expression of c-Jun along with markedly reduced levels of O1 and Krox-20 expression (Fig. 7D, left), clearly indicating that dedifferentiation required JNK but not MEK-ERK activity. An important observation was that SP600125 prevented c-Jun expression and dedifferentiation without preventing the loss of Krox-20 induced by cAMP deprivation, further suggesting that a reduction of Krox-20 expression was not sufficient for dedifferentiation. As specificity controls, we show that SP600125, but not U0126, prevented the induction of c-Jun expression in a dose-dependent manner and that U0126, but not SP600125, reduced the levels of ERK phosphorylation (Fig. 7D, left). As an additional control for the specific involvement of JNK in dedifferentiation, we show that preincubation of SCs with SP600125 did not prevent the induction of Krox-20 or the reduction of c-Jun expression induced by cAMP treatment (Fig. 7E), suggesting that JNK was required for the reversal but not for the initiation of differentiation by cAMP.
The non-requirement of MEK-ERK for dedifferentiation was confirmed by the observation that another broadly used MEK inhibitor, PD98059, did not prevent O1 loss after the removal of the cAMP stimulus (Supplemental Fig. 3). We did not find evidence indicating that SCs undergoing dedifferentiation would express higher levels of phosphorylated ERK (Figs. 6B and 7D, left) or that the extent of ERK phosphorylation would correlate with increased c-Jun or reduced Krox-20 expression (Figs. 6 (B and E) and 7 (A and B)). In conclusion, these results suggest that JNK activity is specifically required for the induction of c-Jun expression in SCs deprived of cAMP stimulation, providing a basis for the requirement of JNK-c-Jun, rather than MEK-ERK, during the onset of dedifferentiation.
DISCUSSION
To better understand how dedifferentiation occurs, it is essential to determine the relationship between the loss of the differentiated characteristics and cell cycle re-entry, two events that usually occur together during the initial stages of regeneration (27). In SCs, we found evidence indicating that proliferation was not required for, and therefore uncoupled to, the onset of dedifferentiation because the cells could undergo repeated cycles of differentiation and dedifferentiation in the absence of mitogenic factors and without entering the cell cycle. Although we found no evidence indicating that RTK and MEK-ERK signaling could promote the transition into an immature state or initiate S phase entry from the differentiated state, we found that not only the initiation but also the reversal of all aspects of differentiation was dependent on cAMP signals. We show that the reversible transition from a differentiated to an immature state relied on a simple on/off switch controlled by cAMP because an increase of cAMP, which enhanced Krox-20 and reduced c-Jun, was sufficient to promote SC differentiation, whereas a decrease in cAMP, which increased c-Jun and reduced Krox-20, was sufficient to promote SC dedifferentiation. It is noteworthy that dedifferentiation required an active signal that was dependent not only on a reduction of intracellular cAMP but also on JNK, rather than MEK-ERK, activity, whereas the initiation of differentiation required cAMP but not JNK activity.
Two main observations from our studies were that a reduction of cAMP was sufficient to reverse all aspects of differentiation and that cAMP presence/absence allowed SCs to shift between alternating differentiated and immature phenotypes, respectively, independently of growth factors and serum. An early study showed that SCs lost galactocerebroside expression when deprived of cAMP-stimulating agents in the presence of serum (24). We herein show that SCs lose not only galactocerebroside expression but also the expression of a variety of myelin markers, even in the absence of mitogens and serum, and that this occurs concurrently with the reacquisition of markers of the immature state, a bipolar morphology and proliferative capacity, all features indicating the full reversal of the differentiated state. We also show that cAMP addition and removal, but not stimulation with growth factors, induces rapid and persistent changes in the levels of Krox-20 and c-Jun, thereby explaining why cAMP, but not growth factors, exerts a prodifferentiating activity in SCs (18) and dedifferentiation occurs after the lowering of cAMP but not growth factor stimulation.
The identification of extracellular signals that drive dedifferentiation, as well as the associated intracellular pathways involved, may render important clues to how this process could be controlled (2). However, little is known about the factors responsible for the initiation of dedifferentiation and how this process is regulated by signaling cascades. We have identified that in SCs, a reduction of cAMP levels drives dedifferentiation at least in part by enhancing the expression of c-Jun in a JNK-dependent manner. JNK is a crucial enhancer of c-Jun expression and activation (37), and it has been shown in other cell types that cAMP elevation decreases JNK activity (38, 39). Along with the changes in the expression of c-Jun, a rapid reduction of Krox-20 expression preceded the onset of dedifferentiation. Surprisingly, we found that SCs would not dedifferentiate under conditions that prevented an increase in c-Jun despite the loss of Krox-20, suggesting that Krox-20 and c-Jun expression are regulated through a different mechanism of action during dedifferentiation and that a reduction of Krox-20 expression is not sufficient for dedifferentiation without an increase in c-Jun. Further investigation is required to better understand the molecular signals controlling c-Jun and Krox-20 expression and activation during dedifferentiation.
The collective data from the present study strongly support the concept that physiological activators of ERK, such as neuregulin-ErbB, are not sufficient to counteract the prodifferentiating effects of cAMP and therefore drive dedifferentiation, which we found to be essentially independent of MEK-ERK activity. This is consistent with our previous study showing that mitogens did not prevent the initiation of differentiation by cAMP (18). However, it is an unexpected observation when compared with data shown by others (7, 32) and thus opens the possibility that neuregulin and ERK initiate myelin breakdown through a mechanism not connected to the up-regulation of c-Jun during dedifferentiation. To confirm our results, we activated ERK by different stimuli, from a variety of growth factors that are effective mitogens for SCs to tumor promoters, and experiments were performed using cultures of adult and postnatal SCs. In addition, we explored possible dose-dependent effects of the growth factors and provided controls that demonstrate the effectiveness of the treatments activating ERK in differentiated SCs. Moreover, we tested the effect of growth factors when given alone and in combination to address possible synergistic effects and also the possibility that a counterbalancing effect of growth factor-activated Akt and PKC would prevent the action of ERK on dedifferentiation. We also expressed an activated form of MEK to selectively activate ERK in SCs and found that this was not sufficient to drive all aspects of dedifferentiation. Most importantly, the inactivation of ERK with different MEK inhibitors provided no indication that MEK-ERK was required for the induction of c-Jun and dedifferentiation under our experimental conditions. This was further supported by the observation that a signal that triggers dedifferentiation (i.e. cAMP removal) did not activate ERK and that no correlation could be established between the magnitude of the ERK signal and dedifferentiation, including expected changes in the levels of Krox-20 and c-Jun.
However, we cannot rule out a role of ERK in SC dedifferentiation. Because the ERK signal might differ considerably according to the type of stimulus, the involvement of ERK might be context-specific, as shown for the induction of dedifferentiation by Mycobacterium leprae infection (6). In a previous study (7), a high and persistent activation of the ERK pathway was induced by overexpressing an oncogenic form of Ras or a tamoxifen-inducible form of Raf. It is highly likely that the potency and kinetics of the induced ERK signal may simply not compare with those of physiological activators of ERK. The high degree of complexity in the regulation of the Raf/MEK/ERK module provides a basis for the involvement of ERK in highly diverse and occasionally contradictory cellular responses (40). Changes in the intensity and the duration of ERK activation can significantly alter cell fate (41). Indeed, the expression of activated Ras or Raf promotes cell growth arrest in SCs (7, 42), which clearly contrasts with the effects of growth factors that use the Ras-ERK pathway as one, but not the only, signaling route that leads to the G1-S transition (11, 26). In this sense, caution should be taken when comparing the effects of oncogenic versus mitogenic ERK signaling, and one should consider that other pathways activated by Ras or Raf may account for the dedifferentiating effect. Because we found that JNK, which is closely related to ERK, was required for dedifferentiation, the possibility exists that at least some of the effects seen on dedifferentiation were due to JNK rather than ERK and/or to an effect of Ras-ERK cross-talking with the JNK pathway because components of the ERK cascade may activate JNK and/or directly increase c-Jun expression (34).
Although neuregulin and RTK signaling had no apparent effects on SC differentiation/dedifferentiation, our results indicated that they had a crucial role on cell cycle re-entry in SCs that had lost their differentiated characteristics because these cells would remain quiescent for a prolonged period of time if not stimulated with growth factors. In addition, our results support the idea that the loss of a differentiated phenotype was a prerequisite for growth factor-initiated S phase entry and that these two processes were not only independent from one another but also controlled by a different mechanism of action. This is in agreement with evidence showing the independence between S phase re-entry and fragmentation of urodele myofibers (43) as well as with experiments on mice exhibiting a targeted disruption of cyclin D1, a G1 cyclin required for SC proliferation (23), which showed that distal SC proliferation was not required for demyelination (44), ensheathment, or the subsequent remyelination of regenerating axons (45).
Because of the differentiating effects of cAMP on cultured SCs, it has been suggested that cAMP controls myelination (14) and that this is mediated by PKA (46). However, more direct evidence was provided recently with the discovery of a novel G-protein-coupled receptor that is required for SC myelination in zebrafish and that it does so in a cAMP-dependent manner (47). We can therefore speculate that dedifferentiation and/or myelin loss may simply result from a reduction of cAMP in SCs, as those observed after injury (48). In support of this, we show that a reduction of intracellular cAMP is sufficient to trigger a dedifferentiating signal that directly impinges on the early transcriptional control of myelin gene expression. However, further investigation is required to test this interesting possibility in models of peripheral nerve injury.
The ability of SCs to dedifferentiate is crucial for the successful regeneration of the peripheral nervous system. SCs display an extraordinary degree of plasticity in their response to injury and were shown to remyelinate axons even in a series of nine repeated nerve crushes (49). Importantly, the autologous transplantation of SCs aimed at nervous tissue repair would not be feasible if SCs did not retain dedifferentiating capacity during adulthood (50). It is not clear why only certain somatic cell types in adult mammals can revert to an immature state that is proliferation-competent, whereas most cell types cannot. Thus, the plasticity displayed by SCs renders them an excellent system for the identification of novel pathways that control cellular differentiation and its reversal with intended applications in cell reprogramming for regenerative biology and medicine.
Supplementary Material
Acknowledgments
We thank R. Berg, L. Kuznesov, and A. Kanaya for technical assistance, M. Alcaraz for graphic design, and Drs. J. Bethea and P. Morton for critical discussion of the manuscript. We also thank Genentech, Inc. for β1-heregulin, Dr. Schachner for hybridoma cells, and Dr. Meijer for Krox-20 antibodies.
This work was supported, in whole or in part, by National Institutes of Health, NINDS, Grant NS009923. This work was also supported by the Miami Project to Cure Paralysis and the Buoniconti Fund.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1, 2, and 3.
- SC
- Schwann cell(s)
- RTK
- receptor tyrosine kinase
- db-cAMP
- N6-2′-O-dibutyryladenosine-3′,5′-cyclic monophosphate
- CPT-cAMP
- 8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphate
- IGF
- insulin-like growth factor
- PMA
- phorbol 12-myristate 13-acetate
- GFAP
- glial fibrillar acidic protein
- MAG
- myelin-associated glycoprotein
- PKA
- protein kinase A
- PCNA
- proliferating cell nuclear antigen
- NGFR
- nerve growth factor receptor.
REFERENCES
- 1.Brockes J. P., Kumar A. (2002) Nat. Rev. Mol. Cell. Biol. 3, 566–574 [DOI] [PubMed] [Google Scholar]
- 2.Straube W. L., Tanaka E. M. (2006) Artif. Organs 30, 743–755 [DOI] [PubMed] [Google Scholar]
- 3.Brockes J. P., Kumar A., Velloso C. P. (2001) J. Anat. 199, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z. L., Yu W. M., Strickland S. (2007) Annu. Rev. Neurosci. 30, 209–233 [DOI] [PubMed] [Google Scholar]
- 5.Guertin A. D., Zhang D. P., Mak K. S., Alberta J. A., Kim H. A. (2005) J. Neurosci. 25, 3478–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapinos N., Ohnishi M., Rambukkana A. (2006) Nat. Med. 12, 961–966 [DOI] [PubMed] [Google Scholar]
- 7.Harrisingh M. C., Perez-Nadales E., Parkinson D. B., Malcolm D. S., Mudge A. W., Lloyd A. C. (2004) EMBO J. 23, 3061–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkinson D. B., Bhaskaran A., Arthur-Farraj P., Noon L. A., Woodhoo A., Lloyd A. C., Feltri M. L., Wrabetz L., Behrens A., Mirsky R., Jessen K. R. (2008) J. Cell. Biol. 181, 625–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodhoo A., Alonso M. B., Droggiti A., Turmaine M., D'Antonio M., Parkinson D. B., Wilton D. K., Al-Shawi R., Simons P., Shen J., Guillemot F., Radtke F., Meijer D., Feltri M. L., Wrabetz L., Mirsky R., Jessen K. R. (2009) Nat. Neurosci. 12, 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrissey T. K., Levi A. D., Nuijens A., Sliwkowski M. X., Bunge R. P. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 1431–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monje P. V., Bartlett Bunge M., Wood P. M. (2006) Glia 53, 649–659 [DOI] [PubMed] [Google Scholar]
- 12.Stevens B., Ishibashi T., Chen J. F., Fields R. D. (2004) Neuron Glia Biol. 1, 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkinson D. B., Bhaskaran A., Droggiti A., Dickinson S., D'Antonio M., Mirsky R., Jessen K. R. (2004) J. Cell. Biol. 164, 385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessen K. R., Mirsky R., Morgan L. (1991) Ann. N.Y. Acad. Sci. 633, 78–89 [DOI] [PubMed] [Google Scholar]
- 15.Morrissey T. K., Kleitman N., Bunge R. P. (1991) J. Neurosci. 11, 2433–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearse D. D., Pereira F. C., Marcillo A. E., Bates M. L., Berrocal Y. A., Filbin M. T., Bunge M. B. (2004) Nat. Med. 10, 610–616 [DOI] [PubMed] [Google Scholar]
- 17.Brockes J. P., Fields K. L., Raff M. C. (1979) Brain Res. 165, 105–118 [DOI] [PubMed] [Google Scholar]
- 18.Monje P. V., Rendon S., Athauda G., Bates M., Wood P. M., Bunge M. B. (2009) Glia 57, 947–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato H., Miyazaki T., Fukai Y., Nakajima M., Sohda M., Takita J., Masuda N., Fukuchi M., Manda R., Ojima H., Tsukada K., Asao T., Kuwano H. (2003) J. Surg. Oncol. 84, 24–30 [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Lara I., González-Moles M. A., Ruiz-Avila I., Bravo M., Ramos M. C., Fernández-Martínez J. A. (1996) Acta. Stomatol. Belg. 93, 29–32 [PubMed] [Google Scholar]
- 21.Monje P. V., Athauda G., Wood P. M. (2008) J. Biol. Chem. 283, 34087–34100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzer J. L., Bunge R. P., Glaser L. (1980) J. Cell. Biol. 84, 767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H. A., Ratner N., Roberts T. M., Stiles C. D. (2001) J. Neurosci. 21, 1110–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobue G., Shuman S., Pleasure D. (1986) Brain Res. 362, 23–32 [DOI] [PubMed] [Google Scholar]
- 25.Schlessinger J. (2000) Cell 103, 211–225 [DOI] [PubMed] [Google Scholar]
- 26.Maurel P., Salzer J. L. (2000) J. Neurosci. 20, 4635–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straube W. L., Brockes J. P., Drechsel D. N., Tanaka E. M. (2004) Cloning Stem Cells 6, 333–344 [DOI] [PubMed] [Google Scholar]
- 28.Jessen K. R., Mirsky R. (2008) Glia 56, 1552–1565 [DOI] [PubMed] [Google Scholar]
- 29.Topilko P., Schneider-Maunoury S., Levi G., Baron-Van Evercooren A., Chennoufi A. B., Seitanidou T., Babinet C., Charnay P. (1994) Nature 371, 796–799 [DOI] [PubMed] [Google Scholar]
- 30.Zanazzi G., Einheber S., Westreich R., Hannocks M. J., Bedell-Hogan D., Marchionni M. A., Salzer J. L. (2001) J. Cell. Biol. 152, 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syed N., Reddy K., Yang D. P., Taveggia C., Salzer J. L., Maurel P., Kim H. A. (2010) J. Neurosci. 30, 6122–6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogata T., Iijima S., Hoshikawa S., Miura T., Yamamoto S., Oda H., Nakamura K., Tanaka S. (2004) J. Neurosci. 24, 6724–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monje P., Marinissen M. J., Gutkind J. S. (2003) Mol. Cell. Biol. 23, 7030–7043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitmarsh A. J., Davis R. J. (1996) J. Mol Med. 74, 589–607 [DOI] [PubMed] [Google Scholar]
- 35.Bennett B. L., Sasaki D. T., Murray B. W., O'Leary E. C., Sakata S. T., Xu W., Leisten J. C., Motiwala A., Pierce S., Satoh Y., Bhagwat S. S., Manning A. M., Anderson D. W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Favata M. F., Horiuchi K. Y., Manos E. J., Daulerio A. J., Stradley D. A., Feeser W. S., Van Dyk D. E., Pitts W. J., Earl R. A., Hobbs F., Copeland R. A., Magolda R. L., Scherle P. A., Trzaskos J. M. (1998) J. Biol. Chem. 273, 18623–18632 [DOI] [PubMed] [Google Scholar]
- 37.Angel P., Hattori K., Smeal T., Karin M. (1988) Cell 55, 875–885 [DOI] [PubMed] [Google Scholar]
- 38.Rao G. N., Runge M. S. (1996) J. Biol. Chem. 271, 20805–20810 [DOI] [PubMed] [Google Scholar]
- 39.Harada Y., Miyatake S., Arai K., Watanabe S. (1999) Biochem. Biophys. Res. Commun. 266, 129–134 [DOI] [PubMed] [Google Scholar]
- 40.Peyssonnaux C., Eychène A. (2001) Biol. Cell. 93, 53–62 [DOI] [PubMed] [Google Scholar]
- 41.Roovers K., Assoian R. K. (2000) BioEssays 22, 818–826 [DOI] [PubMed] [Google Scholar]
- 42.Ridley A. J., Paterson H. F., Noble M., Land H. (1988) EMBO J. 7, 1635–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velloso C. P., Kumar A., Tanaka E. M., Brockes J. P. (2000) Differentiation 66, 239–246 [DOI] [PubMed] [Google Scholar]
- 44.Kim H. A., Pomeroy S. L., Whoriskey W., Pawlitzky I., Benowitz L. I., Sicinski P., Stiles C. D., Roberts T. M. (2000) Neuron 26, 405–416 [DOI] [PubMed] [Google Scholar]
- 45.Yang D. P., Zhang D. P., Mak K. S., Bonder D. E., Pomeroy S. L., Kim H. A. (2008) Mol. Cell. Neurosci. 38, 80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon C., Korade Z., Carter B. D. (2008) J. Neurosci. 28, 3738–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monk K. R., Naylor S. G., Glenn T. D., Mercurio S., Perlin J. R., Dominguez C., Moens C. B., Talbot W. S. (2009) Science 325, 1402–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poduslo J. F., Walikonis R. S., Domec M. C., Berg C. T., Holtz-Heppelmann C. J. (1995) J. Neurochem. 65, 149–159 [DOI] [PubMed] [Google Scholar]
- 49.Thomas P. K. (1970) J. Anat. 106, 463–470 [PMC free article] [PubMed] [Google Scholar]
- 50.Fortun J., Hill C. E., Bunge M. B. (2009) Neurosci. Lett. 456, 124–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.