Abstract
SPRET/Ei mice are extremely resistant to acute LPS-induced lethal inflammation when compared with C57BL/6. We found that in vivo SPRET/Ei mice exhibit strongly reduced expression levels of cytokines and chemokines. To investigate the role of the potent anti-inflammatory glucocorticoid receptor (GR) in the SPRET/Ei phenotype, mice were treated with the GR antagonist RU486 or bilateral adrenalectomy. Under such conditions, both C57BL/6 and SPRET/Ei mice were strongly sensitized to LPS, and the differences in LPS response between SPRET/Ei and C57BL/6 mice were completely gone. These results underscore the central role of GR in the LPS hyporesponsiveness of SPRET/Ei mice. Compared with C57BL/6, SPRET/Ei mice were found to express higher GR levels, which were reflected in increased GR transactivation. Using a backcross mapping strategy, we demonstrate that the high GR transcription levels are linked to the Nr3c1 (GR) locus on chromosome 18 itself. Unexpectedly, SPRET/Ei mice exhibit a basal overactivation of the hypothalamic-pituitary-adrenal axis, namely strongly increased corticosterone levels, ACTH levels, and adrenocortical size. As a consequence of the excess of circulating glucocorticoids (GCs), levels of hepatic gluconeogenic enzymes are increased, and insulin secretion from pancreatic β-cells is impaired, both of which result in hyperglycemia and glucose intolerance in SPRET/Ei mice. We conclude that SPRET/Ei mice are unique as they display an unusual combination of elevated GR expression and increased endogenous GC levels. Hence, these mice provide a new and powerful tool for the study of GR- and GC-mediated mechanisms, including immune repressive functions, neuroendocrine regulation, insulin secretion, and carbohydrate metabolism.
Keywords: Bacterial Toxins, Cytokine, Diabetes, Mouse Genetics, Nuclear Receptors
Introduction
The Mus spretus species has been used to establish several inbred strains, including SPRET/Ei, SEG/Pas, and STF/Pas. M. spretus or Algerian mice and the Mus musculus or house mice diverged about 1.5 million years ago, which results in a great amount of genetic polymorphisms present in the genome of these wild-derived inbred strains compared with the common laboratory M. musculus strains. The ability to produce interspecific crosses and backcrosses between M. spretus and M. musculus, despite their evolutionary divergence (1) and the high degree of sequence variation between them, allows accurate genetic mapping of complex traits (2). Furthermore, due to their high sequence variation, M. spretus strains provide a great amount of additional phenotypic variation (3). As an example, when compared with the standard laboratory mouse strain C57BL/6, SPRET/Ei mice exhibit a strong and dominant resistance against the lethal effects of several inflammatory mediators, such as lipopolysaccharide (LPS) and TNF (4, 5). Both can induce an exaggerated inflammatory response mediated partly by the activation of potent transcription factors such as NFκB and AP1. The LPS-induced response is called endotoxemia, which is a commonly used model for studying sepsis.
Sepsis and septic shock remain an important cause of death in intensive care units despite the availability of various therapeutics (6), such as glucocorticoids (GCs)3 (7). The fact that around 750,000 cases of severe sepsis occur annually in the United States (8) underscores the need for further extensive research. Synthetic GCs (e.g. dexamethasone), which are among the most potent anti-inflammatory molecules, are widely used to treat several inflammatory diseases (9). The biological action of GCs is exerted primarily through activation of the cytoplasmic glucocorticoid receptor (GR), which belongs to the nuclear hormone receptor superfamily (10). GR plays an important role during development and is involved in numerous physiological and pathological processes, including maintenance of homeostasis, behavior, regulation of central nervous system functions, and cell proliferation and survival (11). In addition, GR modulates the immune response mainly by transrepressing NFκB and AP1 (9, 12) and to a lesser extent by transactivational induction of anti-inflammatory proteins such as Mkp1 and Gilz (13). The GR-mediated actions reduce the expression of pro-inflammatory mediators, such as cytokines, chemokines, adhesion molecules, and enzymes (14). Although GCs are among the most commonly used drugs in the clinic to relieve inflammation and various immune disorders (15, 16), therapeutic use of GCs is hampered by the occurrence of GC resistance (17) and several adverse side effects of GCs (18). The latter include diabetes, impaired wound healing, skin and muscle atrophy, hypertension, and osteoporosis (19).
Secretion of GCs (corticosterone (CS) in rodents; cortisol in humans) is controlled by the hypothalamic-pituitary-adrenal (HPA) axis, which shows circadian activity and a strong increase in activity in response to stress. The activation of the HPA axis is initiated with the release of corticotrophin-releasing hormone (CRH) and arginine-vasopressin (AVP) from the hypothalamus. These neurohormones bind to specific receptors to act synergistically on the corticotropic cells of the anterior pituitary gland and induce the secretion of adrenocorticotropic hormone (ACTH), a cleavage product of pro-opiomelanocortin (POMC), in circulation (20, 21). Next, ACTH stimulates the adrenal glands to synthesize and release GCs. By binding to GR, circulating GCs exert negative feedback at the level of the pituitary and the hypothalamus and centrally at the level of the hippocampus, leading to repression of hypothalamic CRH and pituitary POMC expression, which eventually results in the return to homeostasis (21, 22). Alterations in the number of GR molecules or their sensitivity can influence HPA axis activity and, in particular, can regulate hormone levels by mediating the strength of GC feedback inhibition (23, 24).
To gain greater insight into GC physiology in vivo, several GR mutant mice have been generated. Study of loss-of-function mice (25–29) revealed pivotal roles for GR in lung maturation, the negative feedback control of the HPA axis, and carbohydrate metabolism. Moreover, studies on a gain-of-function GR knock-in mouse and a mutant mouse overexpressing the GR have been described (30, 31) and confirmed the importance of tight regulation of GR expression and consequently HPA axis activity for the control of physiological and pathological processes. For example, GR-overexpressing mice showed increased resistance to endotoxemia as well as increased suppression of the HPA axis, leading to reduced CS and ACTH levels (30).
In this study we investigated the molecular mechanism mediating the strong resistance of SPRET/Ei mice to LPS-induced lethal inflammation. We show that the expression of a broad set of inflammatory genes is impaired in SPRET/Ei mice in basal condition as well as upon LPS challenge. Studying the LPS response after GR blockage or adrenalectomy revealed that this reduced gene expression profile is mediated by GR, which appeared to be overexpressed in SPRET/Ei mice. Moreover, transcriptional activity of GR-inducible genes is enhanced in these mice. However, in contrast to GR-overexpressing mutant mice (30, 31), SPRET/Ei mice display overactivation of the HPA axis, leading to a large increase in ACTH and CS levels. The excess of endogenous GC in the SPRET/Ei mice provides an interesting tool for studying the regulation of the neuroendocrine system. Finally, as a consequence of the chronically elevated GC levels, glucose tolerance is severely altered in SPRET/Ei mice. Thus, the SPRET/Ei inbred mouse strain, displaying both high GR and CS levels and activity, will be useful for further elucidation of the mechanisms of GR in physiological and pathological processes.
EXPERIMENTAL PROCEDURES
Mice
Normal and adrenalectomized (adx) C57BL/6J mice were purchased from Janvier (Le Genest-St. Isle, France). SPRET/Ei mice were obtained from The Jackson Laboratories (Bar Harbor, ME) and bred in our facility. Bilateral adrenalectomy of SPRET/Ei mice was performed under isoflurane anesthesia. The drinking water of adx mice was supplemented with 0.9% NaCl. The mice were kept in individually ventilated cages under a constant dark-light cycle in a conventional animal house and received food and water ad libitum. All mice were used at the age of 8–12 weeks, and adx mice were used two weeks after surgery. Animal experiments were approved by the institutional ethics committee for animal welfare of the Faculty of Sciences, Ghent University, Belgium.
Reagents
LPS from Salmonella abortus equii and RU486 (Mifepristone), a GR and progesterone receptor antagonist, were purchased from Sigma. Mice were injected intraperitoneally with LPS in 0.25 ml of pyrogen-free phosphate-buffered saline (PBS) or with 5 mg of RU486 in 0.05 ml of DMSO. Dexamethasone (Dex, a GR synthetic ligand) was purchased from Medini, and macrophage colony-stimulating factor was from Peprotech (ImmunoSource, eBioscience).
Hormone and Cytokine Measurements
Mice were housed individually for at least 3 days before blood sampling to obtain basal, nonstressed hormone levels. Blood was collected from the retro-orbital plexus at 6:00 p.m. within 30 s of handling the cage. Serum and EDTA plasma for measurement of CS and ACTH, respectively, were immediately separated by centrifugation and frozen at −20 °C. Concentrations of CS and ACTH were determined using commercially available radioimmunoassay kits with 125I-corticosterone (COAT-A-COUNT, Diagnostic Products Corp., Los Angeles, CA) and 125I-ACTH (MP Biomedicals Europe), respectively, according to the manufacturers' instructions. For evaluation of stress-induced release of CS and ACTH, blood samples were collected after withholding food for 20 h. Serum IL-6 was determined with a 7TD1 bioassay (32), and IFNβ levels were assessed using a commercial ELISA kit (PBL Biomedical Laboratories). Serum samples were also assayed for several cytokines using Luminex technology (Bio-Rad) following the manufacturer's protocol.
Quantitative Trait Loci (QTL) Mapping
To map the loci responsible for the high GR mRNA levels in SPRET/Ei mice, an interspecific backcross between female (C57BL/6 x SPRET/Ei) F1 mice and male C57BL/6 mice was set up. Livers from 172 N2 backcross mice were excised at the age of 8 weeks and snap-frozen in liquid nitrogen. RNA was isolated, and cDNA was used to perform quantitative real time PCR (qPCR) analysis for GR. Two housekeeping genes, Gapdh and Tbp, were used for normalization. A genome scan was performed with 81 microsatellite markers. Coverage of the genome was estimated by taking the position of the marker loci on the Mouse Genome Data base mouse genetic map obtained from The Jackson Laboratory and applying a swept radius of 20 centimorgan (33). After the first screening, the density of markers was increased on chromosome 18, which was shown to be linked to the trait. Linkage analysis was performed with the MapManager QTX (34) software program using the “marker regression” and “simple interval mapping” tools. The latter analysis evaluates the association between the phenotype and the expected contribution of hypothetical quantitative trait loci at multiple analysis points between each pair of adjacent marker loci. The analysis point yielding the most significant association may be regarded as the location of the putative quantitative trait loci. Significance levels were determined by performing a permutation test, which empirically determines likelihood ratio statistic (LRS) thresholds corresponding to suggestive linkage (genome-wide type I error probability of 0.63), significant linkage (error probability of 0.05), and highly significant linkage (error probability of 0.001). PCR was performed on 100 ng of tail genomic DNA.
qPCR
Tissues samples were collected in RNA Later (Qiagen), and RNA was isolated with the RNeasy Mini kit (Qiagen) according to the manufacturer's instructions. RNA concentration was measured with the Nanodrop 1000 (Thermo Scientific), and 1 μg of RNA was used to prepare cDNA with Superscript II (Invitrogen). qPCR was performed using the Roche LightCycler 480 system (Applied Biosystems). The best performing housekeeping genes were determined by Genorm for each organ (35). Results are given as relative expression values normalized to the geometric mean of the housekeeping genes.
Western Blot Analysis
Proteins in whole tissue lysates (50 μg) were separated by electrophoresis on a 7.5% gradient SDS-polyacrylamide gel and transferred onto nitrocellulose filters. The membranes were incubated overnight at 4 °C with primary antibodies against GRα (1:1000; Santa Cruz, sc-1004, Europe) and actin (1:10000; MP Biomedicals, Europe) as an internal control. After being washed with Tris-buffered saline containing 0.1% Tween 20, the blots were incubated for 1 h at room temperature with anti-rabbit antibody (1:10,000, IRDye 800, LI 926-32211, The Netherlands) and anti-mouse antibody (1:15000, IRDye 680, LI 926-32220, Westburgh, The Netherlands). Normalization was done using the Odyssey 2.1 software.
In Vitro Stimulation of Bone Marrow-derived Macrophages with Dexamethasone
Bone marrow cells were isolated from femurs and tibias of mice. To derive macrophages, after centrifugation (5 min, 1200 rpm, 4 °C) cells were resuspended in 50 ml DMEM (Invitrogen) supplemented with 10% fetal calf serum, 100 units/ml penicillin/streptomycin, 2 mm l-glutamine, 1 mm sodium pyruvate, and 20 ng/ml mouse recombinant macrophage colony-stimulating factor. Bone marrow cells were cultured in bacterial Petri dishes for 7 days, and every 2 days the medium of the adherent cells was refreshed. After 7 days of differentiation, bone marrow-derived macrophages were detached with endotoxin- and enzyme-free cell dissociation buffer (Sigma) and counted by trypan blue exclusion, and the final concentration was adjusted to 1 × 106 cells/ml in DMEM medium supplemented with growth factors. Next, cells were plated out in 6-well plates at 1 × 106 cells/well, and 1 μm Dex was added. Six hours after stimulation, macrophages were lysed with TRIzol (Invitrogen) for RNA isolation.
Weight of Adrenal Gland and Histology of Adrenal Gland, Pancreas, and Pituitary Gland
For analysis of adrenal gland weight, adult mice of each species were evaluated at 2–3 months of age. Whole body weight was measured, and bilateral adrenals were dissected free of surrounding fat. The weight of both adrenals was determined and corrected for body weight to generate the corresponding adrenal weight index values.
Adrenals and pancreases were fixed in 4% paraformaldehyde overnight before embedding in paraffin. Sections of 4 μm were cut and stained with hematoxylin/eosin, and pancreatic sections were also stained by an immunoperoxidase technique for insulin and glucagons. Primary antibodies included rabbit anti-insulin (1:1000) and rabbit anti-glucagon (1:1000), which were purchased from Santa Cruz and Zymed Laboratories Inc., respectively. HRP-labeled anti-rabbit secondary antibody (1:1000) and diaminobenzidine were purchased from Dakopatts. Sections were incubated with primary antibodies overnight at 4 °C and with the secondary antibody for 60 min at room temperature. After the sections were incubated with the chromogen diaminobenzidine, the sections were counterstained with hematoxylin.
Pituitaries were carefully isolated under the stereomicroscope and immediately fixed using paraformaldehyde (4%). Pituitaries were embedded in agarose (Lonza, 2% in PBS) before sectioning with the Vibratome (HM650V, Microm, Walldorf, Germany). Pituitaries were attentively positioned to cut 50-μm sections through all lobes and cleft. Vibratome sections were stored in PBS at 4 °C until additional processing by immunofluorescence. Sections were permeabilized with two 30-min incubations of Triton X-100 (0.1% in PBS; PBST). Aspecific binding sites were preadsorbed with 20% normal goat serum (DakoCytomation, Glostrup, Denmark) in PBST. Sections were incubated overnight at room temperature with a primary antibody: rabbit/rat-anti-ACTH (1/5000; from Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA). For negative controls, the primary antibody was omitted. After thorough washing in PBST, sections were incubated for 1.5 h with an Alexa Fluor 555-conjugated antiserum, goat anti-rabbit (Molecular Probes, Eugene, OR; 1:1000 in PBST). Nuclei were counterstained with the DNA binding dye ToPro3 (Invitrogen). Sections were mounted on glass slides using Vectashield (Vector Laboratories, Burlingame, CA), gently covered with glass coverslips, and stored at −80 °C until analysis. Sections were scanned using a confocal laser scanning microscope (LSM 510, Zeiss, Zaventem, Belgium) in multitrack mode. Specifications for Alexa Fluor 555 were excitation was at 543 nm and emission at 560–615 nm through a NFT545, and for ToPro-3, excitation was at 633 nm, and emission was above 650 nm.
Glucose and Insulin Tolerance Tests
Glucose tolerance in mice was assessed after a 16-h fast. Serial blood samples were taken 0, 30, 60, 120, 180, and 360 min after intraperitoneal injection of 2 g of glucose per kg of body weight. Blood glucose was measured using One-Touch glucose strips and a blood glucose meter (LifeScan Scotland Ltd.). Serum insulin was measured using a commercial kit (rat/mouse insulin ELISA kit; Linco Research, Millipore). Insulin tolerance was determined in fasted animals by injecting insulin intraperitoneal (1 units/kg body weight Actrapid Penfill; Novo Nordisk). Ten minutes later, blood glucose was measured (0 min), and a bolus of glucose was given intraperitoneally to the animals. Blood glucose was then determined at the same time intervals used in the glucose tolerance test.
Dex/CRH Test
The combined Dex/CRH test was performed following the protocol described by Heuser et al. (36). Basal evening CS levels of the animals were obtained 2 weeks before the Dex/CRH test. On the day of testing, all animals received a Dex intraperitoneal injection (50 μg/kg body weight; Medini) at 11 a.m. Six hours later blood was isolated from the retro-orbital plexus, and the animals were intraperitoneally injected with CRH (0,15 mg/kg body weight; rat/human CRH from Sigma). A second blood sample was obtained 30 min after CRH challenge.
Statistics
Survival curves (Kaplan-Meyer plots) were compared by a log-rank test, and final outcomes were by a χ2 test. Data were expressed as the means ± S.E. Statistical significance of differences between groups was evaluated with Student's t tests with 95% confidence intervals and with one-way or two-way analysis of variance. Error bars in the figures represent the mean ± S.E. *, **, and *** represent p < 0.05, p < 0.01, and p < 0.001, respectively.
RESULTS
LPS-resistant SPRET/Ei Mice Show Broad Defects in Gene Expression upon LPS Stimulation
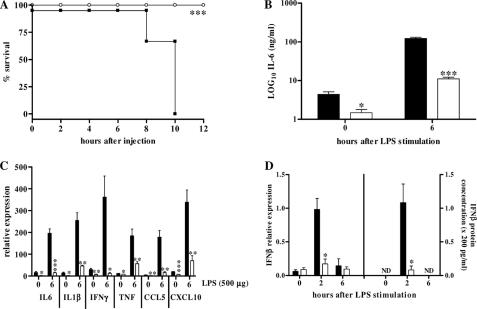
Mahieu et al. (4) previously reported that inbred SPRET/Ei mice, derived from M. spretus, exhibited a dominant resistance against LPS-induced lethality when compared with the commonly used laboratory mouse strains, such as C57BL/6. We repeated these findings; SPRET/Ei mice are indeed highly resistant to a dose of 500 μg of LPS, in contrast to the control C57BL/6, all of which died within 10 h due to LPS-induced lethal shock (Fig. 1A). Next, IL-6 levels, which are good markers to assess sensitivity in acute endotoxemia (37), were measured in circulation 0 or 6 h after LPS stimulation. The significantly lower IL-6 levels in SPRET/Ei confirmed the significant protection of SPRET/Ei mice to LPS compared with C57BL/6 mice (Fig. 1B).
FIGURE 1.
SPRET/Ei mice are very resistant to LPS lethality and show impaired basal and LPS-induced gene expression. Survival (A) and serum IL-6 levels in C57BL/6 and SPRET/Ei mice after intraperitoneal injection of 500 μg of LPS (B) are shown. Mortality was monitored for 60 h (no further deaths occurred). Black bars, C57BL/6 mice (n = 6); white bars, SPRET/Ei mice (n = 4). C, LPS-induced gene expression in liver shows reduced levels of GR-transrepressed genes in SPRET/Ei mice compared with control C57BL/6 mice. C57BL/6 (n = 4, black bars) and SPRET/Ei mice (n = 4, white bars) were injected with 500 μg of LPS intraperitoneally, and mRNA was isolated 0 and 6 h after injection. qPCR was used to measure mRNA levels of a set of GR-repressed genes encoding IL-6, IL-1β, IFNγ, TNF, CCL5, and CXCL10. D, shown are IFNβ mRNA (left) and protein (right) levels in C57BL/6 (n = 3, black bars) and SPRET/Ei (n = 3, white bars) mice at different time points after LPS (500 μg) stimulation. ND, not detectable. Significance was calculated for difference between C57BL/6 and SPRET/Ei.
To study the LPS hyporesponsiveness of SPRET/Ei mice in more detail, a dose of 500 μg of LPS was given intraperitoneal, and the expression of several genes was measured in livers and lungs 0 and 6 h after injection. In both organs of SPRET/Ei mice, induction of all measured pro-inflammatory cytokines and chemokines was compromised in SPRET/Ei mice. There were large differences between SPRET/Ei and C57BL/6 mice in the expression levels of these genes in both basal and LPS-stimulated conditions (Fig. 1C; other pro-inflammatory mediators showed similar results as well as in lungs). Previously, we documented a defective IFNβ production in LPS-stimulated SPRET/Ei mice (4), which was confirmed here at mRNA and protein levels (Fig. 1D). This broad defect in gene expression indicates that the LPS resistance of SPRET/Ei mice is likely to be mediated by the increased activity of a broad anti-inflammatory molecule.
SPRET/Ei Mice Show Increased GR Levels and Increased GR-mediated Transactivation
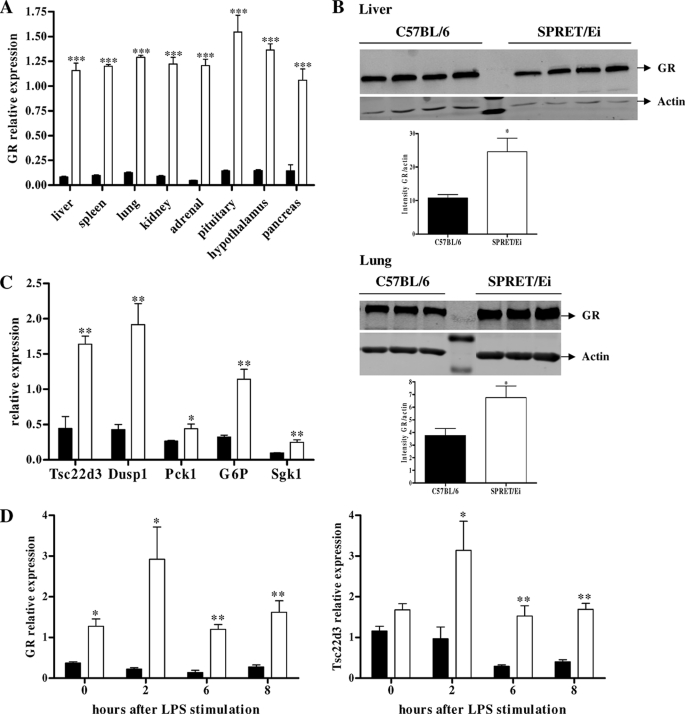
Based on the fact that GCs and GR repress inflammation and the observed reduced expression of numerous cytokines in SPRET/Ei mice upon LPS challenge, we assessed total GR mRNA expression in multiple organs from SPRET/Ei and C57BL/6 mice. The basal GR mRNA levels were significantly higher in all tested organs of SPRET/Ei mice compared with the levels in the control C57BL/6 mice (Fig. 2A). In addition, we determined the GR protein levels in several organs by Western blot analysis. We found significantly higher GR levels in SPRET/Ei livers, but the difference with C57BL/6 was not as prominent as the difference in mRNA levels (Fig. 2B; the proteins levels in spleen and kidney showed similar differences (results not shown)).
FIGURE 2.
GR levels and activity are enhanced in SPRET/Ei mice. A, shown is qPCR analysis of GR mRNA expression in various tissues of C57BL/6 (black bars; n = 5) and SPRET/Ei (white bars; n = 5). B, GR protein levels were measured by Western blot analysis in livers (upper panels) and lungs (lower panels) of C57BL6 and SPRET/Ei mice. The GR (94 kDa) bands were normalized to the intensities of the actin (42 kDa) bands. C, analysis of the mRNA expression of several GR-inducible genes (Tsc22d3, Dusp1, Pck1, G6p, and Sgk) in the liver of unstimulated C57BL/6 (n = 4, black bars) and SPRET/Ei mice (n = 4, white bars) is shown. D, shown are GR (left panel) and Gilz (right panel) mRNA levels in C57BL/6 (n = 3, black bars) and SPRET/Ei (n = 3, white bars) in response to LPS (500 μg). ***, significance levels for differences between C57BL/6 and SPRET/Ei.
We also examined whether the strong GR expression in SPRET/Ei mice was reflected in increased expression of GR-dependent genes by determining the relative mRNA levels of genes coding for Tsc22d3 (Gilz), Dusp1 (Mkp1), Pck1, G6p, and Sgk1. The significantly stronger expression of all these genes in SPRET/Ei mice (Fig. 2C) is consistent with increased transactivation activity of GR in vivo.
Furthermore, we determined the relative expression levels of GR at different time points after LPS challenge, which indicated that the differential expression is enhanced in response to LPS. In addition, the increased GR activity in SPRET/Ei mice was even more pronounced upon LPS stimulation as the difference in Gilz transcription levels between C57BL/6 and SPRET/Ei mice augmented after LPS (Fig. 2D).
Next, we wondered whether other M. spretus lines exhibit the same phenotype as SPRET/Ei mice. Therefore, we tested SEG/Pas mice for their LPS sensitivity and cytokine levels upon LPS stimulation as well as their GR/GC levels and associated GC-mediated phenotypes. These results clearly showed that SEG/Pas mice were susceptible to a high dose of LPS, although to a lesser extent than the C57BL/6 mice (supplemental Fig. S1A). The LPS sensitivity of SEG/Pas mice was consistent with increased levels of pro-inflammatory mediators after LPS challenge (supplemental Fig. S1B). In addition, intermediate levels of GR were found in different organs of SEG/Pas mice when compared with C57BL/6 and SPRET/Ei mice (supplemental Fig. S2), which reflects their intermediate LPS sensitivity.
SPRET/Ei Mice Lose LPS Resistance upon Blocking GR
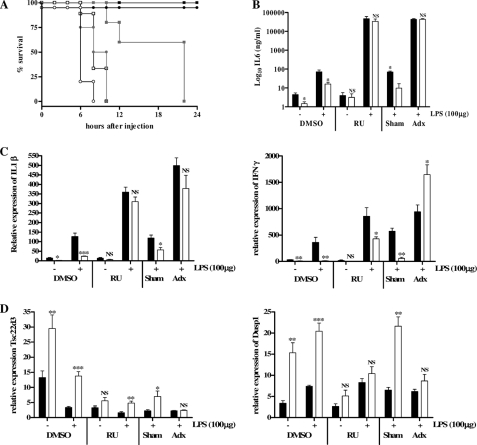
To examine whether GR plays a role in the LPS resistance of SPRET/Ei mice, we treated C57BL/6 and SPRET/Ei mice with RU486 (mifepristone), a non-selective GR antagonist. Additionally, we adx (removal of the adrenal glands) SPRET/Ei and C57BL/6 mice. We first determined the lowest lethal dose of LPS in RU486-challenged and in adx C57BL/6 mice, as it was reported that disruption of the GC-GR axis by bilateral adrenalectomy or by RU486 sensitizes mice to LPS- and TNF-induced inflammation (38). This proved to be 5 μg of LPS (data not shown). This dose of LPS was injected intraperitoneally in SPRET/Ei and C57BL/6 mice after RU486 administration or adx. Irreversible blocking of GR with RU486 or eliminating GCs by adrenalectomy rendered SPRET/Ei mice as sensitive to low doses of LPS as C57BL/6 mice (Fig. 3A). Thus, the strong LPS resistance of SPRET/Ei was completely abolished by both treatments, suggesting that GR signaling confers the extreme resistance of SPRET/Ei mice against the toxic effects of LPS.
FIGURE 3.
Injection of RU486, a GR antagonist, and adrenalectomy sensitize SPRET/Ei mice to LPS and completely abolish the LPS resistance of SPRET/Ei. A, shown is survival of C57BL/6 and SPRET/Ei mice after LPS (5 μg) challenge. Both mouse strains were divided in three groups of which one was treated with DMSO (control) and the other with 5 mg of RU486, and the third group was adrenalectomized. Mortality was monitored for 30 h (no further deaths occurred). Black square, LPS control C57BL/6 (n = 4); gray square, LPS/RU486 (RU)-treated C57BL/6 (n = 5); □, LPS/adx C57BL/6 (n = 9); filled circle, LPS control SPRET/Ei (n = 3); gray circle, LPS/RU486-treated SPRET/Ei (n = 4); ○, LPS/adx SPRET/Ei (n = 5). B, IL-6 concentration in serum after injection of 100 μg of LPS in DMSO-treated, RU486-treated, and adx C57BL/6 (black bars; n = 4 in each group) and SPRET/Ei (white bars; n = 4 in each group) mice is shown. C and D, shown is relative mRNA expression of two pro-inflammatory cytokines (Il-1β, left graph; IFNγ, right graph; C) and two GR-inducible genes (Tsc22d3, left graph; Dusp1, right graph; D). These levels were determined using qPCR analysis of liver samples of DMSO-treated, RU486-treated, and adx C57BL/6 (black bars; n = 4 in each group) and SPRET/Ei mice (white bars; n = 4 per group) after challenge with 100 μg of LPS. Significance was calculated for differences between C57BL/6 and SPRET/Ei. NS, no significant difference.
To further document this protective effect of GR in SPRET/Ei, we measured cytokine levels in circulation 6 h after challenge with 100 μg of LPS. We did not use the low dose of 5 μg of LPS, as this dose is not lethal for C57BL/6 mice, and the LPS-induced difference in cytokine expression between the two species would not be visible. Cytokine levels were measured using Luminex technology, and as expected, the serum levels of all tested pro-inflammatory cytokines upon LPS stimulation was impaired in SPRET/Ei mice, but after blocking GR by RU486 treatment or performing adx, the levels reached similar high levels in both mouse strains (Fig. 3B; levels of other cytokines showed similar results but are not shown). Similarly, basal and LPS-induced mRNA expression of IL-1β and IFNγ was reduced in SPRET/Ei mice, but the same LPS induction profile was observed in both species when GR was blocked or adrenals removed (Fig. 3C; levels of other cytokines are not shown). Although SPRET/Ei mice displayed persistent reduced IFNγ mRNA levels in response to RU486 and LPS, the -fold change induction compared with the LPS-stimulated condition is much greater in SPRET/Ei than in C57BL/6 mice. Notably, LPS-induced cytokine levels also strongly increased in the control C57BL/6 mice upon GR blockage, consistent with the enhanced LPS sensitivity. These results indicate that the GC-GR axis is essential in repressing pro-inflammatory gene induction after LPS injection and that the reduced cytokine induction in SPRET/Ei mice is entirely due to an increased activity of the CS-GR axis. Taken together, these data confirm the major role of GR for protection against an excessive inflammatory response.
We also investigated the expression profile of two GR-inducible genes, Tsc22d3 (Gilz) and Dusp1 (Mkp1), both of which have anti-inflammatory properties. As already depicted in Fig. 2C, SPRET/Ei mice showed significantly higher basal mRNA levels compared with the control mice. We found that this difference was preserved after LPS challenge (see also Fig. 2D) and that after GR blockage or adx, the expression of both genes decreased in both C57BL/6 and SPRET/Ei mice without differences in expression (Fig. 3D). Although the induction of Tsc22d3 in SPRET/Ei mice remained higher than in C57BL/6 mice in response to RU486, there was a strong decrease of the Tsc22d3 mRNA levels in SPRET/Ei mice compared with the non-RU486-treated condition. These findings suggest that the high transcription levels of GR-inducible genes in SPRET/Ei depend on increased GR-mediated transactivation.
The High GR mRNA Levels in SPRET/Ei Are Genetically Linked to the GR Locus on Chromosome 18
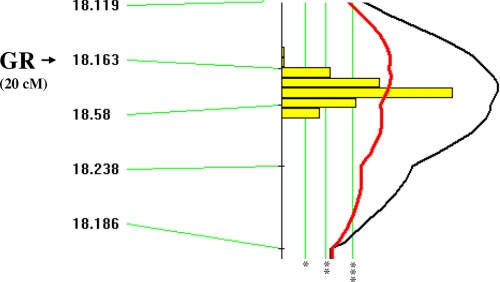
To map the loci conferring the high GR mRNA levels in SPRET/Ei mice, an interspecific backcross was set up, as described under “Experimental Procedures.” GR expression and genotype data were introduced into the MapManager QTX program (34). Linkage analysis was performed by “marker regression,” which identified highly significant linkage of the high SPRET/Ei GR transcription levels to loci on chromosome 18 (Table 1). Next, the GR expression data were randomly permuted 5000 times among the progeny. Suggestive linkage corresponded to an LRS value of 6.0, significant linkage to an LRS value of 11.1, and highly significant linkage to 16.9. By using these empirically determined criteria for significance, we found a highly significant linkage between chromosome 18 and the increased levels of GR transcription in SPRET/Ei mice. To locate the putative quantitative trait loci more precisely on chromosome 18, a simple interval mapping analysis was performed, and this showed strongest linkage to the markers D18Mit163 and D18Mit58 located on chromosome 18 (Fig. 4). This localization was rather precise, as can be deduced from the 95% confidence intervals (Table 1). Because these markers segregate with the Nr3c1 locus, which encodes the GR gene, a sequence variation in the Nr3c1 locus is probably responsible for the high GR expression levels observed in SPRET/Ei mice. This result was confirmed by R/ quantitative trait loci, an additional mapping program (data not shown).
TABLE 1.
Linkage of genetic markers located on chromosome 18 to the high transcription levels of GR in SPRET/Ei mice
| Markera | Positionb | nc | LRS | %d | p valuee | 95% CIf |
|---|---|---|---|---|---|---|
| D18Mit119 | 16 | 170 | 21.5 | 12 | <10−5 | 26 |
| D18Mit163 | 20 | 80 | 21.9 | 12 | <10−5 | 26 |
| D18Mit58 | 24 | 158 | 47.7 | 24 | <10−5 | 13 |
| D18Mit238 | 31 | 164 | 39.9 | 21 | <10−5 | 15 |
| D18Mit186 | 45 | 168 | 16.7 | 9 | 0.00004 | 33 |
a Microsatellites.
b Expressed in centimorgans, according to the Mouse Genome Informatics website.
c Population size.
d Observed variance attributed to this locus as a percent of total trait variance.
e Point-wise p values; the probability of obtaining the observed LRS value by chance.
f Confidence interval according to Darvasi and Soller (82).
FIGURE 4.
High levels of GR mRNA in SPRET/Ei are genetically linked to the Nr3c1 (GR) locus on chromosome 18. Simple interval mapping using the MapManager QTX program reveals that a locus on chromosome 18 is strongly linked with the increased GR levels. The black line indicates the LRS value (see also Table 1), and the red line represents an additive linkage. *, suggestive level (genome wide type I error probability of 0.63); **, significant level (genome wide type I error probability of 0.05); ***, highly significant level (genome wide type I error probability of 0.001). cM, centimorgan.
GR polymorphisms have been associated with variability of mRNA stability and altered transcriptional activity by GR (for review, see Ref. 39). Because we found genetic linkage of high SPRET/Ei GR mRNA levels with the GR locus on chromosome 18, we sequenced the coding region, 3′-untranslated region (UTR), and 2000 bp upstream of the transcription start site of exon 1A to identify polymorphisms unique to the SPRET/Ei genome. In total we identified 64 single nucleotide polymorphisms (SNPs), 9 insertions, and 5 deletions in the SPRET/Ei sequence compared with the C57BL/6 sequence. The sequence variations in the SPRET/Ei DNA sequence were confirmed by the Sanger Institute, who recently made the whole-genome sequence of the SPRET/Ei strain available (Welcome Trust Sanger Institute Mouse Genomes Project).
SPRET/Ei Mice Show Increased Activity of HPA Axis
It is well known that the induction of GCs is strictly regulated by the HPA axis. Because GR plays an important role in the negative feedback control of the HPA axis (40), increased GR expression normally leads to down-regulation of CS by the adrenals (41).
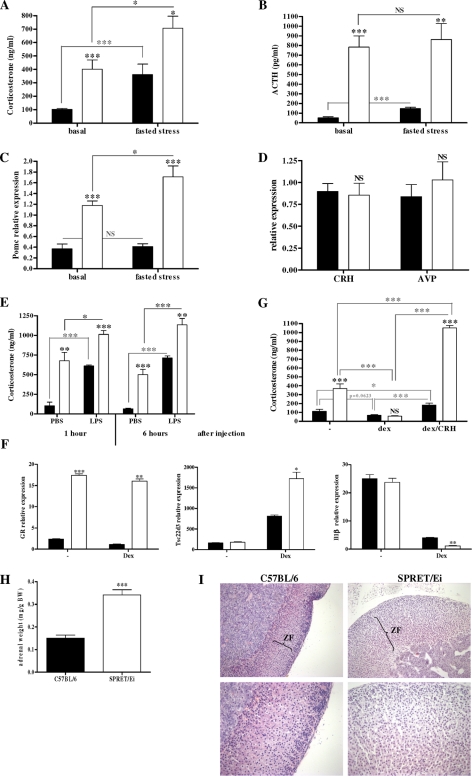
To check whether SPRET/Ei mice also exhibit reduced activity of the HPA axis, we measured basal serum CS and plasma ACTH levels. Unexpectedly, we found that SPRET/Ei mice had significantly increased basal CS (4-fold) and ACTH (16-fold) levels compared with C7BL/6 mice (Fig. 5, A and B). According to the high levels of circulating ACTH, the expression of POMC mRNA in the anterior lobe of the pituitary gland was significantly higher in SPRET/Ei mice than in the control C57BL/6 mice (Fig. 5C). However, hypothalamic Crh and Avp mRNA levels were similar in both genotypes (Fig. 5D). These data indicate that the HPA axis function differs markedly between the two mouse strains and reveal that the HPA axis in SPRET/Ei up-regulates CS production by enhancing release of ACTH. Additionally, this suggests that there might be a defective feedback repression at the level of the pituitary.
FIGURE 5.
SPRET/Ei mice exhibit increased HPA axis activity. A, circulating CS levels were measured in serum from unstimulated C57BL/6 (black bars; n = 24) and SPRET/Ei mice (white bars; n = 17) and in C57BL/6 (n = 6) and SPRET/Ei mice (n = 8) after 20 h of fasting. B, circulating plasma ACTH levels from unstimulated C57BL/6 (black bars; n = 13) and SPRET/Ei mice (white bars; n = 14) and in C57BL/6 (n = 5) and SPRET/Ei mice (n = 4) after 20 h of fasting are shown. C, shown are relative Pomc mRNA levels in pituitary glands from C57BL/6 (n = 8) and SPRET/Ei mice (n = 7) and in C57BL/6 (n = 4) and SPRET/Ei mice (n = 4) after 20 h of fasting. D, relative Crh and Avp mRNA levels in the hypothalamus from C57BL/6 (n = 4) and SPRET/Ei mice (n = 4) are shown. E, shown are serum CS levels in mice injected intraperitoneally with PBS or LPS: C57BL/6 (black bars; n = 4) and SPRET/Ei mice (white bars; n = 4). F, qPCR analysis of GR, Tsc22d3, and Il-1β in untreated or Dex-stimulated bone marrow-derived macrophages from C57BL/6 (black bars; n = 3) and SPRET/Ei (white bars; n = 3) is shown. G, evening CS levels and CS levels 2 weeks later upon Dex and Dex/CRH challenge in C57BL/6 (n = 8) and SPRET/Ei mice (n = 8) are shown. H, mean adrenal weight of 2–3-month-old female and male C57BL/6 (black bars; n = 11) and SPRET/Ei mice (white bars; n = 7). Adrenal weight is expressed as mg/g of body weight (BW). I, histological sections of adrenal glands show low (×20; upper panels) and high (×40; lower panels) power views of hematoxylin/eosin-stained adrenal tissue sections derived from C57BL/6 (left panels) and SPRET/Ei mice (right panels). The upper panels show the extent of zona fasciculata (ZF; arrows), which reveals a marked expansion of the adrenal cortex in SPRET/Ei mice. The lower panels display the degree of hypertrophy in SPRET/Ei mice compared with the control mice. ***, significance levels calculated for differences between C57BL/6 and SPRET/Ei, unless otherwise noted; NS, no significant difference.
CS and ACTH hormones and POMC mRNA levels were measured after induction of food deprivation stress. This led to an increase in circulating CS and ACTH levels in both C57BL/6 and SPRET/Ei mice, but the levels remained significantly higher in SPRET/Ei mice (Fig. 5, A and B, right), indicating that the response to stress is normal in SPRET/Ei mice. The function of the adrenal glands was further investigated in SPRET/Ei mice under experimental stress conditions, namely LPS-induced endotoxemia. It has been reported that LPS stimulates various levels of the HPA axis (42). Therefore, having observed increased CS production in SPRET/Ei mice, we investigated CS levels in response to LPS challenge. When LPS was injected in C57BL/6 mice, we observed a significant increase in the release of CS 1 and 6 h after LPS administration but not after saline injection. A similar up-regulation was seen in SPRET/Ei mice, but the levels remained significantly higher in SPRET/Ei mice than in the control animals (Fig. 5E).
The elevated levels of GCs in SPRET/Ei mice raised the question of whether increased CS rather than increased GR levels mediate the LPS protective effects in SPRET/Ei. Therefore, we performed an in vitro experiment using bone marrow-derived macrophages, which were left untreated or stimulated with Dex. mRNA expression analysis of GR demonstrated that SPRET/Ei macrophages also contain increased GR transcription levels compared with C57BL/6 cells. In addition, in response to equal amounts of Dex SPRET/Ei macrophages showed increased GR transactivation and transrepression action, as indicated by the higher mRNA levels of Tsc22d33 and the lower levels of Il-1β, respectively (Fig. 5F). These findings suggest that a role for the SPRET/Ei GR protein cannot be excluded.
We next tested whether the alteration in the levels of the main components of the HPA axis had any functional consequences for the activity and reactivity of the HPA axis. As already mentioned, when subjected to food deprivation stress, both species mounted a similar CS response (Fig. 5, A and B), which suggests that SPRET/Ei mice display normal induction of HPA axis activity in response to stress. Next, the negative feedback regulation was investigated by a Dex suppression test, which showed that Dex was equally effective in suppressing CS secretion in both mouse strains. A subsequent CRH challenge, however, resulted in a significantly stronger increase of CS in SPRET/Ei animals (Fig. 5G). These dynamic tests of the HPA axis suggest that the endogenous CS excess in SPRET/Ei mice results from increased sensitivity of pituitary corticotropic cells to CRH rather than from a defect in negative feedback inhibition. Furthermore, we performed ACTH staining of sections of the anterior pituitary of C57BL/6, SEG/Pas, and SPRET/Ei mice, which indicated that no corticotrophic adenoma could be found in the pituitary gland of SPRET/Ei mice as ACTH-positive cells were homogenously distributed in SPRET/Ei pituitaries. These ACTH stainings confirm the higher ACTH levels in SPRET/Ei mice compared with C57BL/6 mice (supplemental Fig. S3).
Because the CS levels were very high in SPRET/Ei mice, we looked at the size and morphology of their adrenals, as adrenal cortical hyperfunction is a known consequence of increased ACTH secretion and leads to increased CS concentration (43). The ratio of adrenal weight to body weight ratio in SPRET/Ei mice was twice that of C57BL/6 mice (Fig. 5H), which is consistent with the higher CS levels in SPRET/Ei mice. The higher adrenal weight is not an artifact caused by the smaller body weight of SPRET/Ei mice because differences were significant even when uncorrected for variations in body weight. Histological examination of adrenal glands from SPRET/Ei mice confirmed these findings. We found that the adrenal architecture of SPRET/Ei mice was markedly altered when compared with C57BL/6 mice, with clear cortical thickening. In particular, the zona fasciculata of the adrenal cortex, which is responsible for CS production (44), was thicker, whereas the zona glomerulosa and medullary cells appeared relatively unaffected. Further histological examination indicated that the zona fasciculata is wider because of cell hypertrophy, indicated by an increased volume of eosinophilic cytoplasm per cell and decreased number of cells per field (Fig. 5I). Evidently, SPRET/Ei mice suffer from adrenal hypertrophy. Altogether, our results suggest that the high serum levels of CS observed in SPRET/Ei mice are a consequence of marked morphological alteration of the adrenal cortex, probably due to higher ACTH production, which is in turn due to high sensitivity to CRH of the pituitary glands in SPRET/Ei mice.
Again, we measured some of these parameters in SEG/Pas mice and compared these with the levels observed in C57BL/6 and SPRET/Ei mice. This showed that SEG/Pas mice display an intermediate level of HPA axis activity, as indicated by their intermediate levels of CS, POMC, ACTH, and adrenal weight (supplemental Fig. S4, A–D).
High GC-GR Activity Leads to Glucose Intolerance and Impaired Insulin Secretion in SPRET/Ei Mice
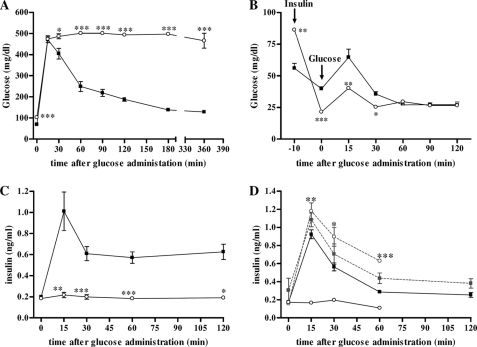
Chronic exposure to elevated levels of GCs leads to metabolic dysfunctions, including hyperglycemia and insulin resistance (45). Furthermore, animals overexpressing GR in pancreatic tissue manifest diabetes at 12–15 months of age (46). Therefore, we expected to observe similar undesirable side effects of high circulating CS in SPRET/Ei animals. Indeed, these mice showed significantly elevated fasting blood glucose levels (102.8 ± 14.06 mg/dl; n = 9) compared with C57BL/6 mice (69.4 ± 9.75 mg/dl; n = 10; p < 0.0001). Next, a glucose tolerance test showed that SPRET/Ei mice are glucose intolerant because they showed impaired clearance of glucose, which was injected intraperitoneal By contrast, C57BL/6 animals showed a glucose peak 15 min after glucose administration, but the glucose levels returned to base-line levels within 120 min (Fig. 6A).
FIGURE 6.
SPRET/Ei mice are glucose-intolerant and secrete no insulin after glucose administration due to their elevated levels of GCs. A, shown is a glucose tolerance test response of C57BL/6 (■; n = 10) and SPRET/Ei mice (○; n = 9) to intraperitoneal insulin injection. B, shown is an insulin tolerance test response of C57BL/6 (■; n = 10) and SPRET/Ei mice (○; n = 9) to intraperitoneal insulin injection (time point −10 min) followed by glucose injection (time point 0). C, serum insulin levels were determined at several time points after glucose injection in C57BL/6 (■; n = 6) and SPRET/Ei mice (○; n = 5). ***, significance levels for differences between C57BL/6 and SPRET/Ei. D, serum insulin levels were determined at several time points after glucose injection in C57BL/6 (black square, control, n = 5; gray square, adx, n = 3) and SPRET/Ei (open circle and full line, control, n = 5; open circle and dashed line, ADX, n = 3). Significance was calculated for differences between control and adx groups of both mouse strains.
GCs exert their diabetogenic effect by increasing hepatic glucose production, inducing peripheral insulin resistance, and decreasing insulin secretion (47, 48). The former is mediated mainly by GC-mediated induction of gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6-phosphatase (G6P). Indeed, as depicted in Fig. 2B, SPRET/Ei mice display significantly higher basal levels of both enzymes. An insulin tolerance test used to determine insulin resistance in SPRET/Ei mice showed that glucose elimination after intraperitoneal insulin administration was similar to C57BL/6 animals, suggesting that insulin sensitivity was not reduced in SPRET/Ei mice (Fig. 6B). However, this indicates a possible impairment of insulin synthesis and/or secretion by β-cells in the endocrine pancreas of SPRET/Ei mice. To assess the GC-mediated inhibitory effect on pancreatic β-cell function, we determined serum insulin levels after glucose administration. This test clearly showed that almost no circulating insulin levels could be detected upon glucose injection in SPRET/Ei mice, in contrast to C57BL/6 mice, which showed a normal insulin response to glucose (Fig. 6C). In addition, glucose and insulin levels were measured in adx C57BL/6 and SPRET/Ei mice upon glucose administration. Adx SPRET/Ei mice showed significantly lower-fasted glucose levels compared with normal SPRET/Ei mice, and the difference between C57BL/6 and SPRET/Ei mice was abrogated. Also, adx SPRET/Ei mice displayed normal glucose clearance after glucose injection (data not shown), reflected in an increase in insulin levels, in contrast to sham-operated hyperglycemic SPRET/Ei mice (Fig. 2D). This confirms the causative role of increased GC levels in the glucose intolerance and the impairment in glucose-stimulated insulin secretion in SPRET/Ei mice. In addition, consistent with the intermediate GC serum levels in SEG/Pas mice, glucose clearance was less efficacious in SEG/Pas mice compared with C57BL/6 mice but better than in SPRET/Ei mice, and they produced intermediate levels of insulin in response to glucose (supplemental Fig. S5, A and B).
To determine whether the reduced insulin levels observed in SPRET/Ei mice were associated with morphological changes or increased apoptosis in β-cells (49), we performed a histological examination of pancreatic tissues stained with hematoxylin and eosin derived from both C57BL/6 and SPRET/Ei mice. This revealed that SPRET/Ei mice and the control mice have similar amounts of islets of Langerhans tissue. In addition, the central regions of the islets in both mouse strains consisted of normal insulin-producing β-cells, whereas glucagon-producing cells were located peripherally in the mantle zone (figures not shown). Thus, no morphological differences between the groups were observed as well as no increased apoptosis in SPRET/Ei pancreases was visible (data not shown). This indicates that the morphology of the islets in SPRET/Ei animals was not affected by increased circulatory GCs and GR overexpression in the pancreas.
As there was no reduction in β-cell mass and no alteration in morphology of β-cells in SPRET/Ei, we investigated insulin production in β-cells of SPRET/Ei mice because it was shown that GCs may decrease insulin biosynthesis by reducing the ATP/ADP ratio inside the β-cells (50). We first measured Ins2 mRNA levels in pancreases of fasted mice 30 min after glucose injection. This showed that the expression of insulin mRNA by pancreases of SPRET/Ei mice was not affected but were even a bit higher than in C57BL/6 pancreases (Ins2 mRNA, C57BL/6 0.645 ± 0.44; SPRET/Ei 1.42 ± 0.30; p = 0.0286). In addition, staining for insulin in endocrine pancreatic tissues showed equal amounts of insulin in C57BL/6 and SPRET/Ei mice upon glucose challenge. The normal insulin production together with the reduced glucose-stimulated circulatory insulin levels in SPRET/Ei mice suggested that the excessive amounts of GC disturb insulin secretion in SPRET/Ei mice.
DISCUSSION
Previous results from our group revealed that SPRET/Ei, an inbred strain derived from M. spretus, is extremely resistant to LPS-induced lethal inflammation (4). We believe that this model is of great relevance in the search for new treatments for sepsis, which remains one of the leading causes of death in intensive care units.
We demonstrate that expression of a broad set of pro-inflammatory cytokines and chemokines was reduced in SPRET/Ei mice upon an LPS challenge when compared with the levels in C57BL/6 mice. Notably, the expression of TLR4 was comparable in SPRET/Ei and C57BL/6 bone marrow-derived macrophages (data not shown), which indicates that lower TLR4 levels are not responsible for the reduced LPS sensitivity observed in SPRET/Ei. Moreover, it is well known that GCs exert strong anti-inflammatory properties, mainly by counteracting the production of pro-inflammatory cytokines, such as IFNβ, IL-1β, IL-6, and TNF (for review, see Refs. 9, 14, and 51). This anti-inflammatory effect involves transrepression mechanisms, such as tethering protein-protein interactions, between GR and other transcription factors, particularly NFκB and AP1 (7, 9). Indeed, during severe inflammation, a coordinated response of the neuroendocrine and immune systems is crucial for survival (52, 53).
We studied the GC-GR axis activity in SPRET/Ei mice. SPRET/Ei mice displayed ubiquitous GR mRNA and protein overexpression accompanied by increased transcriptional activity of GR in basal and LPS-stimulated conditions. However, the increase of protein expression levels in SPRET/Ei organs was not as prominent as the higher GR mRNA levels in SPRET/Ei mice. This might be explained by ligand-dependent homologous down-regulation of GR protein levels as GCs levels are strongly augmented in SPRET/Ei mice (54). Thus, it is likely that elevated GC levels in addition to high GR expression in SPRET/Ei mice account for their protective phenotype, as discussed below. It has been reported that enhanced GR signaling protects the organism from an overwhelming immune response. The finding that an increased GR gene dosage improves the resistance of mice to inflammation (30) could mean that the resistance of SPRET/Ei mice to the deleterious effects of endotoxin is due to the increased GR levels and activity. We confirmed this by using a GR antagonist and by eliminating the endogenous ligands of GR by adrenalectomy, as both lead to complete loss of the resistance of SPRET/Ei mice against LPS lethality. Consequently, expression of pro-inflammatory cytokines increased greatly upon blockage of GR and reached similar levels in both mouse strains. These data confirm that the GC/GR axis mediates the repression of cytokine expression and secretion and that the consequent development of endotoxic shock depends on the level of GR. Notably, our data and unpublished work suggest that the increased GR/GC levels are the driving force of the reduced IFNβ expression that we reported earlier (4). Thus, increased GR levels are essential and sufficient to cause the strong LPS hyporesponsiveness of SPRET/Ei animals. Similar results were obtained with GR-overexpressing mutant mice, although the LPS resistance was not as powerful as in the SPRET/Ei mouse strain (30). Besides inhibiting gene transcription by transrepression, GR also exerts anti-inflammatory activities by inducing transcription of GR-responsive genes, such as Tsc22d3 and Dusp1 (9). SPRET/Ei mice showed higher expression levels of both anti-inflammatory genes under both basal and LPS-stimulated conditions, which suggests that these proteins might contribute to the strong LPS resistance of SPRET/Ei mice. It has been reported that these GR-inducible genes, by virtue of the anti-inflammatory activities of their protein, have a strong impact on inflammatory gene expression (13, 55–57). In addition, the prominent role of Dusp1 (Mkp1) in the control of excessive inflammation has been confirmed in Mkp1-deficient mice, which showed decreased resistance to LPS-induced lethal inflammation (56). Moreover, upon GR blockage the expression of these genes was reduced strongly, and the differences between C57BL/6 and SPRET/Ei mice disappeared, indicating that the increased levels observed in SPRET/Ei are indeed mediated by GR. Thus, our findings confirm the strong anti-inflammatory properties of GR and its protective role against development of endotoxic shock.
We tried to identify the genetic basis of the high GR transcription levels observed in SPRET/Ei by performing a genome-wide linkage analysis. Our results indicate that this phenotype is strongly linked to the GR locus on chromosome 18 and suggests that a sequence variation in that region leads to increased levels of GR mRNA. Several gene variants located in the Nr3c1 locus have been shown to result in changes in transcriptional capacity or to affect stability of the mRNA (for review, see Ref. (39)). Hence, we sequenced the promoter, coding region, and 3′-UTR of the Nr3c1 gene and found 78 sequence variations, of which 64 were SNPs and 14 were insertions or deletions. This large genetic variation between M. spretus and M. musculus can be attributed to their evolutionary divergence (3). None of the found variations in the SPRET/Ei sequence corresponds to known GR gene variants, which were described to be associated to varying GR levels and/or function (39). Thus, further functional research is required to identify the polymorphisms contributing to the high GR expression levels in SPRET/Ei mice.
Most of the known GR SNPs also result in changes in the regulation of the HPA axis at different levels (39), as it is known that GR plays an essential role in the negative feedback control of the HPA axis, mainly at the level of the hypothalamus and the pituitary (21, 40). This mechanism ensures proper regulation of serum GC levels and quick return to homeostasis after challenges such as stress and inflammation (58). Consequently, several GR mutants show changes in the basal regulation of the HPA axis. For example, heterozygous and homozygous GR knock-out mice display enhanced HPA axis activity (25, 26, 59, 60), whereas mutant mice with increased GR ligand affinity or GR gene dosage display reduced expression of the main components of the HPA axis (30, 31). These studies indicate a critical role of GR signaling in normal regulation of the HPA axis. Because GR mRNA levels were also significantly elevated in SPRET/Ei hypothalamus and pituitary gland, which are the primary sites of direct negative feedback regulation of the HPA axis, we hypothesized that regulation of the HPA axis is disturbed in SPRET/Ei mice. Nevertheless, SPRET/Ei mice exhibit a strongly activated HPA axis manifested in higher basal ACTH and CS levels. In addition, it is known that adrenal hypertrophy is a consequence of increased ACTH secretion in the absence of normal negative feedback control of the HPA axis by GCs (43). Accordingly, the adrenal glands of SPRET/Ei mice clearly displayed cortical hypertrophy. This suggests that the negative feedback control seems to be disturbed in SPRET/Ei mice, although they express higher GR transcriptional levels. Because hypothalamic CRH mRNA levels are similar in both SPRET/Ei and control mouse strains, we hypothesized that GR-mediated feedback inhibition in SPRET/Ei mice is defective at the level of the pituitary. ACTH stainings of the anterior pituitary gland of SPRET/Ei mice did not show any evidence that the increased ACTH levels might result from corticotrophic adenomas.
However, a Dex suppression/CRH stimulation test, which indirectly measures GR function in pituitary, showed that Dex administration resulted in similar down-regulation of GC levels in C57BL/6 and SPRET/Ei mice, indicating that defective negative feedback is likely not the cause of the increased basal levels of CS observed in SPRET/Ei mice. Interestingly, an additional CRH stimulation resulted in an overshoot of HPA axis activity in SPRET/Ei mice, suggesting hypersensitization of POMC for CRH-mediated transcriptional induction. CRH exerts its actions on corticotroph cells by binding to CRH receptors 1 (CRH-R1). Although CRH-R1 is essential for ACTH release and regulation of the number of CRH-R1 may have an important role in cell responsiveness to CRH, CRH-R1 content in the pituitary does not correlate directly with corticotroph responsiveness (61, 62). This suggests that the levels of CRH-R1 in SPRET/Ei mice might not directly explain their hyperresponsiveness to CRH. In addition, several inhibitory effects on CRH action and release have been described, and decreased expression of these inhibitory molecules might be involved in the HPA axis phenotype of SPRET/Ei mice. For example, (i) CRH-binding protein is known to block CRH-mediated ACTH secretion (63), (ii) the soluble splice variant of CRH-R2 blocks CRH-cellular actions (64), and (iii) opioid peptides act by suppression of the CRH release at the hypothalamic level (65). Conversely, enhanced stimulation of CRH release and CRH-mediated ACTH release by catecholamines (66) and orexins (67), respectively, might also play a role in the overactivation of the SPRET/Ei HPA axis. Our findings indicate that the SPRET/Ei mouse strain is a suitable animal model for studying neuroendocrine regulation and, more specifically, elucidating the molecular mechanisms and/or mediators involved in the increased ACTH secretion and consequently elevated CS levels despite the high GR levels. Furthermore, chronic hyperactivation of the HPA axis system has been linked to stress-related emotional disorders such as anxiety, anorexia nervosa, and depression (68, 69). Thus, the SPRET/Ei mouse strain might be suitable for studying these psychiatric disorders.
Despite the beneficial anti-inflammatory and immunosuppressive properties of GCs, chronic exposure to elevated levels of circulating GCs also leads to metabolic side effects, including insulin resistance, glucose intolerance, and overt diabetes (18, 45). Excess of GCs can induce hyperglycemia (70, 71), not only by perturbing insulin synthesis and secretion in β-cells of the endocrine pancreas and peripheral insulin function, but also by directly affecting the carbohydrate metabolism (72). Based on the high GC levels in SPRET/Ei mice, we anticipated that SPRET/Ei animals would have a similar phenotype. Indeed, increased basal glucose levels and glucose intolerance were observed in SPRET/Ei mice. It has been reported that excess GCs enhance glucose levels directly by activating many genes, such as Pepck and G6p, involved in hepatic gluconeogenesis (73, 74). The expression levels of these gluconeogenic enzymes were increased in the liver of SPRET/Ei mice. Furthermore, long term GC exposure promotes breakdown of protein and fat stores, thus increasing the supply of substrates for gluconeogenesis, such as alanine and glycerol to the liver (75). Moreover, GCs also induce glucose levels indirectly by antagonizing the actions, synthesis, and secretion of insulin (48, 70, 76, 77). However, an insulin tolerance test revealed no difference in insulin sensitivity between C57BL/6 and SPRET/Ei mice. Therefore, we wondered whether SPRET/Ei mice show reduced insulin production or secretion. Indeed, after glucose injection, no insulin production could be detected in blood serum. Notably, these reduced circulatory insulin levels will also contribute to the increased expression of gluconeogenic enzymes, as the negative influence of insulin normally dominates over the positive effects of cyclic AMP and GCs (78). This indicates that SPRET/Ei mice display impaired glucose-stimulated insulin synthesis and/or secretion. Therefore, we assessed the number and cytoarchitecture of the pancreatic islets of Langerhans in SPRET/Ei pancreatic tissues, and both features seemed to be normal in addition to the absence of GC-induced β-cell apoptosis. These features resemble the phenotype of the transgenic mice overexpressing GR specifically in the pancreatic β-cells (46). Next, we measured insulin expression in pancreatic tissues, and this showed that the production of insulin was unaffected in SPRET/Ei mice. As serum insulin levels were reduced and insulin synthesis was normal, we speculated that glucose-mediated insulin secretion was impaired in SPRET/Ei mice. Insulin secretion is mediated through glucose metabolism within the β-cells, providing ATP, which in turn triggers regulation of ATP-dependent potassium channels, membrane depolarization, and opening of voltage dependent Ca2+ channels (for review, see Refs. 79 and 80). This signaling cascade leads to the release of insulin produced in the secretory pathway from intracellular storage vesicles. It is known that GCs interfere in many ways with the insulin secretory process in pancreatic β-cells (70, 77). Most notably, GCs impair β-cell glucose uptake and oxidation, decrease protein kinase A and C activation, and reduce calcium fluxes by permitting repolarizing potassium currents (for review, see Ref. 72). The mechanism responsible for the defective insulin secretion observed in SPRET/Ei mice remains to be elucidated. Hence, the SPRET/Ei mouse strain will contribute to further identification of the GC effects on insulin secretion from β-cells and consequently will increase our knowledge on GC-induced glucose intolerance.
In addition to the unwanted side effects, GC resistance often occurs due to prolonged exposure to excess of endogenous or synthetic GCs. Homologous GR down-regulation, i.e. GCs being responsible for the down-regulation of its receptor, is one of the mechanisms that can explain the development of GC resistance (81). Therefore, the high circulatory GC levels observed in SPRET/Ei mice would be expected to lead to reduced GR levels, serving as a physiological feedback mechanism. Notwithstanding the SPRET/Ei, GR seems to be resistant to ligand-induced down-regulation as SPRET/Ei mice display high GR levels. Thus, the elevated GR levels in the presence of chronic elevated GC levels in SPRET/Ei animals makes them an appropriate model for studying the mechanism of homologous down-regulation of GR. Consequently, the SPRET/Ei GR variant might be helpful for elucidating further the mechanisms of GC resistance, which limits the success of many GC-based therapies (17).
In conclusion, we demonstrate that SPRET/Ei mice display strongly increased GR levels and that this accounts for their extreme resistance to LPS-induced lethal inflammation. The SPRET/Ei phenotype seems to be unique compared with other M. spretus inbred lines, which show intermediate levels of LPS sensitivity, consistent with intermediate GR levels. The high GR transcription levels in SPRET/Ei mice seem to be caused by a polymorphism in the GR locus on chromosome 18. The dynamics of GR expression can be affected not only by SNPs in the coding region of the gene, leading to aberrant mutants, but also by promoter and 3′-UTR variants, which can change the expression levels. Identification of the GR variant in SPRET/Ei will have important implications for further understanding the role of GR in the maintenance of health and homeostasis. The real value of the SPRET/Ei mouse model is that it is to our knowledge the only mouse that combines an overexpression of GR with an overactivation of the HPA axis, which is characterized by elevated ACTH and GC concentrations likely due to increased sensitivity for CRH. In other mice, such as the YGR transgenic mouse, carrying extra copies of GR, compensatory down-regulation of CS is observed that diminishes the effects of the overexpressed GR (30). The fact that in SPRET/Ei this compensatory complementation does not happen is unique and allows studying the effects of both a high GR and CS activity on the physiology of the mice. Our work shows that this physiology (glucose metabolism) is heavily disturbed so that the SPRET/Ei mice have an added value as research tools for researchers interested in GR. We conclude that the SPRET/Ei mouse strain is a valuable complement to GR mutant mice in the investigation of neuroendocrine regulation and the immunosuppressive actions of GR as well as several phenotypes associated with chronically increased GC levels, such as glucose intolerance, impaired insulin secretion, GC resistance, and psychiatric disorders.
Supplementary Material
Acknowledgments
We thank J. Vanden Berghe and W. Burm for technical assistance and Dr. A. Bredan for editing the manuscript. We are grateful to L. Van Geert, D. Roels, and C. Van Laere for animal care. We thank Prof. Dr. X. Montagutelli for providing M. spretus SEG/Pas mice and Prof. Dr. H. Reichardt for helpful advice. The VIB Genetic Service Facility (University of Antwerp; Department of Molecular Genetics) is acknowledged for sequencing the M. spretus GR DNA sequences.
This work was supported by the Fund for Scientific Research, Flanders, the Belgian Interuniversity Attraction Poles program (IAP VI/18), and the Belgische Vereniging tegen Kanker.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- GC
- glucocorticoid
- GR
- glucocorticoid receptor
- CS
- corticosterone
- HPA
- hypothalamic-pituitary-adrenal
- CRH
- corticotrophin-releasing hormone
- AVP
- arginine-vasopressin
- POMC
- pro-opiomelanocortin
- adx
- adrenalectomized
- Dex
- dexamethasone
- QLT
- quantitative trait loci
- LRS
- likelihood ratio statistic
- PCK1
- phosphoenolpyruvate carboxykinase 1
- G6P
- glucose-6-phosphatase
- CRH-R1
- CRH receptors 1
- qPCR
- quantitative real time PCR.
REFERENCES
- 1.Bonhomme F., Martin S., Thaler L. (1978) Experientia 34, 1140–1141 [DOI] [PubMed] [Google Scholar]
- 2.Flint J., Valdar W., Shifman S., Mott R. (2005) Nat Rev. Genet. 6, 271–286 [DOI] [PubMed] [Google Scholar]
- 3.Dejager L., Libert C., Montagutelli X. (2009) Trends Genet. 25, 234–241 [DOI] [PubMed] [Google Scholar]
- 4.Mahieu T., Park J. M., Revets H., Pasche B., Lengeling A., Staelens J., Wullaert A., Vanlaere I., Hochepied T., van Roy F., Karin M., Libert C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 2292–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staelens J., Wielockx B., Puimège L., Van Roy F., Guénet J. L., Libert C. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9340–9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell J. A. (2006) N. Engl. J. Med. 355, 1699–1713 [DOI] [PubMed] [Google Scholar]
- 7.Rhen T., Cidlowski J. A. (2005) N. Engl. J. Med. 353, 1711–1723 [DOI] [PubMed] [Google Scholar]
- 8.Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 9.De Bosscher K., Vanden Berghe W., Haegeman G. (2003) Endocr. Rev. 24, 488–522 [DOI] [PubMed] [Google Scholar]
- 10.Beato M., Herrlich P., Schütz G. (1995) Cell 83, 851–857 [DOI] [PubMed] [Google Scholar]
- 11.Miller W. L., Tyrrell J. B. (eds) (1995) The Adrenal Cortex (Felig P., Saxter J., Frohman L. eds) McGraw Hill, New York [Google Scholar]
- 12.Barnes P. J. (1998) Clin. Sci. 94, 557–572 [DOI] [PubMed] [Google Scholar]
- 13.Ayroldi E., Riccardi C. (2009) FASEB J. 23, 3649–3658 [DOI] [PubMed] [Google Scholar]
- 14.De Bosscher K., Vanden Berghe W., Haegeman G. (2006) Oncogene 25, 6868–6886 [DOI] [PubMed] [Google Scholar]
- 15.Reichardt H. M., Schütz G. (1998) Mol. Cell. Endocrinol. 146, 1–6 [DOI] [PubMed] [Google Scholar]
- 16.Funder J. W. (1997) Annu. Rev. Med. 48, 231–240 [DOI] [PubMed] [Google Scholar]
- 17.Barnes P. J., Adcock I. M. (2009) Lancet 373, 1905–1917 [DOI] [PubMed] [Google Scholar]
- 18.Schäcke H., Döcke W. D., Asadullah K. (2002) Pharmacol. Ther. 96, 23–43 [DOI] [PubMed] [Google Scholar]
- 19.Saag K. G. (2002) Curr. Rheumatol. Rep. 4, 218–225 [DOI] [PubMed] [Google Scholar]
- 20.Autelitano D. J., Blum M., Lopingco M., Allen R. G., Roberts J. L. (1990) Neuroendocrinology 51, 123–130 [DOI] [PubMed] [Google Scholar]
- 21.De Kloet E. R., Vreugdenhil E., Oitzl M. S., Joëls M. (1998) Endocr. Rev. 19, 269–301 [DOI] [PubMed] [Google Scholar]
- 22.Sapolsky R. M., Romero L. M., Munck A. U. (2000) Endocr. Rev. 21, 55–89 [DOI] [PubMed] [Google Scholar]
- 23.Svec F. (1985) Life Sci. 36, 2359–2366 [DOI] [PubMed] [Google Scholar]
- 24.Gómez F., De Kloet E. R., Armario A. (1998) Am. J. Physiol. 274, R420–R427 [DOI] [PubMed] [Google Scholar]
- 25.Cole T. J., Blendy J. A., Monaghan A. P., Krieglstein K., Schmid W., Aguzzi A., Fantuzzi G., Hummler E., Unsicker K., Schütz G. (1995) Genes Dev. 9, 1608–1621 [DOI] [PubMed] [Google Scholar]
- 26.Tronche F., Kellendonk C., Kretz O., Gass P., Anlag K., Orban P. C., Bock R., Klein R., Schütz G. (1999) Nat. Genet. 23, 99–103 [DOI] [PubMed] [Google Scholar]
- 27.Reichardt H. M., Schütz G. (1996) Mol. Med. 2, 735–744 [PMC free article] [PubMed] [Google Scholar]
- 28.Reichardt H. M., Kaestner K. H., Wessely O., Gass P., Schmid W., Schütz G. (1998) J. Steroid Biochem. Mol. Biol. 65, 111–115 [DOI] [PubMed] [Google Scholar]
- 29.Opherk C., Tronche F., Kellendonk C., Kohlmüller D., Schulze A., Schmid W., Schütz G. (2004) Mol. Endocrinol. 18, 1346–1353 [DOI] [PubMed] [Google Scholar]
- 30.Reichardt H. M., Umland T., Bauer A., Kretz O., Schütz G. (2000) Mol. Cell. Biol. 20, 9009–9017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., Ge R., Matte-Martone C., Goodwin J., Shlomchik W. D., Mamula M. J., Kooshkabadi A., Hardy M. P., Geller D. (2009) J. Biol. Chem. 284, 6249–6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. (1986) Proc. Natl. Acad. Sci. U.S.A.. 83, 9679–9683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silver L. M. (ed) (1995) in Mouse Genetics: Concepts and Applications, pp. 227–253, Oxford University Press, London [Google Scholar]
- 34.Manly K. F., Cudmore R. H., Jr., Meer J. M. (2001) Mamm. Genome. 12, 930–932 [DOI] [PubMed] [Google Scholar]
- 35.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002) Genome Biol. 3, RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heuser I., Yassouridis A., Holsboer F. (1994) J. Psychiatr. Res. 28, 341–356 [DOI] [PubMed] [Google Scholar]
- 37.Brouckaert P. G., Everaerdt B., Libert C., Takahashi N., Fiers W. (1989) Agents Actions 26, 196–198 [DOI] [PubMed] [Google Scholar]
- 38.Bertini R., Bianchi M., Ghezzi P. (1988) J. Exp. Med. 167, 1708–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derijk R. H., de Kloet E. R. (2008) Eur. J. Pharmacol. 583, 303–311 [DOI] [PubMed] [Google Scholar]
- 40.Fink G. (1997) in Principles of Medical Biology (Bittar E. E., Bittar M. eds) Vol. 10A, pp. 30–100, Jai Press, London [Google Scholar]
- 41.Tronche F., Kellendonk C., Reichardt H. M., Schütz G. (1998) Curr. Opin. Genet. Dev. 8, 532–538 [DOI] [PubMed] [Google Scholar]
- 42.Gadek-Michalska A., Bugajski J. (2004) J. Physiol Pharmacol. 55, 663–675 [PubMed] [Google Scholar]
- 43.Besser G. M. (1973) Clin. Endocrinol. (Oxf.) 2, 175–186 [DOI] [PubMed] [Google Scholar]
- 44.Paust H. J., Loeper S., Else T., Bamberger A. M., Papadopoulos G., Pankoke D., Saeger W., Bamberger C. M. (2006) Exp. Clin. Endocrinol Diabetes 114, 6–10 [DOI] [PubMed] [Google Scholar]
- 45.Lenzen S., Bailey C. J. (1984) Endocr. Rev. 5, 411–434 [DOI] [PubMed] [Google Scholar]
- 46.Davani B., Portwood N., Bryzgalova G., Reimer M. K., Heiden T., Ostenson C. G., Okret S., Ahren B., Efendic S., Khan A. (2004) Diabetes 53, S51–S59 [DOI] [PubMed] [Google Scholar]
- 47.McMahon M., Gerich J., Rizza R. (1988) Diabetes Metab Rev. 4, 17–30 [DOI] [PubMed] [Google Scholar]
- 48.Andrews R. C., Walker B. R. (1999) Clin. Sci.. 96, 513–523 [DOI] [PubMed] [Google Scholar]
- 49.Ranta F., Avram D., Berchtold S., Düfer M., Drews G., Lang F., Ullrich S. (2006) Diabetes 55, 1380–1390 [DOI] [PubMed] [Google Scholar]
- 50.Philippe J., Missotten M. (1990) Endocrinology 127, 1640–1645 [DOI] [PubMed] [Google Scholar]
- 51.Liberman A. C., Druker J., Perone M. J., Arzt E. (2007) Cytokine Growth Factor Rev. 18, 45–56 [DOI] [PubMed] [Google Scholar]
- 52.Galon J., Franchimont D., Hiroi N., Frey G., Boettner A., Ehrhart-Bornstein M., O'Shea J. J., Chrousos G. P., Bornstein S. R. (2002) FASEB J. 16, 61–71 [DOI] [PubMed] [Google Scholar]
- 53.Yeager M. P., Guyre P. M., Munck A. U. (2004) Acta Anaesthesiol. Scand. 48, 799–813 [DOI] [PubMed] [Google Scholar]
- 54.Dong Y., Poellinger L., Gustafsson J. A., Okret S. (1988) Mol. Endocrinol. 2, 1256–1264 [DOI] [PubMed] [Google Scholar]
- 55.Chi H., Barry S. P., Roth R. J., Wu J. J., Jones E. A., Bennett A. M., Flavell R. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 2274–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Q., Shepherd E. G., Manson M. E., Nelin L. D., Sorokin A., Liu Y. (2005) J. Biol. Chem. 280, 8101–8108 [DOI] [PubMed] [Google Scholar]
- 57.Eddleston J., Herschbach J., Wagelie-Steffen A. L., Christiansen S. C., Zuraw B. L. (2007) J. Allergy Clin. Immunol. 119, 115–122 [DOI] [PubMed] [Google Scholar]
- 58.Herbert J., Goodyer I. M., Grossman A. B., Hastings M. H., de Kloet E. R., Lightman S. L., Lupien S. J., Roozendaal B., Seckl J. R. (2006) J. Neuroendocrinol. 18, 393–411 [DOI] [PubMed] [Google Scholar]
- 59.Ridder S., Chourbaji S., Hellweg R., Urani A., Zacher C., Schmid W., Zink M., Hörtnagl H., Flor H., Henn F. A., Schütz G., Gass P. (2005) J. Neurosci. 25, 6243–6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cole T. J., Myles K., Purton J. F., Brereton P. S., Solomon N. M., Godfrey D. I., Funder J. W. (2001) Mol. Cell. Endocrinol. 173, 193–202 [DOI] [PubMed] [Google Scholar]
- 61.Nikodemova M., Diehl C. R., Aguilera G. (2002) Arch. Physiol. Biochem. 110, 123–128 [DOI] [PubMed] [Google Scholar]
- 62.Aguilera G., Nikodemova M., Wynn P. C., Catt K. J. (2004) Peptides 25, 319–329 [DOI] [PubMed] [Google Scholar]
- 63.Potter E., Behan D. P., Fischer W. H., Linton E. A., Lowry P. J., Vale W. W. (1991) Nature 349, 423–426 [DOI] [PubMed] [Google Scholar]
- 64.Chen A. M., Perrin M. H., Digruccio M. R., Vaughan J. M., Brar B. K., Arias C. M., Lewis K. A., Rivier J. E., Sawchenko P. E., Vale W. W. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2620–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor T., Dluhy R. G., Williams G. H. (1983) J. Clin. Endocrinol. Metab. 57, 592–596 [DOI] [PubMed] [Google Scholar]
- 66.al-Damluji S., Francis D. (1993) Am. J. Physiol. 264, 208–214 [DOI] [PubMed] [Google Scholar]
- 67.Spinazzi R., Andreis P. G., Rossi G. P., Nussdorfer G. G. (2006) Pharmacol. Rev. 58, 46–57 [DOI] [PubMed] [Google Scholar]
- 68.Schatzberg A. F., Rothschild A. J., Langlais P. J., Bird E. D., Cole J. O. (1985) J. Psychiatr. Res. 19, 57–64 [DOI] [PubMed] [Google Scholar]
- 69.Putignano P., Dubini A., Toja P., Invitti C., Bonfanti S., Redaelli G., Zappulli D., Cavagnini F. (2001) Eur. J. Endocrinol. 145, 165–171 [DOI] [PubMed] [Google Scholar]
- 70.Lambillotte C., Gilon P., Henquin J. C. (1997) J. Clin. Invest.. 99, 414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larsson H., Ahrén B. (1999) Diabetologia 42, 936–943 [DOI] [PubMed] [Google Scholar]
- 72.van Raalte D. H., Ouwens D. M., Diamant M. (2009) Eur J. Clin. Invest. 39, 81–93 [DOI] [PubMed] [Google Scholar]
- 73.O'Brien R. M., Granner D. K. (1996) Physiol. Rev. 76, 1109–1161 [DOI] [PubMed] [Google Scholar]
- 74.Vegiopoulos A., Herzig S. (2007) Mol. Cell. Endocrinol.. 275, 43–61 [DOI] [PubMed] [Google Scholar]
- 75.Postic C., Dentin R., Girard J. (2004) Diabetes Metab. 30, 398–408 [DOI] [PubMed] [Google Scholar]
- 76.Ullrich S., Su J., Ranta F., Wittekindt O. H., Ris F., Rösler M., Gerlach U., Heitzmann D., Warth R., Lang F. (2005) Pflugers Arch. 451, 428–436 [DOI] [PubMed] [Google Scholar]
- 77.Delaunay F., Khan A., Cintra A., Davani B., Ling Z. C., Andersson A., Ostenson C. G., Gustafsson J., Efendic S., Okret S. (1997) J. Clin. Invest. 100, 2094–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quinn P. G., Yeagley D. (2005) Curr. Drug Targets Immune Endocr. Metabol. Disord. 5, 423–437 [DOI] [PubMed] [Google Scholar]
- 79.Ashcroft F. M., Proks P., Smith P. A., Ammälä C., Bokvist K., Rorsman P. (1994) J. Cell. Biochem. 55, Suppl., 54–65 [DOI] [PubMed] [Google Scholar]
- 80.Berggren P. O., Larsson O. (1994) Biochem. Soc. Trans. 22, 12–18 [DOI] [PubMed] [Google Scholar]
- 81.Meyer A. S., Schmidt T. J. (1997) J. Steroid Biochem. Mol. Biol. 62, 97–105 [DOI] [PubMed] [Google Scholar]
- 82.Darvasi A., Soller M. (1997) Behav. Genet. 27, 125–132 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.