Abstract
Interleukin 23 (IL-23) is required for autoimmune inflammation mediated by T helper type 17 cells (TH-17 cells) and is implicated in a number of human immune disorders. Here we restricted IL-23 receptor (IL-23R) deficiency to defined cell populations in vivo to investigate the requirement for IL-23 signaling in TH-17 development and function in autoimmunity, inflammation, and infection. In the absence of IL-23, TH-17 development was ‘stalled’ at the early activation stage. TH-17 cells failed to downregulate IL-2 and the tumor necrosis factor receptor superfamily member CD27, to maintain IL-17 production and to upregulate IL-7Rα expression. These defects were associated with reduced proliferation and reduced numbers of effector TH-17 cells that exit lymph nodes destined for the bloodstream and tissues.
Introduction
To establish inflammation in many models of autoimmunity, innate immune cells must produce IL-23. IL-23 shares the p40 subunit with IL-12 but has a unique p19 subunit1, and in recent years it has become clear that many of the previous associations of IL-12 and TH1 cells with autoimmunity can be explained by a requirement for IL-232. Some of the pro-inflammatory functions of IL-23 appear to be related to the recently identified TH-17 cell subset3. TH-17 cells were initially shown to be a major pathogenic cell type in models of autoimmunity including experimental autoimmune encephalitis (EAE)4,5 and collagen-induced arthritis (CIA)5, and have since been identified in several human inflammatory diseases including Crohn’s6 and psoriasis7. Likewise, polymorphisms in the gene for IL-23R, Il23ra, are major susceptibility factors for these disorders8,9.
Despite these associations, the precise role of IL-23 in TH-17 biology has remained elusive. IL-23 receptor is upregulated on T cells after activation in the presence of inflammatory cytokines such as IL-610. Transforming growth factor β (TGF-β) in the presence of proinflammatory cytokines, particularly IL-6, drives the initial differentiation of TH-17 cells11–13, including expression of the TH-17-specific transcription factor RORγt10, which leads to IL-17A (hereafter, IL-17) and IL-17F production11–13. However, continued stimulation of TH-17 cells with antigen in the presence of TGF-β and IL-6 does not lead to pathogenic activity of those cells14. In addition, high IL-10 production is induced by TGF-β and IL-6 stimulation of TH-17 cells, which can lead to inhibition of TH-17-mediated inflammation. In contrast, IL-23 signaling in TH-17 cells can enhance their pathogenic activity4 and promote production of pro-inflammatory factors4, including IL-2215,16. These findings suggest that IL-23-dependent signaling in TH-17 cells is important for their full pathogenic activity. It has been suggested, based largely on in vitro observations, that IL-23 stimulation increases the number of already-differentiated of TH-17 cells, maintains IL-17 production from TH-17 cells, or promotes survival of TH-17 cells. For example, addition of IL-23 during culture of activated or memory T cells results in increases their proliferation1 and the frequency of IL-17+ T cells produced4; IL-23 is also required during restimulation of TH-17 cells (i.e., cells previously stimulated with TGF-β and IL-6, to maintain IL-17 production from the TH-17 cells)12. Similarly, it has been suggested that IL-23 may stabilize the phenotype of TH-17 cells through mechanisms dependent on transcription factor STAT317,18. Two other cytokines thought to be involved in TH-17 differentiation, IL-6 and IL-21, also share the STAT3-dependent signaling pathway with IL-23.
Finally, IL-23 has been suggested to be a survival factor for TH-17 cells19. While all of these data are consistent with the observation of reduced frequencies of IL-17+ T cells in IL-23-deficient mice4, the precise requirements for IL-23 in vivo during the initiation and effector phases of the TH-17-mediated inflammatory response have yet to be investigated. One further complicating factor when interpreting results obtained in IL-23-deficient mice is that IL-23 may also have important pro-inflammatory effects on cells of the innate immune system2,20. In this report we genetically restricted IL-23R deficiency to defined cell populations in vivo to identify the requirement for IL-23 signaling in central nervous system (CNS) autoimmunity, skin inflammation and parasite infection. We showed that generation of normal TH-17, but not TH1, cell effector responses required IL-23R-dependent signaling. In contrast to previously held assumptions that IL-23 merely maintains TH-17 effector function, we found that IL-23 promotes full differentiation of activated T cells into effector TH-17 cells.
Results
Failed CNS accumulation of Il23ra−/− CD4+ T cells
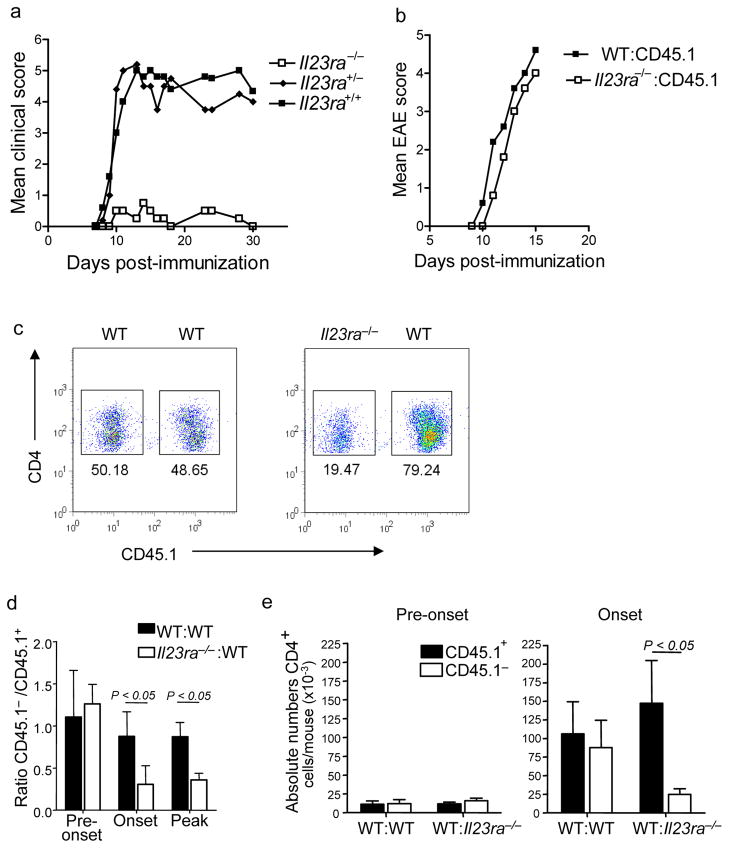
We used mice deficient for the IL-23 receptor21 (Il23ra−/−) to address the functions of IL-23 in TH-17-mediated inflammation in vivo. Similar to the results previously obtained with IL-23-deficient (Il23a−/−) mice2, we found that Il23ra−/− mice were resistant to induction of EAE (Fig 1a) confirming that this receptor is required for IL-23 signaling in vivo. However, this resistance made it impossible to investigate the effects of IL-23 on inflammatory cells in the CNS of these mice. To circumvent this problem, we generated mixed bone marrow chimeric mice, in which 50% of the reconstituting bone marrow was derived from Il23ra−/− CD45.1− donor bone marrow and 50% from wild-type CD45.1+ congenic donor bone marrow (Supplemental Fig 1); thus, wild-type and Il23ra−/− cells could be distinguished on the basis of CD45.1 expression. Eight weeks following transfer of the bone marrow into recipient mice, we found no defect in the ability of Il23ra−/− donor cells to repopulate the T cell, B cell, dendritic cell (DC) and macrophage compartments (data not shown). We then evaluated the mice for susceptibility to induction of EAE compared to Il23ra−/− mice. We found that while mixed Il23ra−/−:wild-type (50%:50%) bone marrow chimeras were fully susceptible to EAE, with only a slight delay in onset of clinical signs compared to wild-type:wildtype (50%:50%) control chimeras (Fig 1b). We then characterized the infiltrating mononuclear cells in the CNS at onset of EAE (day 11 post-immunization) in the chimeric mice. The relative proportions of wild-type and Il23ra−/− macrophages, DCs, and CD8+ T cells were similar, indicating that recruitment and accumulation of these cells in the CNS was not dependent on Il23ra (Supplemental Fig 2). However, we noted a significantly reduced proportion of Il23ra−/− CD4+ T cells compared to wild-type CD4+ T cells at day 11 post-immunization (Fig 1c). We then evaluated the proportions of wild-type and Il23ra−/− CD4+ cells at other time points. Before clinical onset of EAE (day 8–9), a stage when T cells first enter the CNS to establish inflammation, wildtype and Il23ra−/− CD4+ T cells were present at similar levels. However, at onset and during active EAE, the ratio of Il23ra−/− to wild-type CD4+ cells was markedly reduced (Fig 1d). Absolute numbers of CD4+ cells reflected this ratio and further emphasized that although the ratios of wildtype and Il23ra−/− cells were similar before onset of EAE, at this stage there were also very low numbers of cells infiltrating the CNS compared to the numbers that had accumulated by onset of clinical symptoms (Fig 1e). Hence it appeared that wild-type CD4+ cells accumulated normally in the CNS but Il23ra−/− cells did not.
Figure 1. Il23ra−/− CD4+ T cells do not accumulate in the inflamed CNS.
(a) Clinical scores of Il23ra−/−, Il23ra+/− and Il23ra+/+ mice after EAE induction. (b) Clinical scores of mixed bone marrow chimeras following EAE induction. Donor bone marrow genotype is indicated. (c) Flow cytometry of CD4+ cells obtained from the CNS of mixed bone marrow chimeras 11 days post-EAE induction. Genotype of each population is indicated. (d) Proportion of wild-type CD45.1+ or Il23ra−/− CD45.1− CD4+ T cells in the CNS of mixed bone marrow chimeras at indicated time points; data is expressed as mean ratio ± s.d. of CD45.1− to CD45.1+ CD4+ T (wild-type) cells. (e) Absolute numbers of CD4+ T cells in the CNS on day 9 (pre-onset) and day 11 (onset of clinical signs) of EAE. Data shown are each representative of three experiments with 5 mice per group.
CNS Il23ra−/− T cells produce less IL-17
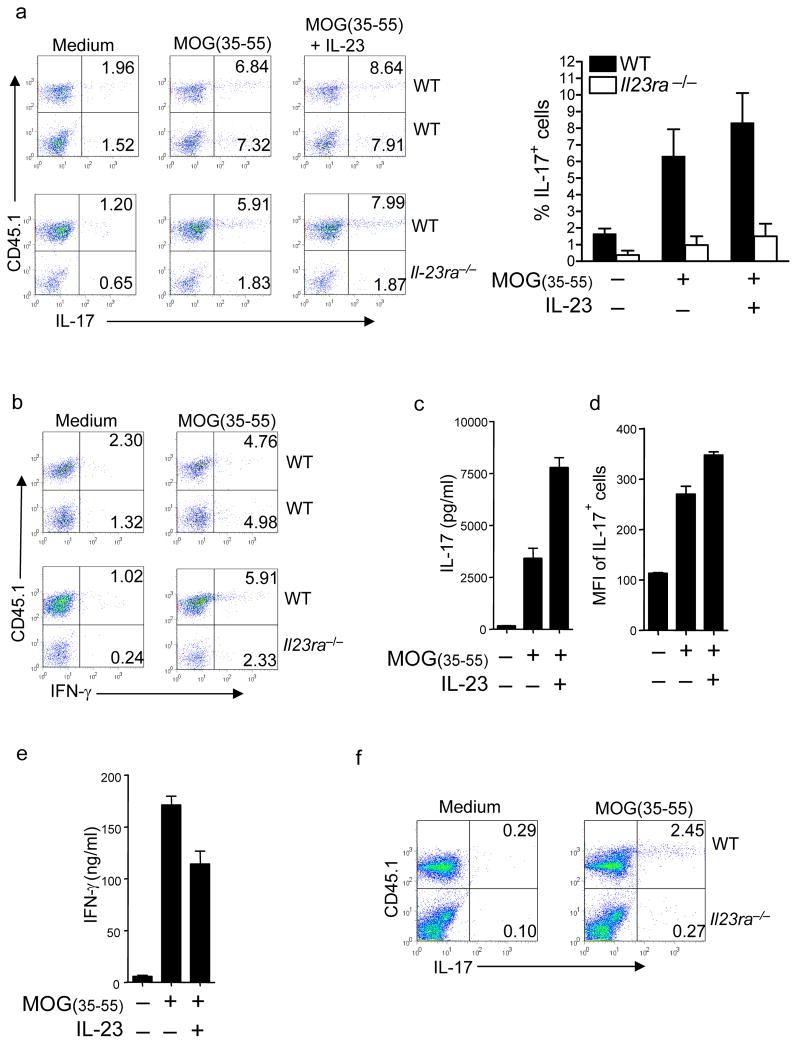
To determine whether there were additional functional defects in the Il23ra−/− cells, we evaluated their ex vivo cytokine responses to MOG(35–55) stimulation in the presence or absence of IL-23 for 24 hours. IL-17 production by CNS Il23ra−/− CD4+ T cells was profoundly reduced at day 11 post-immunization (Fig 2a). IFN-γ production was not increased when IL-23 signaling was absent, indicating that the Il23ra−/− cells had not ‘converted’ to a TH1 cell phenotype (Fig 2b). Addition of IL-23 to the cultures greatly enhanced production of IL-17 into the supernatant by wildtype cells (Fig 2c), and flow cytometry revealed an increase in mean fluorescence intensity per IL-17+ wild-type CD4+ T cell (Fig 2d), confirming that IL-23 promotes TH-17 cell differentiation. Interestingly, IFN-γ production by TH-17 cells was not enhanced by IL-23 (Fig 2e), again suggesting that IL-23 does not enhance TH1 responses. When draining lymph node (dLN) responses were tested, MOG-specific IL-17 production was also found to be severely impaired in Il23ra−/− T cells (Fig 2f). This suggested that the requirement for IL-23 signaling began during earlier differentiation in the lymph node.
Figure 2. Il23ra−/− T cells have reduced IL-17 production after T cell priming.
(a) CD4+ IL-17 production after 24 hour stimulation of CNS cells with MOG(35–55) in the presence or absence of IL-23, CNS cells isolated from mixed bone marrow chimeras 11 days post-EAE induction. (b) CD4+ IFN-γ production after 24 hour stimulation of CNS cells with MOG(35–55) or medium only control, CNS cells isolated from mixed bone marrow chimeras 11 days post-EAE induction. (c) Supernatant IL-17 concentrations after stimulation of CNS mononuclear cells as described in a. (d) Mean fluorescence intensity of IL-17+ wild-type CD4+ CNS cells stimulated as in a. (e) Supernatant IFN-γ concentrations after stimulation of CNS mononuclear cells as in a. (f) Draining lymph node CD4+ T cell IL-17 production in response to 24 hour stimulation with MOG(35–55) in the presence or absence of IL-23, 11 days post-EAE induction. Data shown are from 5 mice per group, representative of at least 3 experiments with similar results.
Il23ra−/− T cells expand but have impaired function
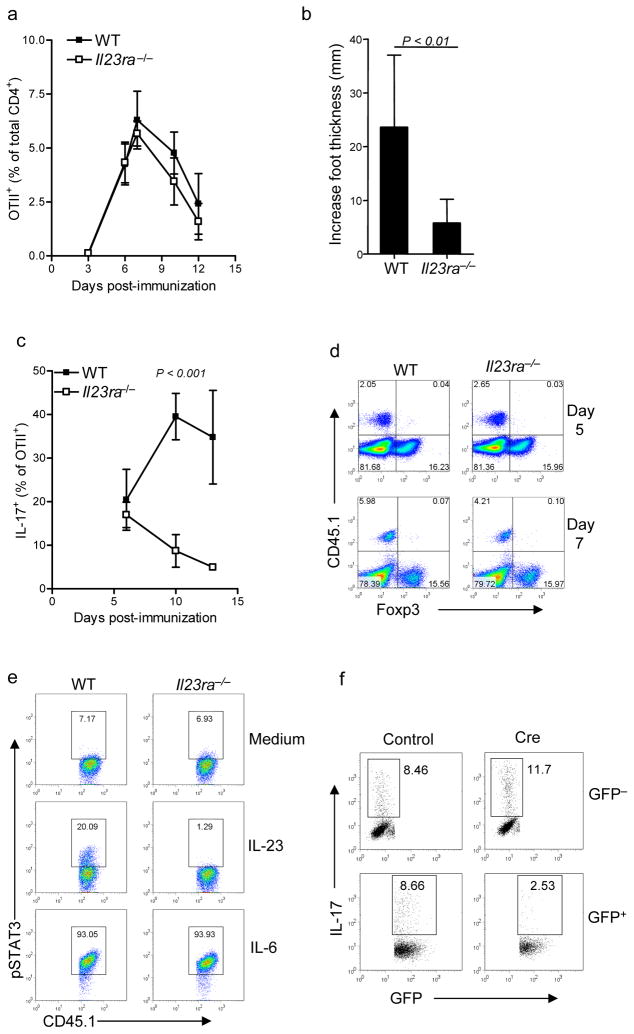
The results thus far suggested that in the absence of IL-23R either fewer MOG-specific CD4+ T cells are present or MOG-specific CD4+ T cells have an altered phenotype compared to wild-type MOG-specific CD4+ T cells. Since the effects of IL-23 were already apparent before T cells left the dLN to enter target organs, it became necessary to directly identify the responding antigen-specific cells in vivo. Because we were unable to track MOG-reactive endogenous CD4+ T cells, we instead made use of the OTII CD4+ T cell receptor transgenic mice that also express the CD45.1+ allele. By generating Il23ra−/− OTII mice and transferring a small number of Il23ra−/− CD45.1+ OTII T cells into wild-type CD45.2 recipient mice, we could investigate the consequences of IL-23R deficiency restricted to CD4+ T cells during activation in an environment in which all other cells were IL-23-responsive. Using such a system, we found that the increase in antigen-specific wild-type and Il23ra−/− cells OTII T cell numbers, on day 7 in the dLN after immunization with OVA(323–339) in complete Freund’s adjuvant (CFA), was IL-23-independent (Fig 3a). The absolute number of OTII cells correlated with their percentage of total CD4+ cells (mean 3.8 × 105 + 1.7 × 105 for wild-type OTII cells compared to 2.7 × 105 + 7 × 104 for Il23ra−/− OTII cells on day 7). We consistently observed a small reduction in frequency of Il23ra−/− cells compared to wild type during the contraction phase, although the difference was not statistically significant (Fig 3a).
Figure 3. Early TH-17 activation is normal but effector responses are impaired in Il23ra −/− .
(a) Frequency of wild-type or Il23ra−/− CD45.1+CD4+OTII T cells, expressed as percentage of total CD4+ T cells (mean ± s.d), following transfer into wild-type recipients and immunization with OVA(323–339). (b) Delayed type hypersensitivity reaction in mice after transfer of wild-type or Il23ra−/− OTII cells. The increase in foot thickness over the contralateral saline-challenged foot is shown. (c) Time course of Intracellular IL-17 in OTII cells from dLN assessed by flow cytometry after ex vivo stimulation with PMA + ionomycin on indicated days post-immunization. (d) Intracellular Foxp3 expression in OTII cells assessed on days 5 and 7 post-immunization (e) Flow cytometry for phosphorylated STAT3 in OTII cells after 15 minute stimulation with indicated cytokines 4 days post-immunization. (f) Flow cytometry of Cre-deleted Stat3flox/flox CD4+ cells following second stimulation with anti-CD3 in the presence of IL-23. IL-17 is shown as percentage of GFP+ and GFP− cells on each plot. All data shown are representative of at least 3 independent experiments having 4 to 5 mice per group.
To determine the functional capactity of the Il23ra−/− OTII TH17 cells, we tested whether they could mediate an IL-17-dependent delayed type hypersensitivity (DTH) reaction22. Compared to recipients of wild-type OTII cells, recipients of Il23ra−/− OTII cells had a markedly reduced DTH swelling response (Fig 3b). This indicated that the effector response in this system was impaired, correlating with findings in the EAE model. We next investigated IL-17 production by Il23ra−/− OTII T cells after immunization. Early IL-17 induction was unaffected by IL-23R deficiency in vivo (Fig 3c), consistent with previously reported in vitro observations that TGF-β and IL-6 alone are sufficient to induce RORγt and IL-17 production10–13. However, we found that Il23ra−/− cells produced less IL-17 compared to wild-type cells at later time points and most apparent from day 10 (Fig 3c). The timing of this decrease in IL-17 production correlated well with the impaired IL-17 response to MOG(35–55) stimulation observed at day 11 in lymph node and CNS in the mixed bone marrow chimeras (see above).
The differentiation of inducible Foxp3+ regulatory T cells (Treg cells) and TH-17 cells appear to be reciprocally regulated11. Recent data has further suggested that IL-23 negatively regulates the generation of Treg cells from naive T cells activated in the gastrointestinal system23. We speculated, therefore, that ‘conversion’ to a Treg cell phenotype may be ‘favored’ by Il23ra−/− OTII cells in response to OVA(323–339) stimulation. However, we detected no increase in Foxp3 expression in lymph node Il23ra−/− OTII cells after immunization with OVA(323–339) in CFA (Fig 3d). Likewise, the proportion of Foxp3+ cells was not affected by IL-23R deficiency in either lymph node or CNS after EAE induction in mixed bone marrow chimeras (Supplemental Fig 3).
Activation of the transcription factor STAT3 activation is crucial for TH-17 development17,24,25, and IL-23 signaling activates STAT318,26. Because other TH-17-associated cytokines, including IL-6 and IL-21, also activate STAT3 we evaluated whether IL-23 could induce phosphorylation of STAT3 in developing TH-17 cells in vivo. After OTII T cell transfer to and immunization of recipient mice, dLN cells were harvested and rested briefly before being stimulated with cytokine. A proportion of wild-type but not Il-23ra−/− OTII T cells responded to IL-23 by phosphorylating STAT3 (Fig 3e) on days 3 to 5 post-immunization, and this response correlated only with a proportion of cells also producing IL-17 on day 5 (data not shown). This suggested that IL-23R may be regulated depending on activation status of the cell rather than being upregulated on all T cells post-activation. In contrast, the majority of both wild-type and Il23ra−/− OTII cells phosphorylated STAT3 in response to IL-6 (Fig. 3e), indicating that there was no inherent difference in ability of the Il23ra−/− OTII cells to activate STAT3 or to respond to IL-6. IL-6 is highly upregulated following immunization with CFA and is detected for several weeks in dLN27, suggesting that there may be additional factors that support pSTAT3-mediating signals from IL-23R.
As already discussed, it is difficult to investigate the specific requirement of IL-23 for STAT3 because other cytokines, especially IL-6, which is most likely required earlier in TH-17 development, also utilize STAT3. We therefore designed an in vitro approach to delete STAT3 expression in TH-17 cells at a later point of development. This was achieved by activating STAT3-floxed (Stat3flox/flox) CD4+ T cells with anti-CD3-antiCD28 in the presence of TGFβ and IL-6 for four days, and then retrovirally expressing Cre recombinase to delete Stat3. Using this system we found that depletion of STAT3 prior to the second round of stimulation of TH-17 cells in the presence of IL-23 resulted in greatly reduced IL-17 production in those cells (Fig 3f; TH-17 cells generated with TGF-β and IL-6 in vitro require the presence of IL-23 during the second round of stimulation with antigen in order to maintain IL-17 production12). Cells that were not successfully deleted of Stat3 (identified as GFP−) still produced IL-17, indicating that loss of IL-17 production was a direct result of loss of Stat3 in the GFP+ cells.
Progression to effector TH-17 cells requires IL-23R
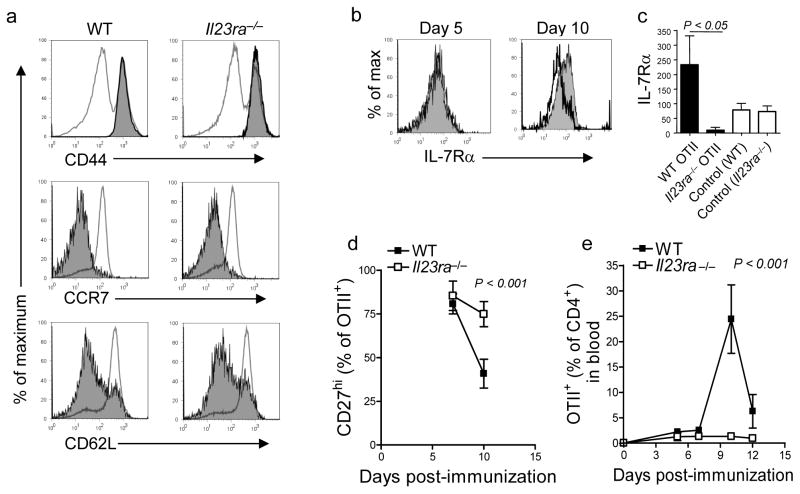
The results thus far suggested that early activation of TH-17 cells may occur normally in the absence of IL-23 and that the defect in function occurs later during the differentiation of effector cells. Expression of several proteins is reflective of the stages of activation of helper T cells. Corresponding with the normal expansion of Il23ra−/− cells, early markers of activation appeared unaffected: both wild-type and Il23ra−/− OTII cells were CD44hi, CCR7lo and CD62Llo following immunization (Fig 4a).
Figure 4. Full differentiation of effector TH-17 cells requires IL-23R in vivo.
(a–e) Analysis of phenotype of wild-type or Il23ra−/− CD45.1+CD4+OTII T cells after transfer into wild-type recipients and immunization with OVA(323–339). (a) Flow cytometry for cell surface expression of CD44, CCR7 and CD62L on wild-type and Il23ra−/− OTII T cells (shaded histograms) overlaid with total CD4+ cells for comparison (clear histograms) at day 7 post-immunization. b) Surface IL-7Rα expression on wild-type (shaded histogram) and Il23ra−/− (clear histogram) OTII cells from dLN. c) RT-PCR analysis of IL-7Rα expression in OTII+ cells sorted by flow cytometry from dLN; values are normalized to ubiquitin expression. As a control, total OTII–CD4+ cells were isolated at the same time from recipients of the indicated OTII cells. Data shown are mean ± s.d. of two groups of samples each containing 5 mice per condition. d) Surface CD27 expression on OTII cells in dLN and blood. e) Frequency of wild-type and Il23ra−/− OTII cells (as proportion of total CD4+ cells) in blood at indicated time points post immunization. Data shown are representative of 3 experiments with similar results.
IL-7Rα expression is required for naïve T cell survival, but is downregulated after TCR activation28. Re-expression of IL-7Rα is thought to be important for effector and memory cell survival29,30, and reflects progression of differentiation. We found that while IL-7Rα was upregulated on wild-type OTII cells at day 10 compared to day 5 post-immunization IL-7Ra was not upregulated in Il23ra−/− OTII cells (Fig 4b). We confirmed this result by qRT-PCR of RNA from wild-type and Il23ra−/− OTII cells in dLNs of mice seven days after immunization (Fig 4c). As the TNF-superfamily costimulatory molecule CD27 has been shown to be downregulated in terminally differentiating effector T cells31, we also evaluated this cell surface marker. We found that in dLNs the percentage of CD27hi wild-type OTII cells decreased after day 7 but that (Fig 4d) the percentage of CD27hi Il23ra−/− OTII cells did not, again suggesting that differentiation of effector cells was impaired.
As activated naive cells differentiate toward becoming effector cells they must leave the lymph node and travel via the circulation to their sites of action in tissues. Evidenced for this stems from the fact that wild-type cells of a differentiated phenotype can be found in the blood (for example, the majority are CD27lo) and the fact that high frequencies of activated effector cells enter the bloodstream as the immune response progresses. Based on the less differentiated phenotype of Il23ra−/− OTII cells we observed in the Lymph node, one would predict that there should be a reduced number of OTII cells present in the blood in the absence of IL-23. Indeed, there was a vast difference in the frequency of activated Il23ra−/− OTII cells compared to wild-type cells in the blood: wild-type OTII cells began leaving lymph node from day 7, with a peak at day 10: at this time point an average of 24.35 + 6.73% (mean + s.d.) of CD4+ T cells in the blood were OTII+ compared to only 4.77 + 0.97% in the lymph node (absolute numbers correlated with frequencies, data not shown). However, this ‘surge’ of activated effector cells in the blood did not occur in the absence of IL-23R (Fig 4e). Thus the impaired DTH effector responses we noted earlier (Fig. 3) was not only the result of reduced effector cytokine production but also reduced numbers of effector cells available to enter tissues. Also, the reduced numbers of Il23ra−/− CD4+ cells in the inflamed CNS of the mixed bone marrow chimeras can thus be attributed to low numbers of effector cells in the blood rather than a failure of those cells to proliferate or survive at that site.
IL-23 promotes TH-17 cell effector cytokine profile
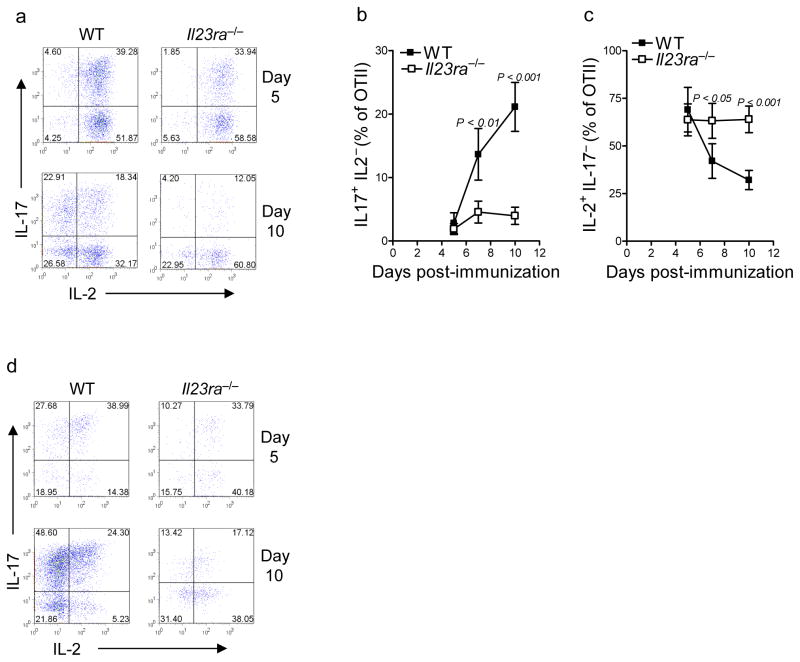
Production of IL-2 occurs early after T cell activation and then normally decreases in response to various differentiation factors, correlating with increased effector cytokine production32,33. IL-2-dependent signaling has also been described to be required for re-expression of IL-7Rα in activated T cells34 and to suppress TH-17 induction35, both via transcription factor STAT5. However, the expression of IL-2 in TH-17 cells developing in vivo has not been well characterized. We observed that the majority of wild-type and Il23ra−/− OTII cells produced IL-2 early after immunization on day 5, and many cells co-produced IL-17, while few produced only IL-17 (Fig 5a–c). As the wild-type response progressed, the frequency of single IL-17+ OTII cells increased (Fig 5b), correlating with a decreased frequency of single IL-2+ cells (Fig 5c). However, the Il23ra−/− OTII cells maintained their original phenotype, with few IL-17+IL-2− cells (Fig 5b) and many IL-2 single positive cells being produced (Fig 5c).
Figure 5. IL-23 promotes effector cytokine profile of TH-17.
(a–d) Intracellular cytokine analysis of wild-type or Il23ra−/− CD45.1+CD4+OTII T cells after transfer into wild-type recipients and immunization with OVA(323–339). (a) Intracellular staining of IL-17 and IL-2 in dLN OTII cells (b) Mean frequency of IL-17+IL-2− cells as proportion of CD4+OTII cells at indicated time points post-immunization. (c) Mean frequency of IL-2+IL-17− cells as proportion of CD4+OTII cells at indicated time points post-immunization. (d) Intracellular staining of IL-17 and IL-2 in blood OTII. Data shown are representative of 4 independent experiments with 4 mice per group.
Despite the high amount of IL-2 production the expression of the high-affinity IL-2 receptor chain, CD25, was not maintained on Il23ra−/− OTII cells (Supplemental Fig 4), suggesting that these cells had limited responsiveness to IL-2. Wild-type OTII cells in the blood consistently showed a more differentiated phenotype than in the lymph node, with greater frequencies of single IL-17+ cells and fewer cells producing IL-2 (Fig 5d). However, Il23ra−/− OTII cells in blood had fewer IL-17+ cells than wild-type, corresponding with the phenotype of the Il23ra−/− cells observed in the CNS in the mixed bone marrow chimeras (Fig 2a).
TH1 effector cells do not require IL-23R
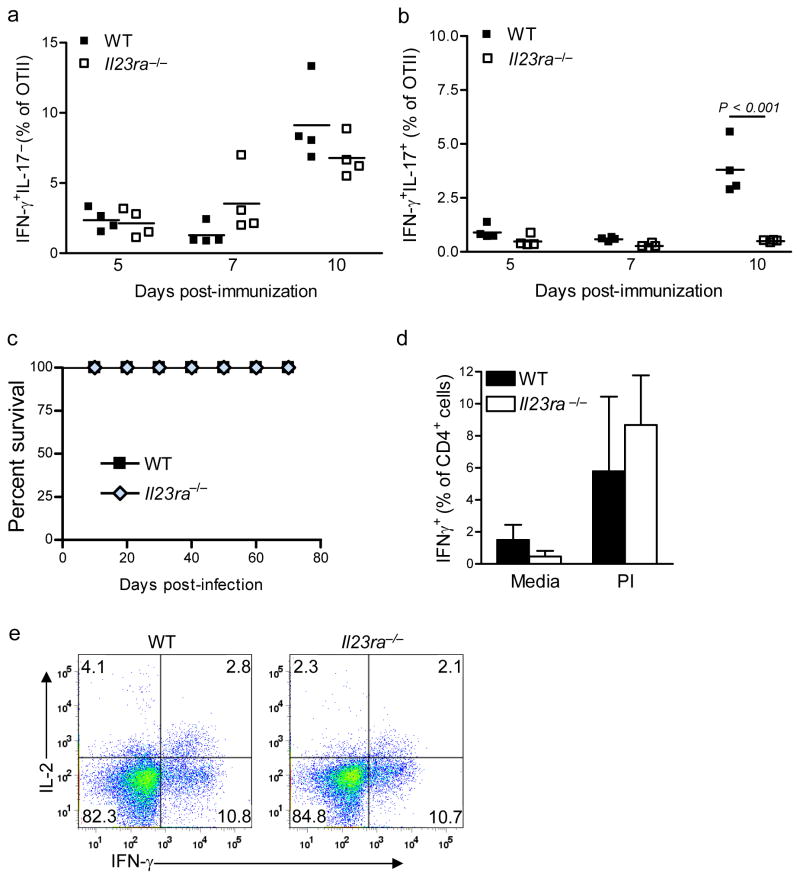
CFA has long been known to induce TH1 responses. It was possible that the reduced IL-17 production we observed in OTII cells lacking IL-23 receptor was due either to differentiation of the naive cells to a TH1 phenotype or to conversion of ‘undifferentiated’ TH-17 cell cells to a TH1 phenotype. We therefore evaluated the percentage of IL23ra−/− and wild-type OTII cells expressing IFN-γ or both IFN-γ and IL-17 after immunization. We found similar frequencies of IFN-γ+IL-17− wild type and Il23ra−/− OTII cells at multiple time points (Fig 6a), which supported our conclusion that TH1 cells were not induced to ‘replace’ the loss of TH-17 cell phenotype after immunization. In addition, compared to wild-type OTII cells we found a reduced percentage of double positive IL-17+IFN-γ+ IL23ra−/− cells (Fig 6b). This result suggested that these double positive cells are similar to IL-23-dependent_IL-17+ TH-17 cells, while IFN-γ+IL-17− cells are dependent on IL-12.
Figure 6. IL-23R is not required for TH1 effector cell development.
(a–b) Intracellular cytokine analysis of wild-type or Il23ra−/− CD45.1+CD4+OTII T cells after transfer into wild-type recipients and immunization with OVA(323–339). a) Frequency of IFN-γ+IL-17− cells as a proportion of CD4+OTII cells on indicated days post-immunization. b) Frequency of IFN-γ+IL-17+ cells as a proportion of CD4+ OTII cells on indicated days post-immunization. Data shown are representative of 3 independent experiments with 5 mice per group. (c–e) Wild-type and Il23ra−/− mice were infected with T. gondii and then followed for survival (c); frequency of IFN-γ+CD4+ cells following ex vivo stimulation of dLN cells from mice 9 days post-infection were also evaluated (d). (e) Intracellular flow cytometry of IFN-γ and IL-2 production by CD4+ cells following ex vivo stimulation of dLN cells from mice 9 days post-infection Data shown are representative of two independent experiments (5 mice per group) with similar results.
The profound defect in the late activation of the Il23ra−/− OTII cells suggested that IL-23 could be required for global T cell differentiation. To check if this was the case, we utilized a TH1- dependent infection model to test the function of Il23ra−/− TH1 effector cells. Production of IFN-γ by T cells is absolutely required for host resistance to the parasite Toxoplasma gondii. Thus, a defective TH1 effector response will cause the host to succumb to this pathogen within 3 weeks of challenge. When wild-type and Il23ra−/− mice were infected with T. gondii and followed for survival, we found no observable difference between strains through day 60 of infection (Fig 6c). This result also confirmed previous findings showing that IL-23p19 is not required for an effective TH1 response to this pathogen36. When the acute response to infection was analyzed we found that the frequency of IFN-γ+CD4+ cells in the dLN was similar between wild-type and Il23ra−/− T cells on day 9 after challenge (Fig 6d), and most of these cells were IL2− (Fig 6e). These results again confirmed that TH1 differentiation is not dependent on IL-23, and that the defects seen were specific to TH-17 effector cell differentiation.
IL-23R signaling is required early during differentiation
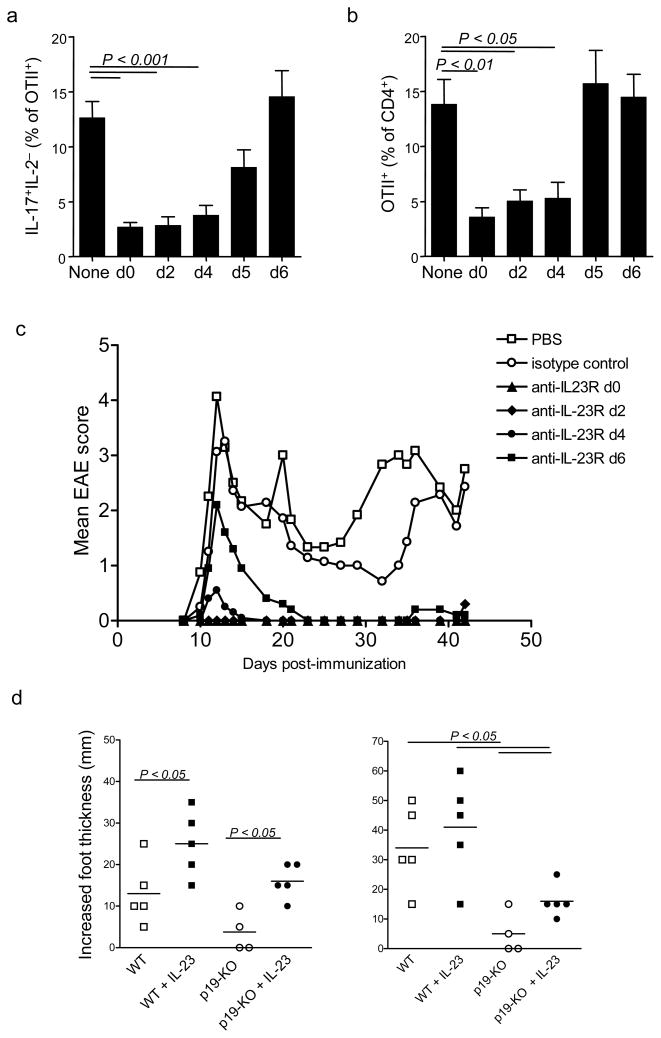
The results so far suggested that IL-23 was driving TH-17 effector differentiation, but it was still not clear where in the process of TH-17 differentiation IL-23 signaling was required. To determine this, we utilized a neutralizing IL23R monoclonal antibody to block IL-23 signaling at specific time points after immunization with OVA-CFA and measured the outcome on day 10 of the response. A single dose of neutralizing anti-IL-23R at the time of immunization recapitulated the phenotype of Il-23ra−/− OTII cells as measured by a reduced proportion of IL-17+IL-2− cells in the dLN (Fig 7a) and reduced frequencies of OTII cells in the blood (Fig 7b). Likewise, adding neutralizing antibody specific for IL-23R either 48 or 96 hours post-immunization blocked effector TH-17 cell differentiation. However, antibody administration at 120 hours or later was ineffective, suggesting that day 4 to 5 is the critical time for receipt of IL-23 signals. These results further support a role for IL-23 in directing differentiation of effector TH-17 cells rather than just maintaining survival of previously-generated effectors,
Figure 7. Timing of IL-23R requirement during TH-17 differentiation.
(a,b) Anti-IL-23R was administered to recipients of wild-type or Il23ra−/− OTII cells at the indicated times postimmunization. Draining lymph nodes were harvested on day 10 for analysis of intracellular IL-17 and IL-2 (a) and frequency of OTII cells in blood was assessed (b). (c) Clinical score of SJL mice administered anti-IL-23R on days indicated after EAE induction. (d) Delayed type hypersensitivity response in footpads of p19-deficient recipients of wild-type OTII cells ten days postimmunization with OVA(323–339). Where indicated, IL-23 was administered with ovalbumin in the footpad at the time of challenge and mice also received IL-23 in the contralateral footpad. Data shown are each representative of at least two experiments with 5 mice per group.
To further confirm that IL-23 signals in CD4+ T cells are required for their differentiation into pathogenic effector cells, we treated SJL mice with a single dose of neutralizing anti-IL-23R at different times after induction of EAE and observed for clinical signs during primary and relapse phases of disease. Treatment of SJL mice on day 0, 2 or 4 after immunization resulted in complete inhibition of EAE signs up to six weeks post-immunization, confirming the long-lasting inhibition of effector cell differentiation (Fig 7c); however, delayed treatment of SJL mice on day 6 after immunization was less effective in inhibiting primary disease. In SJL mice, relapses are triggered by effector TH-17 cells that were activated during the preceding period of disease37. We found that SJL mice treated with anti-IL-23R on day 6 were fully protected from subsequent relapsing disease, showing that while ’delayed’ treatment with anti-IL-23R does not block a first wave of activated cells, the induction of new effector CD4+ T cells could be prevented.
The timing of critical IL-23 function in CD4+ T cells, as demonstrated by neutralizing IL- 23R (described above), suggested that a major site of IL-23 signaling in T cells occurred while they are in the lymph nodes, which is consistent with the fact that this is where most of the activated cells are found at days 4 to 6. However, IL-23 is thought to be highly expressed in sites of tissue inflammation, and IL-23 was able to enhance effector functions in CNS-derived CD4+ T cells (Fig 2). To further confirm that IL-23 can drive differentiation of effector cells during antigen recognition in the tissues, we tested whether exposure to IL-23 in the skin could restore the impaired delayed type hypersensitivity response that occurs when T cells have been activated in the absence of IL-23. To this end, wild-type OTII cells were transferred into IL-23 p19-deficient (Il23a−/−) recipients, which were then challenged to induce DTH at day 10 post-immunization. As expected, the response was impaired compared to wild-type. However, just one administration of recombinant IL-23 in the challenge site resulted in partial restoration of the response (Fig 7d); saline plus IL-23 was used as the contralateral control for OVA plus IL-23, and injection of IL-23 in the absence of antigen did not induce a measurable swelling response in this timeframe (data not shown). Given the low numbers of circulating OTII cells at this timepoint, and the lack of continued IL-23 production, this result is notable. These findings collectively confirm that IL-23 is able to drive effector TH-17 cell function in target organs.
Impaired TH-17 proliferation correlates with failed differentiation
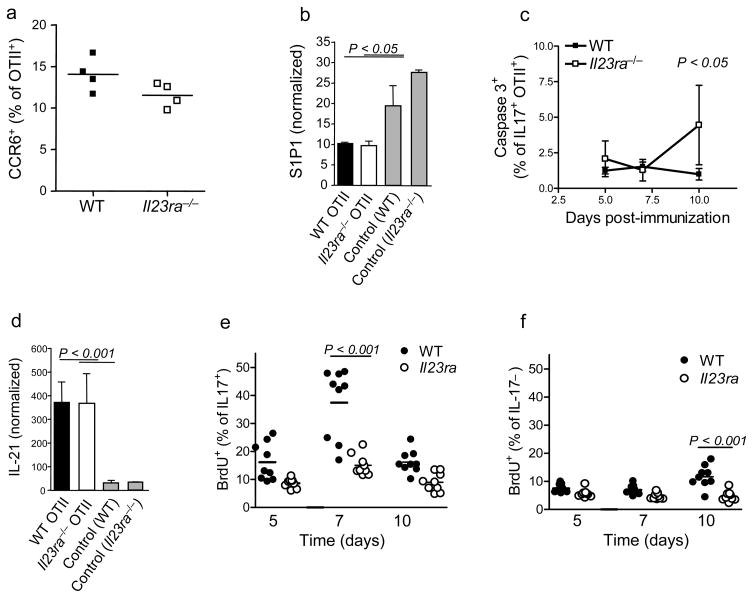
We have thus far shown that IL-23 acts in the lymph node to drive differentiation of effector TH-17 cells, but this does not explain the failure of Il23ra−/− T cells to enter the circulation in large numbers. There are three potential explanations to explain this: Il23ra−/− T cells are unable to egress out of the lymph node, Il23ra−/− IL-17+ T cells die more readily or Il23ra−/− IL-17+ T cells do not expand during the response. In terms of migration, CCR6 has been described as an important chemokine receptor expressed by TH-17 cells6,38. The proportion of wild-type and Il23ra−/− OTII cells expressing CCR6 at day 10 did not differ significantly (Fig. 8a). Sphingosine-1-phosphate (S1P) regulates lymphocyte egress from the lymph node39. Compared to wild-type OTII cells expression of the receptor for sphingosine-1-phosphate, S1P1 was not altered in Il23ra−/− cells on day 7 post-immunization (Fig. 8b). Although this does not preclude differential expression of S1P1 on IL-17+ cells within the total OTII population, together with the expression of CD62L and CCR7 (Fig. 4a), it seems unlikely that the failure of large numbers of Il23ra−/− cells to leave the lymph node was due to a single defect in expression of receptors regulating entry and exit from lymph node. Furthermore, the numbers of Il23ra−/− cells in the lymph node are not increased relative to wild-type, as would be expected if they were accumulating there, again suggesting that migration is not the major defect.
Figure 8. Proliferation of IL-17+ cells is most affected by IL-23R deficiency.
(a) Frequency of OTII+ cells expressing CCR6 in dLN on day 10 post-immunization. (b) S1p1 gene expression in OTII+ cells sorted by flow cytometry from dLN on day 7, values are normalized to ubiquitin. As a control, total OTII–CD4+ cells were isolated at the same time from recipients of the indicated OTII cells. (c) Proportion of IL-17+ cells that were positive for active caspase 3 was assessed by intracellular staining in OTII cells from dLN at indicated times post-immunization. (d) IL-21 expression in OTII+ cells sorted by flow cytometry from dLN on day 7; values are normalized to ubiquitin. (e.f) Analysis of BrdU incorporation by OTII cells 4 hour after in vivo administration of BrdU on indicated days post-immunization; expressed as proportion of IL-17+ (e) and IL-17− (f) OTII cells that were positive for BrdU. Data shown are representative of 2 independent experiments each with 4–5 mice/group, except (e,f) representative of 4 independent experiments with 4 mice per group.
It has been proposed that IL-23 may mediate survival of TH-17 cells19. It is difficult to measure apoptosis in vivo since dying cells are cleared so quickly, but there did not appear to be any difference in the frequencies of caspase 3-positive cells when gating on IL17− cells (data not shown). There was an increase in caspase 3-positive apoptotic cells in the Il23ra−/− IL17+ cohort only on day 10 post-immunization (Fig. 8c). This finding correlates with the reduced expression of IL-7Rα and decreasing frequencies of IL-17+ cells at this timepoint, suggesting that as a consequence of IL-23R deficiency TH-17 cells do become more susceptible to death over time. However, there are already fewer IL-17+ cells by day 7 and very few by day 10, so it seems unlikely that this effect is sufficient to explain the difference in frequencies of cells in the blood at day 10.
In terms of the survival and maintenance of cells of the TH-17 cell phenotype, IL-21 is known to act as an autocrine growth factor for TH-17 development and is induced by IL-6, IL-23 and IL-21 itself18,40,41. To assess whether IL-21 induction was affected by IL-23R deficiency, OTII T cells were purified from draining lymph node on day 7 post-immunization. Expression of IL-21 mRNA was equally increased in both wt and Il23ra−/− OTII T cells compared to control non-transgenic (OTII-) CD4+ cells isolated from the same lymph node (Fig. 8d). Therefore it appears that IL-23 is not required for production of IL-21, and most likely IL-6 and IL-21 itself are sufficient.
Our final hypothesis was that the proliferation of activated IL-17+ Il23ra−/− T cells was reduced, resulting in fewer effector cells. The proportion of OTII cells in cycle at difference timepoints was therefore assessed by measuring bromodeoxyuridine (BrdU) incorporation four hours after administration in vivo. By focusing in on IL-17 production, it was clear that IL-23R deficiency resulted in significantly fewer proliferating IL-17+ cells on day 7 (Fig. 8e). In contrast, Il23ra−/− IL-17− cells were similar to wild-type at earlier timepoints but had significantly reduced frequency of cells in cycle on day 10 (Fig 8f), at which point the Il23ra−/− population also had reduced IL-17 production (Fig 3c). Although single IL-17+ cells had incorporated the most BrdU, the difference in proliferation between wt and Il23ra−/− IL-17+ cells could not be further subdivided based on IL-2 expression within the IL-17+ cells (data not shown). The finding that IL-17+ cells proliferate less on day 7 is highly significant because this time point provides the link between the requirement for IL-23 on day 5 and the large defects that become most apparent on day 10 (reduced IL-17 production, lack of effector cells in blood). Reduced cycling in the absence of IL-23 was also confirmed in the dLN and CNS of mixed bone marrow chimeric mice 11 days postimmunization to induce EAE (Supplemental Fig 5). Proliferation and differentiation of helper T cells are intimately linked32 and as already discussed, activated T cells in the blood have a more differentiated phenotype than those in the lymph node. Taken together, these results strongly support the conclusion that IL-23 drives expansion of TH-17 effector cells.
Discussion
In this study, we have compared the role of IL-23R in the EAE and OTII and DTH models and found concordance of the results. During the initial 5 to 7 days of the response, OTII cells were unaffected by IL-23R deficiency and their frequencies in the blood were similar to wild-type cells. Likewise, at the early time point in the chimera EAE system the ratio of wild-type to Il23ra−/− CD4+ cells in the CNS was unaffected, although numbers were very low. At day 10, there were obvious functional defects apparent in the phenotype of the Il23ra−/− OTII cells, and their frequency in the blood remained low compared to the large increase of wild-type cells. Similarly, at onset of EAE (day 11), it was clear that the number of Il23ra−/− CD4+ cells had not greatly increased in the CNS compared to wild-type cells, and the Il23ra−/− CD4+ cells that were present were not producing IL-17, consistent with a low frequency of Il23ra−/− antigen-specific effector cells available to enter theCNS from the circulation at this time point. Therefore, we can combine results from these two systems to understand the requirements for IL-23 signaling during TH-17 development in the lymph node and function in target tissues.
Our results have identified a far more central function for IL-23 in TH-17 differentiation than previously thought. As expected, early TH-17 development appeared normal in the absence of IL- 23R, with expansion of antigen-specific T cells and induction of IL-17. However, these cells did not progress in their differentiation and did not down-regulate CD27 or re-upregulate IL7Rα. Il23ra−/− cells also failed to generate large numbers of IL-17-producing progeny that could enter the bloodstream and therefore did not infiltrate tissues in large numbers or successfully initiate inflammation. Importantly, neutralizing antibody experiments demonstrated that these effects relied on IL-23 signals received between days 4 to 6, before the appearance of large numbers of effector TH-17 cells. Furthermore, the timing of this requirement for IL-23 signaling also directly preceded the effects on proliferation of IL-17+ cells. Taken together, these results show that rather to increase the number or to maintain already-differentiated TH-17 cells, IL-23 is in fact required for the differentiation process to generate large numbers of effector TH-17 cells.
Studies have demonstrated that IL-23p19-deficient mice have a normal response to both toxoplasma36 and mycobacteria42 infections. However, while IL-23 does not appear to play a central role in protective responses to these infections, the presence of IL-23 can partially compensate for the absence of IL-1236. Our data further confirms that IL-23R is not required for a fulminant TH1 response, and also that IL-23 does not repress an outgrowth of TH1 cells since there was no increase in IFN-γ+ cells in its absence. However we did observe a reduction in IFN-γ+IL-17+ cells. These double-positive T cells are found in many inflammatory sites, but their role in disease pathogenesis is still unclear. Our results suggest that the double IFN-γ IL-17 producers are more TH-17-like than TH1-like, as they were reduced IL-23R-deficient mice, just as are IL-17+ cells. Finally, the normal TH1 response in absence of IL-23R suggests that the defects in activation seen in Il23ra−/− T cells are specific to TH-17-inducing conditions rather than to global activation of T cells.
The number of activated T cells in the lymph node at any given time is a function of the rates of proliferation, death and migration. The results presented here suggest the reduced proliferation rate of Il23ra−/− OTII cells, particularly IL-17+ cells, later in the response results in the failure to generate large numbers of cells that egress into the blood, and together these two factors balance so that the overall number of OTII cells in the lymph node is not greatly altered compared to wild type. Given that the Il23ra−/− population never attains a high frequency of single IL-17+ or CD27lo cells, and that there is not a great increase in apoptotic cells, it seems likely that it is the actual generation of effector cells that is affected, rather than the survival of these effector cells. This would be consistent with the decreased BrdU uptake and reduced proliferation rate in the absence of IL-23, as cell division is known to be linked with cellular differentiation32. We therefore conclude that the major function of IL-23 in TH-17 biology is to drive terminal differentiation; in its absence, TH-17 cells experience ‘arrested development’ leading to impaired function.
The following question then arises: is IL-23 merely driving proliferation of developing TH-17 cells and thus facilitating their differentiation? There are several observations that suggest this is not the case. In the initial description of the predominant role of IL-23 rather than IL-12 in EAE induction, we demonstrated that forced expression of IL-23 in the brain of IL-23-deficient mice was able to restore their susceptibility to inflammation2. Using the OTII model, we have shown here that injection of IL-23 in the site of antigen challenge was able to partially restore the DTH response in IL-23-deficient mice. These two findings suggest that although TH-17 effector generation in the lymph node is greatly impaired in the absence of IL-23, for the few cells that do migrate into the blood, exposure to IL-23 in the periphery may be sufficient to activate effector functions (i.e. drive terminal differentiation) to a sufficient level to induce inflammation. In the DTH model, one injection of soluble cytokine was used and the response was temporary. In the EAE experiments, adenoviral expression resulted in more sustained IL-23 production most likely explaining the greater efficacy in disease induction. In addition, IL-23 enhanced IL-17 production by CNS-derived T cells in a short time period in vitro, and IL-23 has previously been shown to be required for production of other TH-17-associated pro-inflammatory cytokines such as IL-2215,16. Taken together, these studies support the hypothesis that IL-23 can act both in the lymph node and peripheral tissues to drive terminal differentiation of effector cells, and that proliferation of cells developing in the lymph node is only one facet of IL-23 functions in promoting TH-17-mediated inflammation.
The failure of IL-23R-deficient T cells to re-express IL-7Rα after activation may have long-term consequences for these cells. Although it is down-regulated during early activation, IL-7Ra re-expression is required for survival of effector and memory T cells29,30,34. There is currently little data available whether effective TH-17 memory responses can be generated in autoimmune models, but two vaccination studies using the pathogens Mycobacterium tuberculosis and Bordetella pertussis suggested this is possible43,44. Elson et al have reported increased numbers of apoptotic CD4+ T cells following long-term in vivo neutralization of IL-23 in a colitis model, although the mechanisms were not clear19. Indeed, we also observed a slight increase in caspase3+ IL17+ Il23ra−/− cells on day 10, although the low frequency of IL-17+ cells makes it difficult to assess the significance of this observation. Overall then, it seems likely that survival of effector TH-17 cells and/or TH-17 memory formation would be impaired in the absence of signals from IL-23.
TH-17 development is known to depend on STAT3 signaling17,24,25 and expression of the transcriptional regulator RORγt10 and RORα45. It has also been suggested that STAT4 may contribute to TH-17 development, since cells from STAT4 ‘knockout’ mice have partially reduced IL-17 production25, although others report no role for STAT446,47 and we were unable to detect high levels of STAT4 phosphorylation in T cells following stimulation with IL-23 (data not shown). IL-6, IL-21 and IL-23 are all strong activators of STAT3 phosphorylation, and since IL-6 and possibly IL-21 are required for early differentiation of TH-17 cells, it is very difficult to investigate the requirement for STAT3 phosphorylation by IL-23 during later differentiation. However, we were able to confirm that STAT3 is phosphorylated by IL-23 ex vivo in developing TH-17 cells, and also that STAT3 is required for TH-17 production of IL-17 in vitro. Hence we believe that IL-23 acts through STAT3, although there may also be additional pathways activated.
In conclusion, we have shown that IL-23R is required for terminal differentiation and therefore function of TH-17 cells in vivo. These findings also have important implications for targeting IL-23 and its receptor therapeutically in TH-17-mediated diseases in humans. Our results suggest that the beneficial effects of neutralizing IL-23 may not be instantaneous since IL-23 promotes but does not appear to be required for effector functions of already generated TH-17 cells. However, the effects should be long-lasting since ongoing TH-17 generation should be blocked, and potentially TH-17 memory responses will be impaired thereby impacting rates of disease relapse, as we have observed in the EAE model. In addition, the ‘upstream’ position of IL-23 in inflammatory cascade compared to downstream mediators such as TNF allows hope for greater efficacy. In some diseases such as psoriasis, there are strong indications that IL-23 acts not only on TH-17 cells but also innate immune cells, and in these circumstances targeting IL-23 may prove even more effective. The success of anti-IL-12 p40 clinical trials in psoriasis48 suggest this may be the case, but clinical testing of neutralizing IL-23 will specifically address this question.
METHODS SUMMARY
Mice
Generation of Il23ra−/− mice has previously been described21. Mixed bone marrow chimeras were generated as described (Supplemental Fig 1). Il23ra−/− OTII+CD45.1+ mice were generated at Schering Plough Biopharma. All animal procedures were approved by the Schering Plough Biopharma IACUC committee, in accordance with AAALAC guidelines.
Reagents
The following FACS antibodies were purchased from BD Biosciences : CD4 (RM4–5), CD45.1 (A20), CD44 (IM7), CD62L (MEL-14), CD27 (LG.3A10), CD25 (7D4), CCR6 (140706), BrdU (51–23619L) (used with BrdU staining kit from BD according to manufacturer’s instructions), IFN-γ (XMG1.2), IL-17 (TC11-18H10), IL-2 (JES6-5H4) (intracellular cytokine staining performed using Cytofix-cytoperm kit from BD according to manufacturer’s instructions), pSTAT3(pY705) (4-P-STAT3) (used with Phosflow staining kit from BD according to manufacturer’s instructions), caspase 3 active form (C92–605). The following were purchased from eBioscience: CCR7 (4B12), Foxp3 (FJK-16s) (used with Foxp3 staining kit from eBioscience according to manufacturer’s instructions), IL7Rα (A7R34).
EAE
C57BL/6 mice (including Il23ra−/− and mixed bone marrow chimeras) were immunized with 100 μg MOG 35–55 in 200 μl CFA containing 100 μg H37Ra in four sites on the back. SJL mice were immunized with 100 μg PLP 139–151 in 200 μl CFA (IFA containing 20μg Heat Killed Mycobacterium tuberculosis H37Ra (both Difco) in four sites on the back. All mice also received 100 ng Pertussis toxin (List Biological Laboratories) intraperitoneally on day 0 and 2. EAE was assessed according to the following clinical grades: 1: flaccid tail; 2: impaired righting reflex and hindlimb weakness; 3: partial hindlimb paralysis; 4: complete hindlimb paralysis; 5: hindlimb paralysis with partial forelimb paralysis; 6: moribund.
OTII tracking studies
Recipient mice (CD45.2+) received 105 CD45.1+ Il23ra−/−, Il23ra+/+, or a 50:50 mix of Il23ra−/− + Il23ra+/+ OTII CD4+ T cells intravenously one day before immunization with 100μg OVA(323–339) in CFA subcutaneously in the flank. Phenotype of OTII+ cells was assessed by flow cytometry, gating on live CD4+CD45.1+ cells, except caspase 3 analysis where all lymphocytes were included in analysis. For BrdU analysis, mice were injected intraperitoneally with 1mg BrdU (BD Biosciences) four hours before sacrifice. Intracellular phosphorylated STAT3 levels were assessed using BD Phosflow protocol as directed.
T cell stimulation for cytokine analysis
CNS mononuclear cells were isolated as previously described2,4 from mixed bone marrow chimeras with EAE and cells were cultured in complete media (RPMI media containing 10% FCS, supplemented with Pen-Strep, L-Glutamine, HEPES, sodium pyruvate, and 2-ME) with 100 μg/ml MOG(35–55) in the presence or absence of IL-23 (SPB) for 24 hours. Golgiplug (BD Biosciences) was added for the final 4 hours of culture before FACS analysis. For stimulation of OTII T cells ex vivo for cytokine analysis, dLN were harvested at indicated timepoints post-immunization and single cell suspensions obtained. Cells were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin (both Sigma-Aldrich) in the presence of Golgiplug (BD Biosciences) for 4 hours in complete medium followed by FACS staining and analysis. For stimulation of OTII cells ex vivo for STAT 3 phosphorylation analysis, dLN cell suspensions were incubated with complete medium alone, 50ng/ml IL-23 or 50 ng/ml IL-6 for fifteen minutes.
Delayed Type Hypersensitivity
Immunized recipient mice of OTII cells were challenged in one hind footpad with 100μg ovalbumin in saline; the contralateral footpad was injected with saline. Foot thickness was measured using calipers (Mitutoyo) and data shown as increase in thickness over the saline control. Where indicated, 7μg IL-23 was given in addition to the ovalbumin or saline control.
Toxoplasma gondii infection
Aged matched female C57BL/6 or indicated ‘knockout’ mice were infected intra-peritoneally with 20 cysts of the ME-49 strain of T. gondii. Draining lymph nodes were removed from mice at day 9 post-infection and cultured 4 hours with golgistop in the presence or absence of PMA and ionomycin. Cells were then stained with Pacific-blue conjugated anti-CD4, PE-conjugated anti-IL-2, and APC-conjugated IFN-γ before analyzed by flow cytometry for the presence of cytokine producing T cells.
Retroviral STAT3 depletion
Naive (CD62L+CD44−) CD4+ STAT3fl/fl cells were stimulated with anti-CD3 and anti-CD28 for three days in the presence of TGF-β (5ng/mL), IL-6 (10ng/ml) and anti IL-2 (10ug/ml). Cells were washed on day three and left to expand for 4 days in the presence of IL-2. During this time cells were infected with MIGR-GFP (control) or Cre-GFP retrovirus in the presence of 4ug/mL Polybrene. On day 8 cells were restimulated for a further three days in the presence or absence of IL-23 (10ng/mL).
IL-23R neutralization in vivo
Mouse anti-mouse IL-23R monoclonal antibody (21A4) was generated at SPB. A single dose of 200 μg was delivered intraperitoneally on indicated days post-immunization with OVA(323–339) or induction of EAE with PLP(139–151).
Statistics
Where appropriate, ONE-WAY ANOVA (for multiple groups) or Student’s t-test were performed, P values are shown where statistical significance was found, and all data are represented as means + standard deviation (s.d.).
Supplementary Material
Acknowledgments
We would like to acknowledge the assistance of Bela Desai and Steve Jungers with FACS sorting, Caroline Diveu and Rob Kastelein for helpful discussions.
Footnotes
Author contributions: MM and DC designed experiments and wrote the manuscript. MM carried out most experiments with assistance from BJS and YC, CT performed Toxoplasma experiments, AL and JO provided Cre-STAT3 knockdown data, WB and TM performed gene expression analysis.
References
- 1.Oppmann B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23–IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–7. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 8.Duerr RH, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RL, et al. Polymorphisms in the IL-12beta and IL-23R Genes Are Associated with Psoriasis of Early Onset in a UK Cohort. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701140. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 12.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 14.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 15.Liang SC, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 17.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007 doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 19.Elson CO, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 20.Hue S, et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–83. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan JR, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–87. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 23.Izcue A, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell dependent colitis. Immunity. 2008;28:559–70. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris TJ, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–7. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 25.Mathur AN, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–7. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 26.Parham C, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 27.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–6. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 28.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–6. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Kondrack RM, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–15. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritsch RD, et al. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;175:6489–97. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 32.Bird JJ, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–37. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 33.Villarino AV, et al. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J Exp Med. 2007;204:65–71. doi: 10.1084/jem.20061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204:547–57. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–81. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman LA, et al. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173:1887–93. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- 37.Miller SD, et al. Evolution of the T-cell repertoire during the course of experimental immune-mediated demyelinating diseases. Immunol Rev. 1995;144:225–44. doi: 10.1111/j.1600-065x.1995.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 38.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 39.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 40.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 41.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chackerian AA, et al. Neutralization or absence of the interleukin-23 pathway does not compromise immunity to mycobacterial infection. Infect Immun. 2006;74:6092–9. doi: 10.1128/IAI.00621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–9. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 44.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4(+) T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 45.Yang XO, et al. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors RORalpha and RORgamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 48.Krueger GG, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–92. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.