Abstract
Over two decades, significant advances in our understanding of the humoral immune response to HIV-1 infection have been made, yet a tremendous amount of work lies ahead. Despite these advances, strategies to reliably induce antibodies that can control HIV-1 infection are still critically needed. However recent advances in our understanding of the kinetics, specificity and function of early humoral responses offer alternative new approaches to attain this goal. These results along with the new broad NAb specificities, the role for other antibody functions, the increased understanding of HIV-1-induced changes to B cell biology, and results from the RV144 “Thai” trial showing potential modest sterilizing protection by non-neutralizing antibody responses, have renewed focus on the humoral system. In this review, recent advances in our understanding of the earliest humoral responses are discussed, highlighting presentations from the meeting on the Biology of Acute HIV Infection (AHI).
Keywords: B cells, AIDS, antibodies, HIV-1
Introduction
Since the discovery of HIV-1, study of the antibody response to the virus has been integral to the search for mechanisms of virus inhibition and the development of strategies to prevent infection. The initial antibody response to HIV-1 is primarily directed at non-neutralizing epitopes on envelope (Env), while antibody responses that neutralize transmitted and early infection virus isolates are delayed, arising several months after the transmission event [1]. Broadly neutralizing antibody (NAb) responses arise in a minority of patients and are generally not associated with control of viremia [2–4], and in most patients NAb responses lag behind virus escape [5, 6]. More than two decades of research has yielded a small number of human monoclonal antibodies capable of neutralizing a wide range of virus isolates [7–14] and many antibodies with narrow breadth [15]. Furthermore, changes in the humoral immune system have been described in patients with AIDS and include abnormal B cell activation [16], a loss of recall responses to antigens [17] and the development of autoantibodies and other autoimmune manifestations [18, 19]. Passive protection trials in non-human primates have shown that monoclonal antibodies of sufficient titer and quality can be protective against live virus challenge [20, 21] and many vaccine efforts have been aimed at inducing broadly NAbs. Unfortunately, no vaccine candidates have reliably induced antibodies similar to the rare broad human NAbs [22, 23].
Several recent findings have re-energized interest in the potential of protective NAbs against HIV-1 either induced through vaccination or given in passive transfer. In addition to the well-characterized NAbs that bind to Env glycans (2G12 [7]), the Env gp41 membrane proximal external region (MPER) (2F5 [8, 12], 4E10 [11], Z13 [9, 14]), and the Env gp120 CD4-binding site (IgG1b12 [10]), another target for broad NAbs involving the Env gp120 V1/V2 and V3 regions has been identified [13, 24, 25]. This quarternary epitope is expressed on neutralization-sensitive trimeric Env and can be partially blocked by soluble CD4 binding [13, 24]. Type-specific NAbs to this region have been reported [24, 25], and recently two new broad NAbs against this epitope were identified using a high-throughput screen of over 30,000 activated memory B cells from 1800 infected donors [13]. This additional NAb target, coupled with the observation that NAb breadth occurs more frequently than previously thought—up to 20% of subjects ultimately develop a degree of breadth [4]—provides encouragement that such broadly neutralizing NAbs can arise naturally and therefore the right vaccine might also induce a NAb response.
Another hopeful note has been struck by the results of the ALVAC prime/recombinant-Env gp120 boost RV144 Phase III “Thai” trial [26]. In the modified intention-to-treat analysis, a 31% vaccine efficacy was found with suggestive evidence that protection occurred early after vaccination. Thus, the vaccine produced a weakly protective effect against acquisition but not viral control following infection, potentially related to the levels of binding antibodies elicited. Although the magnitude of the effect was small, these results are in stark contrast to the failures of other high profile studies including the VaxGen/AIDSVAX trial [27], the Merck STEP trial [28], and the failed cellulose sulfate microbicide trial [29]. These new study results suggest that the kinds of protective responses desired from vaccines may be achievable.
On September 22nd and 23rd, 2009, a symposium was held in Boston, MA on the Biology of Acute HIV Infection (AHI). The humoral session of the Boston AHI meeting reported on a wide range of aspects of the role of the humoral immune system in acute HIV-1 infection. The talks spanned topics including B cell development/germinal center formation, the evolution of the autologous and broadly NAb responses, and definition of additional responses mediated by antibodies that may play a key role in containment of viral replication. These topics and an overview of related literature constitute the remainder of this review.
B Cell Development and Dysfunction
The development of B cells occurs in the bone marrow with committed lymphocyte precursors that undergo sequential immunoglobulin gene rearrangements [30]. These rearrangements ultimately lead to the expression of surface immunoglobulins with specificity identical to that of the antibody secreted by the same cell [31]. B cells undergo initial positive and negative selection events before transitional 2 B cells emigrate from the bone marrow and become naïve B cells [32]. Once naïve B cells encounter antigen combined with support signals from T cells, naïve B cells mature and become memory and plasma cells which are respectively responsible for recall responses and for secreting high levels of plasma antibodies.
Unlike their T cell counterparts, antibody-secreting cells do not need to circulate in blood to perform their “effector” function. B cells in various maturational stages can circulate in the blood but also can reside in tissues including specialized lymphoid tissues like the spleen, lymph nodes, tonsils, and bone marrow. Similar to T cells, B cells reside in other tissues and comprise a portion of the lymphocytes resident in mucosa-associated lymphoid tissues in the genitourinary and gastrointestinal tracts. As with T cells, it is thought that tissue B cells play a critical role in host defense from pathogens and commensal organisms. A number of studies of HIV-1+ lymphoid tissues noted structural changes to B cell follicles including follicular hyperplasia that correlated with the development of clinical lymphadenopathy [33, 34]. These early studies did not demonstrate destruction of B cell follicles until late in clinical disease [33, 34].
More recent studies have established that gastrointestinal tract CD4+ T cells are a principal site for HIV-1 and SIV replication [35, 36], that depletion of those cells occurs early during infection [37, 38], and that those T cells are not rapidly replaced after anti-retroviral therapy is started [39]. These data, combined with data presented at the AHI meeting, suggest that HIV-1-associated damage to lymphoid tissues in the gastrointestinal tract, along with loss of CD4+ T cell help, may explain the delay in NAb production following HIV-1 transmission [40]. This study focused on the effect of HIV-1 infection on the humoral immune compartment in acute and early HIV-1 infection patients, identified through clinical protocols established by the Center for HIV/AIDS Vaccine Immunology (CHAVI). Patients donated peripheral blood and/or terminal ileal biopsy specimens which were analyzed using immunohistochemistry and quantitative image analysis as well as flow cytometry. Using a variety of markers to identify both primary and secondary germinal centers in terminal ileal tissues, the study found evidence of germinal center damage in >80% of germinal centers identified in specimens from acute/early HIV-1 infection patients compared to <5% from healthy control tissues. Similarly, when germinal centers were analyzed for the presence of secondary follicle formation, indicative of B cell selection and maturation with support from follicular dendritic cells and T cells, two-thirds of follicles studied from healthy tissues showed evidence of secondary germinal center formation compared to one-third in those from HIV-1 infected patients. Together these data showed that, in terminal ileum, the environments that support B cell maturation were severely damaged in HIV-1 infection. These data contrasted with those previously described for other lymphoid tissues, but correlated well with data on early T cell loss following HIV-1 transmission [35–39].
Flow cytometric analysis showed that in terminal ileum and peripheral blood there was a decreased fraction of B cells with a naïve phenotype and a corresponding increase in B cells with a memory B cell and plasma cell phenotype [40]. In particular, the fraction of circulating B cells with a plasmablast/plasma cell phenotype was higher in acute/early HIV-1 infection patients [40], which for some patients was higher than the fraction reported for other models of antigen stimulation such as influenza immunization [41]. The reason for this increase in circulating plasma cells in acute/early HIV-1 infection is not known but it may be due to the high levels of cytokines circulating during early infection [42]. At this time, work is ongoing to determine the significance of the plasmacytosis observed during acute/early HIV-1 infection and to compare this with other disease and vaccination models.
Broadly Neutralizing Antibodies
NAbs can be detected in most patients who are infected with HIV-1 at some point in the first year of infection after seroconversion [43]. This antibody response has been described as ineffective because autologous NAbs often lag behind changes in the virus and rarely target the contemporaneous virus [6]. Alternatively, NAbs have been shown to drive virus escape [44] and may therefore be responsible for control of viremia in some patients after cessation of anti-retroviral therapy [45]. NAbs can fall into two categories—antibodies that bind to variable regions of HIV-1 and that neutralize a relatively narrow group of isolates and those that bind to conserved regions of the virus and that can neutralize a wider range of isolates, including those in different subtypes or from diverse geographical regions [23]. For reasons that are not entirely clear, the initial antibody response to transmitted HIV-1 in most individuals are primarily of a narrow spectrum, while broadly NAbs are produced in only a fraction of infected individuals and when produced, occur later in HIV-1 infection [4].
Recently, a number of new studies describing escape of HIV-1 from NAb pressure have begun to shed light on this phenomenon. All HIV-1-infected individuals produce autologous NAb responses that the virus rapidly evades [5, 6]. In her presentation, Dr. Lynn Morris postulated that identifying NAb specificities that evolve naturally may identify vulnerable regions of HIV-1 that the immune system is able to target. Given the difficulty in trying to elicit broadly NAbs thus far [22, 23], induction of these early NAb responses could serve as a strategy to induce protective NAbs through vaccination. To define the pattern and evolutionary kinetics of these autologous humoral response, autologous NAb activity was examined in a well characterized acute infection cohort, the CAPRISA cohort [2, 46]. Although the kinetics of the autologous NAb responses varied, all subjects developed autologous NAbs, with a delay ranging from 9 weeks post infection up to one year, but these kinetics did not correlate with the rate of disease progression [2]. Autologous NAbs targeted particular regions of the HIV-1 Env in a sequential manner, with the C3 domain of Env being the earliest target followed by the V1/V2 region, and later extending to other regions of the Env complex [47]. The NAb targets were confirmed following back-mutation of later viral variants which were engineered to encode for earlier wild-type sequences that restored NAb sensitivity. In a representative example, 2 waves of NAbs were observed, and backmutation of the later viral constructs demonstrated that that these waves represented two different NAb specificities that developed at distinct time points following infection, first to the C3 domain and then later to the V1/V2 regions of the viral Env. This staged response developed despite the fact that the second NAb epitope was present in the earliest viral isolates, suggesting that immunodominace patterns may drive reproducible antibody targeting of vulnerable regions on the viral Env [47]. In the other individuals studied, the pattern varied in such a way that although the NAbs targeted the predicted areas, escape mutations that conferred escape from NAb recognition occurred at a distance from these regions of the viral Env.
The phenomenon of sequential antibody responses to transmitted HIV-1 has been described in other contexts (figure 1). The earliest detectable humoral responses to transmitted HIV-1 can be detected in plasma donor pools and were described by Tomaras, et al. [1]. These responses consist of immune complexes of antibody and HIV-1 virions that arise on average at 8 days after the onset of viremia, followed in 5 days (on average 13 days after the onset of viremia) by free antibodies directed against Env gp41. In a majority of samples studied, the initial antibody response consisted of simultaneous IgM and IgG antibodies suggesting early class switching associated with HIV-1 infection. Interestingly, compared to Env gp41 antibodies, gp120 antibody responses were delayed occurring on average 28 days after the onset of viremia—this phenomenon occurs despite simultaneous exposure of the immune system to both antigens. While waves of antibody responses to different viral antigens are not unknown (e.g., patterns of antibodies associated with Epstein-Barr virus infections [48]) these antigens are often involved in different stages of the virus life cycle. The reasons for the development of sequential antibody responses to HIV-1 Env are as yet unknown.
Figure 1.
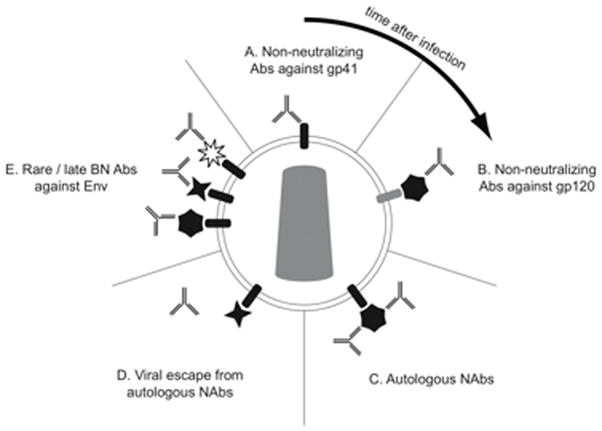
The antibody response to HIV-1 occurs in stages, shown here in a clockwise direction starting at the top. A. The initial antibody response to HIV-1 is non-neutralizing and directed at gp41. B. Soon thereafter arise non-neutralizing antibodies directed against gp120. C. After a delay of weeks to months, autologous neutralizing antibodies arise that apply selection pressure on the virus. D. Viral mutation results in neutralization escape by HIV-1, represented here by a change in the shape of gp120. E. In some patients, antibodies that can neutralize a wide range of HIV-1 isolates arise, represented here by a variety of shapes of gp120. Mixing of envelope shapes on a single virus particle is shown for illustrative purposes only.
Additional data presented by Morris showed that in some patients, broad autologous NAb responses may occur at early time points. One particular subject, CAP206, developed an anti-MPER antibody detectable after only 6 months of infection [49]. This NAb was able to inhibit a wide range of viruses and the activity was absorbable by a peptide with the MPER sequence. Using a number of techniques, the Morris group is pursuing further studies to isolate the antibody to determine whether it was a novel B cell clone that arose spontaneously or if it is the result of affinity maturation of an original autologous NAb response.
To determine the impact of autologous Nabs on antiviral control and viral evolution, the Morris group used quantitative PCR to track fluctuations of wild-type and emerging quasispecies/viral variants[47]. The Morris group was able to demonstrate that following the appearance of the first NAb wave, the overall viral load declined consistent with the decline in the wild-type viral variant levels. This was followed by the emergence of an escape variant that then out-competed the wild-type isolate, resulting in slight increase in the overall viral load. This observed “blip” in overall viral load in parallel with antibody-induced-emergence of alternate viral quasispecies suggests that similar fluctuations in viral load may reflect the emergence and disappearance of viral variants under immune pressure.
Another set of studies focused on HIV-1 subtype C was presented by Cynthia A. Derdeyn of Emory University. Serodiscordant couples from a cohort established in Lusaka, Zambia were recruited and the HIV-1 negative partner was tested every three months until infection occurred; after infection, the partners provided longitudinal samples every three months [50]. Previous work from this cohort demonstrated that autologous NAbs in some patients were detectable at two months after infection, suggesting that the antibody response in this group of subtype C infected individuals might differ from that found in subtype C infection in the CAPRISA cohort or in subtype B infection [51]. The majority of subtype C infections in this cohort appeared to be from a single transmitted virus [52], consistent with similar studies of subtype B infection [53]. Using a single genome amplification technique, the group cloned functional Env genes and generated unmutated pseudoviruses as well as chimeras and mutants that were used in a single-round neutralization assay to study escape [50]. Consistent with reports from other investigators, virus isolates resistant to neutralization with sera at a given time point could be detected at every time point tested, suggesting that the antibody response in these patients lagged behind HIV-1 mutations.
Using chimeric and mutated Envs, a number of interesting findings emerged. No single Env domain or restricted set of Env domains were found to dominate the autologous NAb response. In contrast to subtype B infections, the V3 loop was not a prominent epitope for NAbs but, similar to data from the CAPRISA cohort, evidence of escape mutations in V1/V2, V5, the α2 helix, and the gp41 ectodomain were found. In one patient, changes in glycosylation were found to be associated with escape, both with glycan loss in V1 and glycan gain in V2. Most intriguingly, a pattern of convergent pathways was found for one patient at 28 months after infection, where for one Env isolate changes to V5 conferred resistance while for a different Env isolate resistance was conferred by changes to V1V2 and the gp41 ectodomain. Thus similar to observations by the Morris group, these data from the Lusaka cohort confirm that the NAb response may not always drive HIV-1 mutation in a linear fashion, but that it may select different mutational profiles through sequence, glycosylation, or structural features of the Env depending on other features of a given viral quasispecies. These data suggest that although predictable patterns exist in the kinetics of protein targeting in acute HIV infection, the pathways of escape may be more complicated.
Antibodies to Recruit Innate Immunity
Antibody functions not directly related to their ability to neutralize HIV-1 can play a critical role in prevention of infection. In fact, the constant region of the antibody is able to recruit a number of additional effector functions including the deposition of the complement system, stimulation of cytokine/chemokine secretion, triggering of phagocytosis, immunoregulation, and the recruitment of cytolytic activity [54]. All these activities are triggered following the interaction of the CH2-domain of the antibody with circulating complement or Fc-receptors found on immune cells (figure 2) [55, 56]. Given that Fc-receptors are present on all innate immune cells (including natural killer (NK) cells, dendritic cells (DCs), and monocytes, etc.) as well as on B cells, antibodies also play a central role in orchestrating and providing specificity for the potent antiviral activity of diverse innate immune cells. The roles of Fc-receptor binding and complement fixation are not equally involved in antibody mediated protection of SIV infection, as demonstrated by modifications of NAb IgG1b12 [57]. Sterilizing protection from infection was unaltered by changes to the Fc-region of IgG1b12 that abrogated complement recruitment, where as modifications that resulted in a loss of Fc-receptor engagement decreased the protective antiviral NAb potency, suggesting that the ability of this NAb to recruit the cells of the innate immune system via Fc-receptors may play a critical role in antiviral control [57]. Similarly, the efficacy of some therapeutic antibodies, such as the anti-CD20 antibody rituximab used for the treatment of B cell diseases like non-Hodgkin’s lymphoma and Epstein-Barr virus-driven post-transplant lymphoproliferative disorders, hinge on the capacity of that antibody to recruit the effector functions of innate immune cells such as NK cells [58, 59]. Several studies have shown that antibody-dependent cell-mediated cytotoxicity (ADCC) inducing antibodies can play a dramatic role in the early containment of several infections, however less is known about the role of this humoral immune function in the early control of HIV-1 infection [56].
Figure 2.
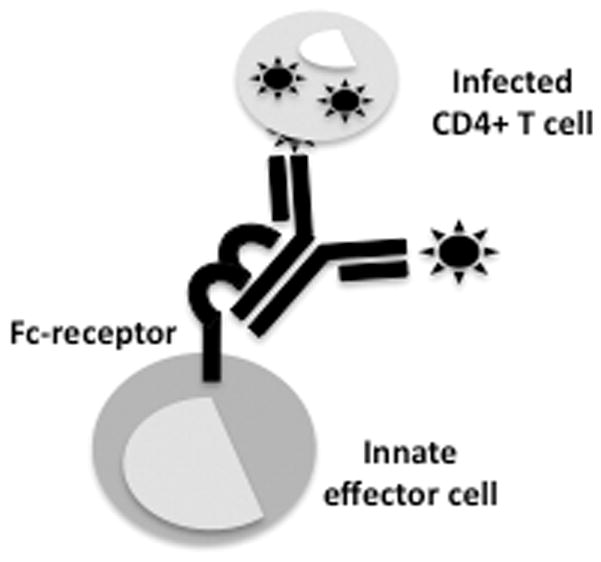
Antibodies act as a bridge that can provide specificity and therefore focus the antiviral activity of innate immune cells to eliminate or contain viral replication following an interaction with an Fc-receptor located on the surface of an innate immune effector cell (including monocytes, NK cells, DCs, neutrophils, and other granulocytes).
To define the role of ADCC inducing antibodies in antiviral control, the ADCC activity of antibodies was compared among HIV-1-infected individuals using D. Forthal’s antibody-dependent cell-mediated virus inhibition (ADCVI) assay [60, 61]. This activity was observed only in a fraction of chronically-HIV-1-infected patients who had active viral replication, whereas strong ADCVI activity was observed in individuals with undetectable viral loads either in the presence of antiretroviral therapy or in those with spontaneous elite control. Using an assay based on multi-parameter flow cytometry, antibodies from elite controllers were also able to recruit NK cell degranulation more effectively and with a broader polyfunctional profile NK cell responses were found compared to assays of antibodies from chronically infected individuals. Differences in the capacity of abs to recruit NK cell activity likely pertain to intrinsic differences in the Abs generated in different patient populations, given that effector (NK) cells and targets are all derived from HIV-negative control subjects. These results strongly suggest that, despite the fact that elite controllers typically exhibit reduced levels of NAbs[62], these individuals do elicit antibodies with properties that enable them to recruit the antiviral activity of NK cells, potentially contributing to their antiviral control.
In contrast to the Elite controllers, only a fraction of acutely HIV-1-infected individuals exhibited ADCC-inducing antibodies at baseline, however, of the individuals that exhibited ADCC activity, the activity was as potent as the antiviral activity of antibodies from elite controllers. Furthermore, ADCC-inducing antibodies from acutely infected individuals were also able to elicit polyfunctional NK cell responses. These data, combined with the fact that antiretroviral therapy was able to partially reconstitute ADCC activity, strongly suggest that persistent viral replication rather than high antigen loads, as is seen in acute HIV infection, may be responsible for a loss of ADCC-inducing activity in chronic progressive HIV-1 infection.
In addition to these changes in the quality of antibody-mediated recruitment of innate immune activity, effective ADCC is contingent on the presence of a functional effector cell bearing an Fc-receptor(s) [56]. Previous work has shown that ADCC activity declines with progressive infection, and while the antibody response has a diminished capacity to inhibit viral replication, it is also plausible that waning ADCC activity may also be attributable to changes in Fc-receptor expression in cells over the course of HIV-1 infection. In fact, defining the pattern of Fc-receptor changes that occur throughout HIV-1 infection may provide us with clues regarding the mechanism by which innate immune recruiting antibodies may mediate their antiviral control. Furthermore, it is possible that ADCC inducing antibodies may be particularly important during acute infection when viral loads decline dramatically following peak viremia [63]. Indeed, Fc-receptor was dramatically altered on the surface of innate immune cells at different stages of HIV infection, with a reduction of Fc-receptor expression on innate immune cells in chronic, progressive HIV-1 infection. In contrast, FcγR1 expression was significantly enhanced on monocytes in acute HIV-1 infection. However it is still unknown whether this elevation in FcγR1 is upregulated following all viral infections or only in response to HIV-1 specifically, or whether innate immune recruiting capacity is involved in the clearance and control of the virus early in infection.
Conclusion
Over two decades, significant advances in our understanding of the humoral immune response to HIV-1 infection have been made, yet a tremendous amount of work lies ahead. Despite these advances, we are still confronted with the task of creating strategies to reliably induce antibodies that can control HIV-1 infection. Antibodies against HIV-1 can display a wide array of reactivities including rare broadly neutralizing antibodies against gp41 MPER, potent CD4 binding site antibodies, as well as antibodies against the glycan shield and other conserved epitopes. These types of antibodies are, of course, the primary targets of current HIV-1 vaccine development. However, to date these antibodies have not been found early in infection nor have they been readily elicited by HIV-1 vaccine candidates. In contrast, antibodies that are commonly elicited by both vaccination and infection have not been considered capable of mediating protection, including weakly-neutralizing CD4 antibodies, co-receptor binding site and hypervariable region antibodies, as well as gp41 non-neutralizing antibodies. Recent developments in our understanding of autologous neutralizing antibodies as well as Fc-receptor-mediated anti-HIV-1 activity offer new approaches to attain protection from HIV-1 transmission at the mucosal surface. Moreover, data from the RV144 “Thai” trial suggests a modest level of sterilizing protection likely to have been elicited by non-neutralizing Abs, raising the hypothesis that potentially narrower breadth and/or innate immune recruiting capacities may have contributed to the prevention of acquisition. Intense efforts are underway to dissect both the cellular and humoral immune response in this vaccine trial to define potential correlates of protection. These results along with the new broadly NAb specificities, the potential role for other antibody functions, and the increased understanding of HIV-1-induced changes to B cell biology have renewed focus on the humoral system and efforts to recruit it as a whole rather than just its parts.
Acknowledgments
This work was supported by a Collaboration for AIDS Vaccine Discovery grant from the Bill and Melinda Gates Foundation; by the Center For HIV/AIDS Vaccine Immunology (CHAVI) Grant, U19 AI067854; and an NIH R01 AI080289.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- AIDS
acquired immunodeficiency syndrome
- Env
envelope
- HIV-1
human immunodeficiency virus type 1
- MPER
membrane proximal external region
- NAb
neutralizing antibody
- PBMC
peripheral blood mononuclear cells
Footnotes
The authors have no conflicts of interest.
References
- 1.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray ES, Moore PL, Choge IA, et al. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. Journal of virology. 2007;81:6187–96. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen X, Parks RJ, Montefiori DC, et al. In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth. Journal of virology. 2009;83:3617–25. doi: 10.1128/JVI.02631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nature Medicine. 2009;15:866–70. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 5.Frost SD, Trkola A, Gunthard HF, Richman DD. Antibody responses in primary HIV-1 infection. Curr Opin HIV AIDS. 2008;3:45–51. doi: 10.1097/COH.0b013e3282f310ae. [DOI] [PubMed] [Google Scholar]
- 6.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4144–9. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calarese DA, Scanlan CN, Zwick MB, et al. Antibody Domain Exchange Is an Immunological Solution to Carbohydrate Cluster Recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 8.Conley AJ, Kessler JA, 2nd, Boots LJ, et al. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41–2F5, an anti-gp41 human monoclonal antibody. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:3348–52. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson JD, Brunel FM, Jensen R, et al. An affinity-enhanced neutralizing antibody against the membrane-proximal external region of human immunodeficiency virus type 1 gp41 recognizes an epitope between those of 2F5 and 4E10. Journal of virology. 2007;81:4033–43. doi: 10.1128/JVI.02588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roben P, Moore JP, Thali M, Sodroski J, Barbas CF, 3rd, Burton DR. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. Journal of virology. 1994;68:4821–8. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stiegler G, Kunert R, Purtscher M, et al. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Research & Human Retroviruses. 2001;17:1757–65. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- 12.Trkola A, Pomales AB, Yuan H, et al. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. Journal of virology. 1995;69:6609–17. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker LM, Phogat SK, Chan-Hui PY, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–9. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zwick MB, Labrijn AF, Wang M, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. Journal of virology. 2001;75:10892–905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore JP, Cao Y, Qing L, et al. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. Journal of virology. 1995;69:101–9. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. The New England journal of medicine. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 17.De Milito A. B Lymphocyte Dysfunctions in HIV Infection. Current HIV Research. 2004;2:11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- 18.Kaye BR. Rheumatologic manifestations of infection with human immunodeficiency virus (HIV) Ann Intern Med. 1989;111:158–67. doi: 10.7326/0003-4819-111-2-158. [DOI] [PubMed] [Google Scholar]
- 19.Kopelman RG, Zolla-Pazner S. Association of human immunodeficiency virus infection and autoimmune phenomena. Am J Med. 1988;84:82–8. doi: 10.1016/0002-9343(88)90012-5. [DOI] [PubMed] [Google Scholar]
- 20.Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nature medicine. 2000;6:207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 21.Baba TW, Liska V, Hofmann-Lehmann R, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature medicine. 2000;6:200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 22.Burton DR, Desrosiers RC, Doms RW, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–236. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 23.Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14943–8. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorny MK, Stamatatos L, Volsky B, et al. Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. Journal of virology. 2005;79:5232–7. doi: 10.1128/JVI.79.8.5232-5237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honnen WJ, Krachmarov C, Kayman SC, Gorny MK, Zolla-Pazner S, Pinter A. Type-specific epitopes targeted by monoclonal antibodies with exceptionally potent neutralizing activities for selected strains of human immunodeficiency virus type 1 map to a common region of the V2 domain of gp120 and differ only at single positions from the clade B consensus sequence. Journal of virology. 2007;81:1424–32. doi: 10.1128/JVI.02054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. The New England journal of medicine. 2009 doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 27.Pitisuttithum P, Berman PW, Phonrat B, et al. Phase I/II Study of a Candidate Vaccine Designed Against the B and E Subtypes of HIV-1. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2004;37:1160–1165. doi: 10.1097/01.qai.0000136091.72955.4b. [DOI] [PubMed] [Google Scholar]
- 28.Sekaly R-P. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? The Journal of Experimental Medicine. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Wijgert JH, Shattock RJ. Vaginal microbicides: moving ahead after an unexpected setback. AIDS (London, England) 2007;21:2369–76. doi: 10.1097/QAD.0b013e3282ef83fd. [DOI] [PubMed] [Google Scholar]
- 30.Pillai S, Cariappa A, Moran ST. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunological Reviews. 2004;197:206–18. doi: 10.1111/j.0105-2896.2003.097.x. [DOI] [PubMed] [Google Scholar]
- 31.Julius MH, Janeway CA, Jr, Herzenberg LA. Isolation of antigen-binding cells from unprimed mice. II. Evidence for monospecificity of antigen-binding cells. Eur J Immunol. 1976;6:288–92. doi: 10.1002/eji.1830060410. [DOI] [PubMed] [Google Scholar]
- 32.Su TT, Guo B, Wei B, Braun J, Rawlings DJ. Signaling in transitional type 2 B cells is critical for peripheral B-cell development. Immunological Reviews. 2004;197:161–78. doi: 10.1111/j.0105-2896.2004.0102.x. [DOI] [PubMed] [Google Scholar]
- 33.Racz P, Tenner-Racz K, Kahl C, Feller AC, Kern P, Dietrich M. Spectrum of morphologic changes of lymph nodes from patients with AIDS or AIDS-related complexes. Progress in allergy. 1986;37:81–181. doi: 10.1159/000318442. [DOI] [PubMed] [Google Scholar]
- 34.Tenner-Racz K, Racz P, Gartner S, et al. Ultrastructural analysis of germinal centers in lymph nodes of patients with HIV-1-induced persistent generalized lymphadenopathy: evidence for persistence of infection. Progress in AIDS pathology. 1989;1:29–40. [PubMed] [Google Scholar]
- 35.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 36.Veazey RS, Marx PA, Lackner AA. The mucosal immune system: primary target for HIV infection and AIDS. Trends in immunology. 2001;22:626–33. doi: 10.1016/s1471-4906(01)02039-7. [DOI] [PubMed] [Google Scholar]
- 37.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. The Journal of experimental medicine. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. The Journal of Experimental Medicine. 2004;200:761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. Journal of virology. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levesque MC, Moody MA, Hwang KK, et al. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med. 2009;6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. Journal of virology. 2009;83:3719–33. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moog C, Fleury HJ, Pellegrin I, Kirn A, Aubertin AM. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. Journal of Virology. 1997;71:3734–41. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 45.Montefiori DC, Hill TS, Vo HT, Walker BD, Rosenberg ES. Neutralizing antibodies associated with viremia control in a subset of individuals after treatment of acute human immunodeficiency virus type 1 infection. Journal of virology. 2001;75:10200–7. doi: 10.1128/JVI.75.21.10200-10207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Loggerenberg F, Mlisana K, Williamson C, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore PL, Ranchobe N, Lambson BE, et al. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog. 2009;5:e1000598. doi: 10.1371/journal.ppat.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okano M, Thiele GM, Davis JR, Grierson HL, Purtilo DT. Epstein-Barr virus and human diseases: recent advances in diagnosis. Clin Microbiol Rev. 1988;1:300–12. doi: 10.1128/cmr.1.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gray ES, Madiga MC, Moore PL, et al. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. Journal of virology. 2009;83:11265–74. doi: 10.1128/JVI.01359-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rong R, Li B, Lynch RM, et al. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog. 2009;5:e1000594. doi: 10.1371/journal.ppat.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li B, Decker JM, Johnson RW, et al. Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1. Journal of virology. 2006;80:5211–8. doi: 10.1128/JVI.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haaland RE, Hawkins PA, Salazar-Gonzalez J, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jefferis R, Lund J. Interaction sites on human IgG-Fc for FcγR: current models. Immunol Lett. 2002;82:57–65. doi: 10.1016/s0165-2478(02)00019-6. [DOI] [PubMed] [Google Scholar]
- 55.Nimmerjahn F, Ravetch JV. Analyzing antibody-Fc-receptor interactions. Methods Mol Biol. 2008;415:151–62. doi: 10.1007/978-1-59745-570-1_9. [DOI] [PubMed] [Google Scholar]
- 56.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 57.Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 58.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–34. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 59.Jefferis R. Glycosylation of antibody therapeutics: optimisation for purpose. Methods Mol Biol. 2009;483:223–38. doi: 10.1007/978-1-59745-407-0_13. [DOI] [PubMed] [Google Scholar]
- 60.Forthal DN, Landucci G, Phan TB, Becerra J. Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1. Journal of virology. 2005;79:2042–9. doi: 10.1128/JVI.79.4.2042-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. Journal of virology. 2001;75:6953–61. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bailey JR, Lassen KG, Yang HC, et al. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol. 2006;80:4758–70. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pantaleo G, Cohen OJ, Schacker T, et al. Evolutionary pattern of human immunodeficiency virus (HIV) replication and distribution in lymph nodes following primary infection: implications for antiviral therapy. Nature Medicine. 1998;4:341–5. doi: 10.1038/nm0398-341. [DOI] [PubMed] [Google Scholar]


