Abstract
Many therapies designed to reduce food intake and body weight act, in part, by blocking the dopamine transporter (DAT) – a protein responsible for clearing extracellular dopamine (DA) after release thereby terminating its action. Here, we found that a single injection of the drug trodusquemine (MSI-1436) decreased food intake in rats. To assess the effects of MSI-1436 on DAT function, fast-scan cyclic voltammetry was used to measure DA concentration changes in the ventral striatum. DA release was evoked by electrical stimulation of the ventral tegmental area every 5 minutes. After 3 baseline measurements, rats were injected with MSI-1436 (10mg/kg), the known DAT blocker bupropion (80mg/kg) or saline and evoked DA release and reuptake were monitored for an additional hour. Neither saline nor MSI-1436 caused a significant change in the magnitude of evoked release from baseline values whereas bupropion caused a significant increase. In addition, neither saline nor MSI-1436 significantly increased DA decay rates while such an increase was observed with bupropion. Thus, over a time course when MSI-1436 suppresses food intake it does not affect DAT function. The results support MSI-1436 as an anti-obesity treatment which spares DAT.
Keywords: obesity, striatum, voltammetry, motivation, reward
The prevalence of obesity in the United States has reached epidemic proportions and costs billions of dollars for medical treatment on a yearly basis (Finkelstein et al., 2004). Not surprisingly, biomedical research has focused on anti-obesity treatments, although with very limited success. Amphetamines were the first class of drugs to be approved by the United States Food and Drug Administration (FDA) to treat obesity (Colman, 2005). Among a variety of actions (Sulzer et al., 2005), amphetamine acts on biogenic amine (e.g. neurotransmitters dopamine (DA), norepinephrine, serotonin) transporters to reduce reuptake and prolong neurotransmitter action. Amphetamine effects include not only weight loss, but increased locomotor activity and addiction. More recently, anti-depressants that block plasma membrane transporters for the reuptake of biogenic amines have been used as anti-obesity agents. Indeed, sibutramine, which has FDA approval for the treatment of obesity, blocks the reuptake of biogenic amines and reduces body weight, in part, by increasing locomotor behavior (Golozoubova et al., 2006). Bupropion, another anti-depressant that causes a reduction in body weight in humans (Harto-Truax et al., 1983) and animals (Billes and Cowley, 2007) also inhibits biogenic amine reuptake rates (Stamford et al., 1989).
The neurotransmitter DA and its release sites in the striatum and nucleus accumbens play critical roles in aspects of reward (Berridge and Robinson, 2003; Di Chiara and Bassareo, 2007; Roitman et al., 2005) and addiction (Volkow et al., 2007). Brief (less than 5s; phasic) activations of DA neurons and increases in extracellular DA are evoked by primary rewarding stimuli (Roitman et al., 2008; Schultz, 2007) and stimuli predictive of reward (Phillips et al., 2003b; Roitman et al., 2004; Schultz, 2007). Once DA is released into the extracellular space, it diffuses from its release site and its extracellular actions are primarily terminated by DA reuptake via the DA transporter (DAT). The DAT regulates the half-life of phasically released DA (Cragg and Rice, 2004) and thus manipulations of the DAT can have profound effects on the duration of DA action and behavior (Cagniard et al., 2006; Jones et al., 1998; Jones et al., 1999; Salahpour et al., 2008; Stuber et al., 2005). Drugs that reduce DAT function increase general locomotor behavior (Luscher and Ungless, 2006). Thus, any drug with an affinity for the DAT that is developed to combat obesity may acutely reduce feeding and body weight by increasing general locomotor behavior and temperature but that acute reduction can be compensated for with increased food intake (Billes and Cowley, 2008).
Trodusquemine (MSI-1436) is a novel aminosterol originally isolated as a natural compound from the liver of the dogfish shark (Squalus acanthias) (Rao et al., 2000). Previous work in rodents has demonstrated that peripheral and central administration of MSI-1436 reduces food intake and body weight (Ahima et al., 2002). However, its mechanism of action remains unclear. Recently, in vitro binding and cell-based assays indicated that MSI-1436 binds to the DAT (Lantz et al.). It remains unknown whether MSI-1436 causes a functional change in the rate of DA reuptake via the DAT in vivo. Here, we assessed whether MSI-1436 altered the rate of DA uptake via the DAT using fast-scan cyclic voltammetry in the whole animal. This technique samples the extracellular concentration of DA on a millisecond timescale and can therefore dissociate drug effects on DA release and reuptake (Stamford et al., 1989). First, we assessed the effects of MSI-1436 on acute food intake and compared it with a drug known to reduce food intake but also known to block the DAT – bupropion. Second, we characterized the action of MSI-1436 on phasic DA release and reuptake via the DAT and compared its effects to bupropion.
Experimental procedures
Experiment 1: food intake
Subjects
Adult, male Sprague-Dawley rats (n=31), weighing approximately 400g were individually housed in Plexiglas cages with wire tops. Animals had access to food (Purina Rat Chow) and water ad libitum and maintained on a 12 hr light: dark cycle with lights on at 06:20h. Animals were handled in accordance with all ethical standards of the National Institutes of Health (NIH) and the University of Illinois at Chicago (UIC). All procedures were approved by the Institutional Review Committee for the Use of Animal Subjects at the University of Illinois at Chicago (UIC).
Procedure
For three days preceding drug injection, animals and food were weighed in the 20 min prior to onset of the dark cycle and all animals were injected with 0.9% saline solution (1 ml/kg; i.p.). Food intake was measured after the first and 24 hours of the onset of the dark cycle. On the experimental day, animals and food were weighed, as described previously, but then injected with MSI-1436 (10 mg/kg in 0.9% saline; i.p.; n=13; gift from Genaera Corporation; Plymouth Meeting, PA) or bupropion (80mg/kg in 0.9% saline; i.p.; n=18; Sigma-Aldrich, St. Louis, MO). Doses were chosen based on prior work with each drug. MSI-1436 has been shown to significantly reduce 24hr food intake with a 10mg/kg dose (Ahima et al., 2002) and bupropion has been shown to reduce food intake with an 80mg/kg dose (Billes and Cowley, 2007). Data collection during the dark cycle was made under red light conditions to avoid disrupting the normal circadian behavior of the rats.
Experiment 2: fast-scan cyclic voltammetry
Subjects
Adult male Sprague-Dawley rats (n=12; Charles River Laboratories, Wilmington, MA) weighing approximately 300g were individually housed in Plexiglas cages with wire tops. Animals had access to food (Purina Rat Chow) and water ad libitum and maintained on a 12 hr light:dark cycle with lights on at 06:20h. Animals were handled in accordance with all ethical standards of the NIH and UIC. The experimental protocol was approved by the Institutional Review Committee for the Use of Animal Subjects at UIC.
Surgery
Animals were anesthetized with an injection of a mixture of Ketamine (100 mg/kg, i.m) and Xylazine (50 mg/kg, i.m.) and placed in a stereotaxic instrument. A midline incision was made to expose the skull, and coordinates of suture lines (bregma, lambda) were measured. A guide cannula (Bioanalytical Systems, West Lafayette, IL) over the striatum (AP: +1.3mm, ML: +1.3mm, relative to bregma) and an Ag/AgCl reference electrode placed contralateral to the guide cannula were implanted. Stainless steel skull screws and dental cement were used to secure all items. A bipolar stimulating electrode was then placed just dorsal to the ventral tegmental area (VTA)/substantia nigra pars compacta (SNc) (−5.2 AP, 1.0 ML from bregma and 7 mm ventral from the dural surface). All coordinates were derived from a rat stereotaxic atlas (Paxinos and Watson, 1986). A detachable micromanipulator containing a glass-sealed carbon-fiber electrode (75-100 μm exposed tip length, 7 μm diameter, Goodfellow, Oakdale, PA) was inserted into the guide cannula, and the electrode was lowered into the ventral striatum. The bipolar stimulating electrode was then lowered in 0.2mm increments until DA release was detected at the carbon-fiber electrode in response to a stimulation train (60 biphasic pulses, 60 Hz, 120 μA, 2 ms per phase). The stimulating electrode was then fixed with dental cement and the carbon-fiber electrode was removed. These procedures have been used extensively (Day et al., 2007; Phillips et al., 2003b; Roitman et al., 2004).
Voltammetric recordings
The carbon fiber was held at −0.4 V against Ag/AgCl between voltammetric scans and then driven to +1.3 V and back in a triangular fashion at 400 V/s for each voltammetric measurement. The application of this triangle waveform causes oxidation and reduction of chemical species that are electroactive within this potential range, producing a change in current at the carbon-fiber. Specific analytes (including DA) are identified by plotting these changes in current against the applied potential to produce a cyclic voltammogram. The current arising from electrode processes (double-layer charging, etc.,) was removed by using background-subtraction. The background period (1000 ms) was taken as the minima during the 5 s before electrical stimulation of the VTA. The position of the microelectrode was then optimized by monitoring electrically evoked (24 biphasic pulses, 60 Hz, 120 μA, 2 ms per phase) DA release. Experiments were conducted when an electrode placement yielded robust (>30:1 signal to noise) electrically evoked DA release. We electrically evoked DA release every five minutes and characterized: 1) peak [DA] evoked by stimulation; 2) the latency to peak from the start of stimulation; 3) the latency for [DA] to return to 50% of its peak (halflife); and 4) the rate of signal decay for the first 3.5 s following the start of stimulation. Data were statistically analyzed using commercially available software (Statistica, Tulsa, OK; GraphPad Prism, La Jolla, CA). After 3 stimulations, rats were injected with MSI-1436, bupropion or saline and voltammetric recordings were made for an additional 12 stimulations.
Signal identification and separation
Signal identification and separation was accomplished using principal component analysis which has been described previously (Day et al., 2007; Heien et al., 2004; Heien et al., 2005). Stimulation of the VTA leads to two well-characterized electrochemical events: an immediate but transient increase in [DA] and a delayed but longer-lasting basic pH shift. To separate these signals, a training set was constructed from representative, background-subtracted cyclic voltammograms for DA and pH. This training set was used to perform principal component analysis on data collected during the drug session. Principal components were selected such that at least 99.5% of the variance in signal was accounted for by the model. After use, carbon-fiber electrodes were calibrated in a solution of known [DA] to convert observed changes in current to differential concentration.
Results
MSI-1436 and bupropion differentially effect food intake
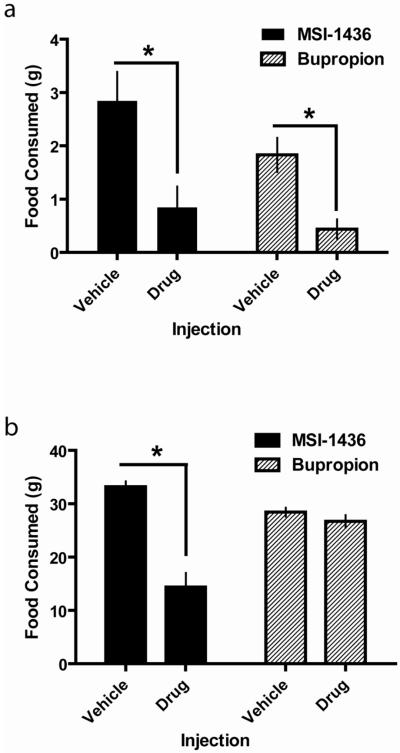
A single injection of MSI-1436, at the dose used in these studies, has been shown to decrease food consumption and body weight over days (Zasloff et al., 2001). MSI-1436 rapidly accumulates in the brain (Zasloff et al., 2001) and is more effective in reducing food intake and body weight when administered centrally (Ahima et al., 2002). Previous studies investigated MSI-1436 action on feeding 24hr post-injection. Here, to determine if MSI-1436 had acute effects on food intake and over the same time-course as the assay for DA signaling, we measured food intake during the first hour of the dark phase – when rats consume their largest meal. The effects of MSI-1436 on acute (first hour dark phase) food intake were compared to the effects of bupropion using a 2-way ANOVA (Injection (vehicle or drug) X Drug (MSI-1436 or bupropion). As shown in Figure 1a, there was a significant main effect of Injection (F(1,58) = 22.8; p<0.001). The absence of a main effect of drug or a significant interaction suggests that MSI-1436 and bupropion altered acute food intake to similar degrees.
Figure 1.
Acute and 24hr food intake are differentially affected by MSI-1436 and burporion. (a.) Both MSI-1436 (black bars) and bupropion (hatched bars) suppress food intake during the first hour of the dark phase. Rats were injected with either vehicle or drug just prior to lights off and food intake was measured 1hr later. (b). MSI-1436 (black bars) but not bupropion (hatched bars) suppresses food intake for 24hr post-injection. For the same rats as in (a), food intake was measured 24hr after injection with either vehicle or drug. Bars represent group means and error bars denote +1SEM. *p<0.05 for vehicle vs. drug injection.
Amount of food consumed over 24hrs was examined using a 2-way ANOVA (Injection (vehicle or drug) X Drug (MSI-1436 or bupropion). As shown in Figure 1b, MSI-1436 and bupropion differentially affected 24hr food intake. There were significant main effects of Injection (F(1,58) = 49.5; p<0.001) and Drug (F(1,58) = 6.7; p<0.05) as well as a significant interaction (F(1,58) = 34.3; p<0.001). A post-hoc Tukey HSD test revealed that only MSI-1436, relative to vehicle, caused a significant reduction in 24hr food intake (p<0.001).
MSI-1436 does not alter the dynamics of evoked DA release or reuptake
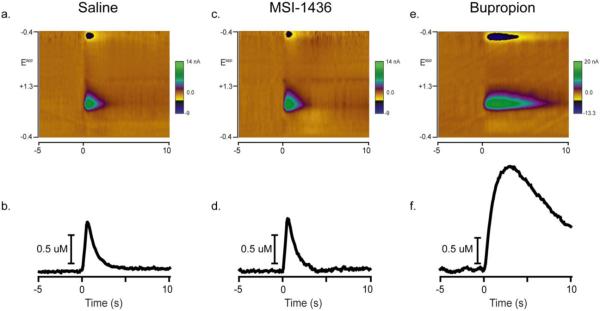
DA release was evoked once every 5 minutes and peak [DA] and rate of reuptake were characterized in rats injected with either saline, MSI-1436 or bupropion. Figure 2 shows examples of evoked DA release and reuptake from each of the three groups 1hr after injection. While DA was evoked by electrical stimulation of the ventral tegmental area in all three rats, similar peak DA concentrations and rates of reuptake were observed 1hr after saline (Figure 2a,b) and MSI-1436 (Figure 2c,d) injections. However, 1 hr after bupriopion treatment, peak DA concentration was over double in magnitude and the rate of reuptake appeared slower relative to saline and MSI-1436 injected rats. Multiple factors can play a role in the absolute magnitude of DA release and reuptake rate. Different carbon fiber electrodes were used across rats. Moreover, stimulating electrodes placed in the ventral tegmental area could potentially recruit more or fewer DA fibers across rats. In spite of this, the evoked peak in DA concentration during the baseline period was not significantly different across groups (406.7±106, 463.9±168 and 354.2±41 nM for saline, MSI-1436 and buproprion, respectively). To normalize the data, peak DA concentration was averaged across the 3 baseline stimulations and set to 100% for each rat.
Figure 2.
Representative examples of evoked DA release and reuptake 1hr after injection. At time 0s, a stimulation train (biphasic, 2ms/phase, 24 pulses, 60 Hz, 120 μA) is applied to the ventral tegmental area while voltammetric recordings are made for the 5s before and 10s after stimulation onset. (a.) Evoked DA release and reuptake 1hr after i.p. injection of saline. A colorplot displays all aspects of the voltammetric recording. Time is the abscissa, the electrode potential is the ordinate and current changes are encoded in color. At the onset of stimulation, current is detected at ~0.6V (green) on the positive and ~−0.2V (blue) on the negative going scan. This signature is indicative of DA (Phillips et al., 2003a). (b.) Using principal component analysis, DA concentration over time is extracted from the voltammetric data in a. (c.) Colorplot of evoked DA release and reuptake 1hr after i.p. injection of MSI-1436. (d.) DA concentration over time extracted from the voltammetric data in c. (e.) Colorplot of evoked DA release and reuptake 1hr after i.p. injection of bupropion (note the change in the colorplot scale). (f.) DA concentration over time extracted from the voltammetric data in e.
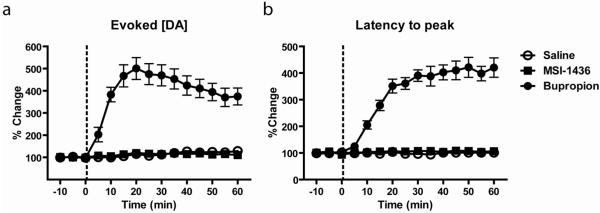
Evoked DA release consists of rising and falling phases and the rate of DA reuptake affects both (John and Jones, 2007). We first determined whether the different injections affected the peak concentration of DA evoked by the same stimulation over the course of the experiment as well as the latency from initiation of stimulation to the peak (Figure 3). For peak DA (Figure 3a), a 2-way ANOVA found significant main effects of time (F(14,135)=15.8; p<0.0001) and drug (F(2,135)=523.0; p<0.0001) as well as a significant interaction (F(28,135)=12.2; p<0.0001). A post-hoc Dunnet’s test comparing all time points for all three types of injection against the very first baseline sample of the saline group revealed that only bupropion caused a significant increase in peak DA concentration. Specifically, bupropion caused an increase in peak DA beginning at the very first post-injection time point (5 min post-injection) and the significant increase lasted through the duration of the experiment. For latency to peak (Figure 3b), a 2-way ANOVA found significant main effects of time (F(14,135)=28.6; p<0.0001) and drug (F(2,135)=897; p<0.0001) as well as a significant interaction (F(28,135)=26.9; p<0.0001). A post-hoc Dunnet’s test comparing all time points for all three injections against the very first baseline sample of the saline group revealed that only bupropion caused a significant increase in latency. Specifically, bupropion caused an increase in latency to peak beginning at 10 min post-injection and lasting through the duration of the experiment.
Figure 3.
Evoked DA release is altered by bupropion but not by MSI-1436 or saline. DA release was evoked once every 5min for 15 samples. (a.) The magnitude of evoked release (peak height) was normalized for each rat and expressed as percent change from the average of the 3 baseline samples. The known reuptake blocker bupropion (filled circles) caused a significant increase in peak height 5min after injection through the duration of the experiment. Injection of either saline (open circles) or MSI-1436 (closed squares) had no effect on peak height. (b.) The latency to peak DA concentration following stimulation onset was normalized for each rat and expressed as percent change from the average of the 3 baseline samples. Bupropion (filled circles) caused a significant increase in latency to 5min after injection through the duration of the experiment. Injection of either saline (open circles) or MSI-1436 (closed squares) had no effect on peak height. In both graphs, data points represent group means and error bars denote ±1S.E.M.
While the rising phase of evoked DA is comprised of release and reuptake, the falling phase is due to reuptake alone. Many drugs that reduce food intake and body weight increase DA concentration by reducing the rate of DA reuptake via the DAT (Stamford et al., 1989; Wellman, 2005). Here, we examined the effects of saline, MSI-1436 and bupropion on the rate of DA reuptake following evoked DA release. The peak [DA] evoked by electrical stimulation was set to 100% and data were expressed as %change from peak [DA]. The latency For DA concentration to fall to 50% (Halflife) of its peak was determined and is shown in Figure 4. With respect to Halflife, a 2-way ANOVA found significant main effects of time (F(14,135)=3.6; p<0.001) and drug (F(2,135)=102.4; p<0.0001) as well as a significant interaction (F(28,135)=3.1; p<0.0001). A post-hoc Dunnet’s test comparing all time points for all three types of injection against the very first baseline sample of the saline group revealed that only bupropion caused a significant increase in Halflife. Specifically, bupropion caused an increase in Halflife beginning at 20 min post-injection and lasting through the duration of the experiment.
Figure 4.
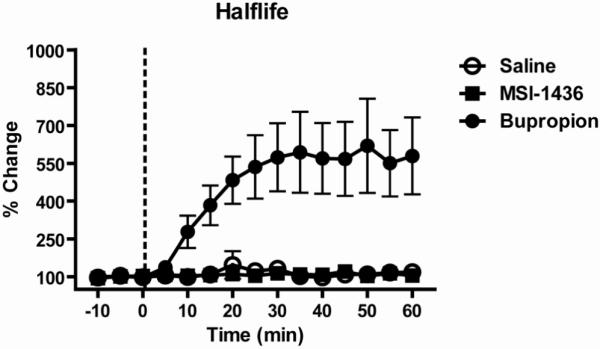
Bupropion, but not MSI-1436 or saline, decreases the rate of decay from peak evoked DA release. The time for evoked DA release to fall to 50% of its peak value (Halflife) was measured once every 5min. Halflife was normalized for each rat and expressed as percent change from the average of the 3 baseline samples. The known reuptake blocker bupropion (filled circles) significantly increased Halflife from 20 min after injection through the duration of the experiment. Injection of either saline (open circles) or MSI-1436 (closed squares) had no effect on Halflife. Data points represent group means and error bars denote ±1S.E.M.
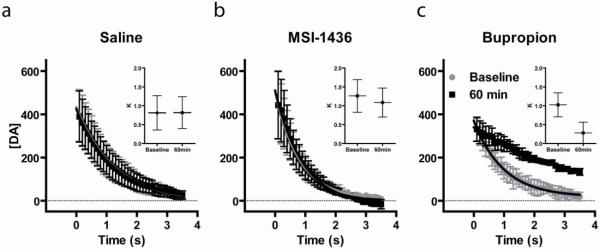
The rate of DA reuptake via the DAT is concentration dependent. Therefore, to assess whether each type of injection altered the rate of reuptake, the portion of the reuptake curve for the last baseline sample and 60min sample were compared over the same concentration range. Similar analyses for the rate of DA reuptake have been previously reported (Britt and McGehee, 2008; Cheer et al., 2004). As can be seen in Figure 5, average reuptake curves for saline and MSI-1436 did not differ whereas bupropion caused a change in the rate of reuptake. These data were fit with a single exponential decay function to obtain time constants (K) and their 95% confidence intervals for each curve (see inserts). Only buproprion caused a change in K that exceeded the 95% confidence interval prior to injection. Thus, using multiple analyses, MSI-1436 was similar to saline in that it had no effect on the rate of DA reuptake via the DAT which was in sharp contrast to the known DAT blocker bupropion.
Figure 5.
Bupropion, but not MSI-1436 or saline, altered indices of the rate of DA reuptake. The falling phase of evoked DA for a baseline (gray filled circles) and the 60 min (black filled squares) samples was fitted with a single exponential decay for saline (a), MSI-1436 (b) and bupropion (c). The rate constant (K; horizontal lines) and 95% confidence interval (whiskers) was obtained from each fit (inserts).
Discussion
Several commercially available drugs that reduce food intake and body weight in humans target central catecholamine signaling including altering the rate of DA reuptake via the DAT (Wellman, 2005). While these drugs have desirable effects on food intake their mechanism of action can limit their usefulness. For example, they may promote a decrease in food intake by increasing locomotor behavior and thermogenesis – an indirect mechanism of action that may produce only transient changes in body weight (Billes and Cowley, 2008). MSI-1436 is a drug that has been shown to potently reduce food intake and body weight following a single injection in both normal rodents and in rodent models of obesity (Zasloff et al., 2001). This finding was replicated and extended here to demonstrate both an immediate and prolonged effect of MSI-1436 on food intake. In vitro and cell-based assays point to multiple mechanisms by which MSI-1436 may exert its anorectic effects including the identification of the DAT as a target (Lantz et al.). We tested whether MSI-1436 alters the rate of DA reuptake via the DAT using in vivo FSCV. While a known DAT blocker, bupropion, clearly increased the magnitude of evoked DA release, increased the latency for evoked DA to reach its peak and decreased the rate of DA reuptake (as indicated by Halflife and K), MSI-1436 had no effect on any of these measures. Indeed, on measures of evoked DA release and reuptake, MSI-1436 was no different from saline injection. Thus, we show that MSI-1436 reduces food intake within 1hr of injection but does not affect DAT function over this same time course.
Rats typically consume a large meal at the onset of darkness during a light-dark cycle (Roitman et al., 2001). We have shown here that a peripheral injection of MSI-1436 was effective in reducing food intake normally observed during the first hour of the dark phase. The DAT blocker bupropion also suppressed food intake in the first hour of the dark phase. However, suppression of food intake by bupropion was transient and compensated for by increased intake such that by 24hr post injection, food consumption was no different from saline treatment. These data highlight the limitations of DAT blockers alone for the treatment of obesity. Unlike, bupropion, MSI-1436 caused a lasting suppression in food intake which suggested that MSI-1436, in part, had non-overlapping mechanisms of action with bupropion. The prolonged suppression in food intake was unlikely to be the result of malaise as MSI-1436 failed to condition a taste aversion in previous studies (Ahima et al., 2002). Moreover, the typical compensatory responses to food deprivation are not exhibited in MSI-1436 treated rodents (Ahima et al., 2002).
The known DAT blocker bupropion increased both the magnitude of evoked release and the rate of decay. Many drugs that reduce food intake, including the few that have been approved by the FDA for clinical use, reduce the rate of reuptake of biogenic amines. Non-selective DA reuptake inhibitors such as mazindol and cocaine reduce the number of feeding bouts in rats (Cooper and van der Hoek, 1993). In addition, highly selective DA reuptake inhibitors have very similar effects on food intake (van der Hoek and Cooper, 1994). More recently, the FDA-approved appetite suppressant phentermine has been shown to increase extracellular DA concentration in the nucleus accumbens of freely moving rats (Baumann et al., 2000) possibly via a reduction in the rate of DA reuptake via the DAT (John and Jones, 2007). Drugs that are commonly abused all increase dopamine concentration in the ventral striatum (Cheer et al., 2007; Pierce and Kumaresan, 2006). While not all drugs that block the DAT are abused, abuse potential remains a possibility for obesity treatments that interact with the dopamine transporter. Thus, therapeutics that spare the DAT are advantageous.
MSI-1436 had no effect on measures of DA release or reuptake. Thus, while the assay used here was highly sensitive to changes in the rate of DA reuptake, the data strongly support the idea that a peripheral dose of MSI-1436 that affects food intake does not alter DA reuptake in the ventral striatum. It is important to note that other biogenic amines and other DA terminal regions were not tested in this study. The scope of this investigation was limited to DAT function in the ventral striatum. Nevertheless, MSI-1436 clearly spared DAT function in the ventral striatum – an area critical for feeding behavior (Szczypka et al., 2001). Bupropion and phentermine increase locomotor behavior and thermogenesis (Billes and Cowley, 2008; Golozoubova et al., 2006). This response is mediated, in part, by the action of these drugs on the DAT and their ability to increase extracellular levels of DA (Baumann et al., 2000; John and Jones, 2007; Rowley et al., 2000; Stamford et al., 1989). Interestingly, MSI-1436 does not increase locomotor behavior (Ahima et al., 2002) and, coupled with the observation here that MSI-1436 does not alter DA reuptake rates, strongly suggests a mechanism of action that is independent of increasing DA signaling.
We have definitively shown that MSI-1436 causes no change in the rate of DA reuptake via the DAT. Instead, MSI-1436 appears to act on specific hypothalamic targets. Expression of the immediate-early gene c-fos (Fos) is commonly used to map potential drug targets. Peripheral administration of MSI-1436 caused a significant elevation of Fos in the paraventricular nucleus (PVN) of the hypothalamus – a region of the brain strongly associated with feeding behavior and energy regulation (Spiegelman and Flier, 2001). In addition, administration of MSI-1436 directly into the PVN is highly effective in suppressing food intake (Ahima et al., 2002). MSI-1436 is effective in suppressing orexigenic neuropeptide expression in the hypothalamus. Finally, recent work has shown that MSI-1436 selectively inhibits protein-tyrosine phosphatase 1B (PTP1B) (Lantz et al.). PTP1B dephosphorylates the insulin receptor (IR) and also dephosphorylates insulin receptor substrate 1 (IRS1) (Goldstein et al., 2000). PTP1B expression and activity are increased in obese and insulin-resistant humans, and these elevations have also been documented in rodent models of obesity (Ahmad et al., 1997; Di Paola et al., 2002). Thus, MSI-1436 presents as an anti-obesity treatment that selectively targets homeostatic mechanisms and spares aspects of catecholamine signaling and locomotor behavior. Compounds of this nature need further development but are suggestive of anorectic treatments that are not dependent on alterations of DA signaling.
Acknowledgements
This work was supported by DA025634 (to MFR) and a grant from the Genaera Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima RS, Patel HR, Takahashi N, Qi Y, Hileman SM, Zasloff MA. Appetite suppression and weight reduction by a centrally active aminosterol. Diabetes. 2002;51:2099–2104. doi: 10.2337/diabetes.51.7.2099. [DOI] [PubMed] [Google Scholar]
- Ahmad F, Azevedo JL, Cortright R, Dohm GL, Goldstein BJ. Alterations in skeletal muscle protein-tyrosine phosphatase activity and expression in insulin-resistant human obesity and diabetes. The Journal of clinical investigation. 1997;100:449–458. doi: 10.1172/JCI119552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse (New York, N.Y. 2000;36:102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Billes SK, Cowley MA. Catecholamine reuptake inhibition causes weight loss by increasing locomotor activity and thermogenesis. Neuropsychopharmacology. 2008;33:1287–1297. doi: 10.1038/sj.npp.1301526. [DOI] [PubMed] [Google Scholar]
- Billes SK, Cowley MA. Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology. 2007;32:822–834. doi: 10.1038/sj.npp.1301155. [DOI] [PubMed] [Google Scholar]
- Britt JP, McGehee DS. Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J Neurosci. 2008;28:1672–1681. doi: 10.1523/JNEUROSCI.4275-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Annals of internal medicine. 2005;143:380–385. doi: 10.7326/0003-4819-143-5-200509060-00013. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, van der Hoek GA. Cocaine: a microstructural analysis of its effects on feeding and associated behaviour in the rat. Brain research. 1993;608:45–51. doi: 10.1016/0006-8993(93)90772-f. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends in neurosciences. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature neuroscience. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Current opinion in pharmacology. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Paola R, Frittitta L, Miscio G, Bozzali M, Baratta R, Centra M, Spampinato D, Santagati MG, Ercolino T, Cisternino C, Soccio T, Mastroianno S, Tassi V, Almgren P, Pizzuti A, Vigneri R, Trischitta V. A variation in 3′ UTR of hPTP1B increases specific gene expression and associates with insulin resistance. American journal of human genetics. 2002;70:806–812. doi: 10.1086/339270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein EA, Fiebelkorn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obesity research. 2004;12:18–24. doi: 10.1038/oby.2004.4. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. The Journal of biological chemistry. 2000;275:4283–4289. doi: 10.1074/jbc.275.6.4283. [DOI] [PubMed] [Google Scholar]
- Golozoubova V, Strauss F, Malmlof K. Locomotion is the major determinant of sibutramine-induced increase in energy expenditure. Pharmacology, biochemistry, and behavior. 2006;83:517–527. doi: 10.1016/j.pbb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Harto-Truax N, Stern WC, Miller LL, Sato TL, Cato AE. Effects of bupropion on body weight. The Journal of clinical psychiatry. 1983;44:183–186. [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Analytical chemistry. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proceedings of the National Academy of Sciences of the United States of America. 2005 doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Joseph JD, Barak LS, Caron MG, Wightman RM. Dopamine neuronal transport kinetics and effects of amphetamine. Journal of neurochemistry. 1999;73:2406–2414. doi: 10.1046/j.1471-4159.1999.0732406.x. [DOI] [PubMed] [Google Scholar]
- Lantz KA, Hart SG, Planey SL, Roitman MF, Ruiz-White IA, Wolfe HR, McLane MP. Inhibition of PTP1B by Trodusquemine (MSI-1436) Causes Fat-specific Weight Loss in Diet-induced Obese Mice. Obesity (Silver Spring, Md. doi: 10.1038/oby.2009.444. [DOI] [PubMed] [Google Scholar]
- Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS medicine. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press Inc.; San Diego, California: 1986. [Google Scholar]
- Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med. 2003a;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003b;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and biobehavioral reviews. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Rao MN, Shinnar AE, Noecker LA, Chao TL, Feibush B, Snyder B, Sharkansky I, Sarkahian A, Zhang X, Jones SR, Kinney WA, Zasloff M. Aminosterols from the dogfish shark Squalus acanthias. Journal of natural products. 2000;63:631–635. doi: 10.1021/np990514f. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, van Dijk G, Thiele TE, Bernstein IL. Dopamine mediation of the feeding response to violations of spatial and temporal expectancies. Behavioural brain research. 2001;122:193–199. doi: 10.1016/s0166-4328(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nature neuroscience. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley HL, Butler SA, Prow MR, Dykes SG, Aspley S, Kilpatrick IC, Heal DJ. Comparison of the effects of sibutramine and other weight-modifying drugs on extracellular dopamine in the nucleus accumbens of freely moving rats. Synapse (New York, N.Y. 2000;38:167–176. doi: 10.1002/1098-2396(200011)38:2<167::AID-SYN8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4405–4410. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends in neurosciences. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J. Dissociation of the actions of uptake blockers upon dopamine overflow and uptake in the rat nucleus accumbens: in vivo voltammetric data. Neuropharmacology. 1989;28:1383–1388. doi: 10.1016/0028-3908(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Progress in neurobiology. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RD. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- van der Hoek GA, Cooper SJ. The selective dopamine uptake inhibitor GBR 12909: its effects on the microstructure of feeding in rats. Pharmacology, biochemistry, and behavior. 1994;48:135–140. doi: 10.1016/0091-3057(94)90509-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Archives of neurology. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Modulation of eating by central catecholamine systems. Current drug targets. 2005;6:191–199. doi: 10.2174/1389450053174532. [DOI] [PubMed] [Google Scholar]
- Zasloff M, Williams JI, Chen Q, Anderson M, Maeder T, Holroyd K, Jones S, Kinney W, Cheshire K, McLane M. A spermine-coupled cholesterol metabolite from the shark with potent appetite suppressant and antidiabetic properties. Int J Obes Relat Metab Disord. 2001;25:689–697. doi: 10.1038/sj.ijo.0801599. [DOI] [PubMed] [Google Scholar]