Summary
Long-term changes in the hypothalamic pituitary adrenal (HPA) axis as a result of early life stress could be related to the development of substance use disorders during adulthood. In this study, the neuroendocrine, physiologic (HR), and subjective responses to corticotropin releasing hormone (CRH) and the Trier Social Stress Task (TSST) in individuals with cocaine dependence, with (n=21)/without early life stress (n=21), non-dependent individuals with early life stress (n=22), and a control group were examined (n=21). CRH increased cortisol and ACTH levels in all groups. However, a significant effect of early life stress on ACTH was observed indicating that the increase in ACTH was greatest in subjects with a history of childhood stress. Post-hoc analysis indicated the early life stress/non-cocaine dependent individuals exhibited significantly higher levels of ACTH as compared to the early life stress/cocaine-dependent group. Despite the elevated ACTH response there was no difference between the groups in the cortisol response to CRH. The TSST produced a significant elevation in ACTH and cortisol all study groups. No significant group differences were observed. The subjective stress and peak heart rate responses to the TSST were greatest in cocaine-dependent subjects without early life stress. In response to CRH, subjective stress and craving were positively correlated in cocaine-dependent subjects regardless of early life stress history, while stress and craving following the TSST were correlated only in cocaine-dependent subjects without a history of early life stress. Findings support previous studies demonstrating that subjects with a history of childhood adversity exhibit elevated ACTH and blunted cortisol levels in response to stress. In contrast, HR and subjective stress in response to the TSST were greatest in cocaine-dependent subjects without a history of early life stress, suggesting that childhood adversity may desensitize autonomic and subjective responding to social stress in adults with cocaine-dependence.
Keywords: hypothalamic-pituitary-adrenal (HPA)axis, cocaine dependence, early life stress, trauma, Trier Social Stress Task, corticotropin releasing hormone (CRH), stress
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is critical for maintaining homeostasis in response to physiological threat or challenge. However, persistent activation of the HPA axis as a consequence of physical, emotional, sexual abuse, and/or neglect, can produce long-term neurobiological changes which may underlie the development of adult psychiatric disorders such as anxiety, depression, or substance use disorders (Mullen et al., 1996; Heim and Nemeroff 2001; Furukawa et al., 1999). The consequence of early life stress can be observed at the molecular level. For example, rodents exposed to postnatal stressors such as maternal separation, exhibit elevated corticotropin releasing hormone (CRH) mRNA in the hypothalamus, central amygdala, and increased CRH receptor levels in the locus coeruleus (Plotsky and Meaney 1983; Plotsky et al., 2005). Interestingly, these same areas have been implicated in stress-induced anxiety and relapse in individuals with substance use disorders (Erb et al., 2001; Smith and Aston-Jones 2008; Erb and Stewart 1999). Therefore, increased responsivity of CRH circuitry may be one of the long-term changes associated with exposure to adversity during childhood. In some individuals this may manifest as maladaptive coping skills, such as drug seeking as an attempt to “self-medicate” in the face of aversive environmental stimuli (Hyman et al., 2007).
An association between early life stress and the subsequent development of substance use disorders is well documented. Two prospective studies that followed participants over 20 years documented that childhood trauma is a significant predictor of binge drinking and stimulant abuse (Jasinski et al., 2000; Widom et al., 1999). Furthermore, childhood trauma increases the probability of relapse in cocaine-dependent women (Hyman et al., 2008). Given that cocaine-dependent individuals frequently report anxiety and nervousness prior to relapse and report twice as many “daily hassles”, as compared to non-dependent individuals (Back et al., 2005), we hypothesized that subjects with cocaine dependence and a history of early life stress would exhibit a enhanced disruption in HPA hormonal, subjective, and physiological response to stress as compared to cocaine dependent subjects without early life stress and a control group. In the present study, ACTH, cortisol, heart rate (HR) and subjective responses to CRH and the Trier Social Stress Task were examined in cocaine-dependent subjects and control subjects with varying levels of exposure to early childhood stress.
Methods
Participants
Data for the present study were drawn from a larger study on gender differences in stress and cue reactivity among cocaine dependent and non-dependent control participants. Cocaine dependent individuals and healthy controls (smokers and non-smokers) were recruited from a 50 mile radius of the Charleston area, primarily via media advertisements over a 48-month period. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, and received Institutional Review Board approval. Informed consent was acquired prior to study participation. Current cocaine dependence was assessed using the Structured Clinical Interview for DSM-IV (SCID-IV), which permits accurate diagnosis of lifetime and current psychiatric disorders using DSM-IV criteria (First et al., 1994). General exclusion criteria included (1) current major depressive disorder (MDD) (2) current post traumatic stress disorder (PTSD); (3) history of or current medical conditions that might interfere with safe conduct of the study or impact HPA activity; (4) history of or current psychotic, eating, or bipolar affective disorders; (5) synthetic glucocorticoid or exogenous steroid therapy within one month of testing; (6) current benzodiazepine, antipsychotic, antidepressant, antianxiety, mood-stabilizers, beta-blocker and other medication use that might interfere with HPA axis activity or psychophysiologic measurement; (7) pregnancy, nursing, or ineffective means of birth control; (8) body mass index ≥ 35; or (9) DSM-IV criteria for substance dependence except caffeine, nicotine or marijuana within the past 60 days. Demographic characteristics are listed in Table 1.
Table 1.
Comparison of group demographics between subjects in the Early Life Stress, Cocaine-Dependent (ELS/CD), Early Life Stress, Non-Cocaine-Dependent (ELS/Non-CD), Non-Early Life Stress, Cocaine-Dependent (Non-ELS/CD), and Non-Early Life Stress, Non-Cocaine-Dependent (Non-ELS/Non-CD) groups
| Variable1 | ELS | Non ELS | p-value3 | ||
|---|---|---|---|---|---|
| CD | Non CD | CD | Non CD | ||
| n | 21 | 22 | 21 | 21 | - |
| Age | 37.3 ± 9.4 |
36.1 ± 11.5 |
40.3 ± 11.5 |
36.9 ± 12.1 |
NS |
| Body Mass Index | 26.0 ± 4.3 |
27.7 ± 4.3 |
24.2 ± 3.0 |
26.6 ± 4.1 | NS |
| Number of early traumatic experiences2 |
19.2 ± 5.7 |
19.0 ± 8.4 |
5.3 ± 5.5 | 5.2 ± 2.3 | <0.001 |
| Male, n (%) | 9 (42.9) | 10 (45.5) | 11 (52.4) | 10 (47.6) | NS |
| Some college, n (%) |
9 (42.9) | 19 (95.0) | 12 (60.0) | 16 (76.2) | <0.01 |
| Employment, n (%) | 9 (42.9) | 17 (81.0) | 11 (55.0) | 15 (71.4) | 0.05 |
| Caucasian, n (%) | 14 (70.0) | 10 (45.4) | 7 (33.3) | 15 (71.4) | 0.03 |
| Married, n (%) | 2 (9.5) | 5 (23.8) | 2 (10.0) | 3 (14.3) | NS |
| Smokers, n (%) | 19 (90.5) | 15 (68.2) | 14 (66.7) | 11 (52.4) | NS |
| Social Anxiety Disorder |
0 (0.0) | 1 (4.6) | 2 (9.5) | 1 (4.8) | -- |
| Cannabis Use Disorder |
2 (9.5) | 0 (0.0) | 1 (4.8) | 0 (0.0) | -- |
Descriptive statistics are listed as Mean ± Standard deviation unless otherwise noted.
Represent the total number of childhood traumas reported by the ETI-SR.
Two Sided Wilcoxon Rank Sums test for continuous measures and Pearson’s Chi-Square test for categorical measures (Fishers Exact test used when appropriate).
Measures
Early life stress was assessed using the Early Trauma Inventory-Self Report (ETI-SR) (Bremner et al., 2000). The ETI-SR is a 62-item self-report assessment of four domains of childhood trauma and adversity (i.e., sexual, physical, emotional, and general), as well as the frequency, onset, and emotional impact of the event on the respondent. Subjects are asked to inventory the frequency of events ranging from emotional events such as “parents failing to understand your needs” to extremely traumatic events such as rape. Thus, the ETI encompasses a broad range of early life adverse experiences. There are several methods that can be used to score the ETI; however a simple sum of the number of events across each domain has been demonstrated as a valid measure of childhood stress (Scher et al., 2001; Bremner et al., 2007). The median split of the total ETI score was used to estimate the magnitude of the differences in the outcomes between subjects reporting categorically high (above the median) and low (below the median) levels of stress, and has been used successfully in several studies as a method to assess the severity of childhood adversity (Hyman et al., 2008; Lee et al., 2005; Roy et al., 2007). Subjects were grouped into the ELS/CD (early life stress with current cocaine dependence), ELS/non-CD (early life stress without current cocaine dependence), non-ELS/CD (no early life stress with current cocaine dependence) or non-ELS/non-CD (no early life stress without current cocaine dependence).
Procedures
Study procedures were conducted at the Clinical and Translational Research Center (CTRC) of the Medical University of South Carolina. Subjects were admitted to an inpatient unit at approximately 2000 hr the evening prior to testing. Subjects were asked to remain abstinent at least three days prior to their study visit. Abstinence was assessed using self-report, urine drug screen (Roche Diagnostics), and breathalyzer tests (AlcoSensor III, Intoximeters, Inc). Subjects dependent on nicotine were provided with a nicotine patch.
Since this was part of a larger study examining cue and stress reactivity, each subject participated in the Trier Social Stress Task (TSST), cocaine cue-exposure (drug paraphernalia) and CRH administration. On the morning of testing, an indwelling intravenous catheter was inserted in the forearm of the non-dominant hand. Participants were connected to electrodes for heart rate measurement. Between 1340h and 1400h, subjects completed either the TSST or the cue protocol. Specifically, subjects were exposed either to cocaine-related paraphernalia for ten minutes or they completed the TSST. The TSST and cocaine cue tasks were counterbalanced so that half of the subjects participated in the TSST on day 1 and half completed the TSST on day 2. All subjects received CRH at 1700 on the first day of the study. Although half of the subjects performed the TSST on the day after the CRH challenge, the TSST was never performed immediately after CRH. A comparison of responding between these tasks is presented in a separate manuscript (Waldrop et al., 2009). There was a 60 minute rest period prior to CRH administration.
Two baseline assessments of subjective, heart rate, and neuroendocrine parameters were obtained ten minutes apart to provide for a stable baseline index for challenge response comparisons. Pre-session subjective scales included the Craving/Distress/Mood Scale, a modification of the Within Session Rating Scale designed to rapidly assess craving and other mood feeling states (including stress) during the test session (Childress et al., 1986). This 100-mm visual 10-point Likert scale is anchored with adjectival modifiers (“not at all” to “extremely”). This scale was also utilized at each of the post-task assessment time points.
Trier Social Stress Task (TSST)
Subjects were asked to give a simulated job-talk to a panel of three confederates, and were told that the entire task would be recorded. After the five minute job-talk, subjects were asked to complete serial subtraction of two-digit numbers from a four-digit number aloud for five minutes. Each time an error was made, the participant was instructed to start over. Confederates were instructed not to provide any verbal or non-verbal feedback (e.g., smiles, nods). Immediately following the TSST, subjective ratings and heart rate measurements were obtained and blood was collected for ACTH and cortisol assay. Neuroendocrine samples, subjective ratings, and physiologic measurements were further assessed at 5-minute, 30-minute, 60-minute, and 120-minutes post task.
CRH Challenge
At 1700h (day 1), ovine CRH (1ug/kg; provided by Ferring Pharmaceuticals) was administered via IV push over a one-minute period. Immediately following CRH administration, subjective ratings and heart rate measurements were obtained and blood was collected for ACTH and cortisol assay. Neuroendocrine samples, subjective ratings, and physiologic measurements were further assessed at 5-minute, 30-minute, 60-minute, and 120-minutes post task.
Plasma Hormone Analysis
Blood samples were collected in EDTA-prepared tubes and immediately placed on ice. Plasma was obtained by centrifugation under refrigeration and the serum sample frozen at −70° C until assayed in duplicate. Allegro HS-ACTH system (Nichols Institute Diagnostics), which has an intra-assay c.v. of 6% with a sensitivity of 1 pg/ml, was used for ACTH assays. Cortisol was assayed using the Roche Diagnostic Elecsys 2010 immunoassay analyzer and kits based on an electrochemiluminescence competitive immunoassay having a functional sensitivity of 0.29 µg/dL and intra-assay variability of less than 2%. GCRC personnel collected all samples and Rockefeller University personnel performed the analysis.
Statistical Analysis
Categorical demographic data were analyzed by using frequency tests, p-values are two-sided Fisher Exact values, while continuous data were analyzed by two-way analysis of variance (ANOVA): (early life stress × cocaine status). Neuroendocrine (ACTH and cortisol) and physiological (heart rate) outcomes were analyzed using three-way analysis of covariance (time × early life stress × cocaine status) (ANCOVA) with repeated measures. All longitudinal analyses included race, gender, and baseline as covariates in the model. Non-normality of the outcomes and residuals was addressed via log10 transformations. Peak change was calculated as the percent change in response over baseline: (max response – baseline response) / baseline response × 100. The peak change was compared between all four groups by a one-way ANOVA and Bonferroni post-hoc test. Subjective measures of stress and craving were summarized by calculating the area under the curve (AUC) using the trapezoid rule and also by frequency tests of the number of responders. For frequency analysis, subjects were considered responders if they exhibited any increase in craving or stress post task, as compared to baseline. Since craving was absent in the non-cocaine dependent subjects, craving data were only compared between the cocaine-dependent groups (ELS/CD and non-ELS/CD). The subjective stress response to the TSST and CRH was compared between groups. Group differences in AUC were conducted using the nonparametric Kruskal-Wallis test. Correlation analysis was performed on peak change data using Spearman’s rank correlation coefficient (rs) by experimental group. All tests were two-tailed and p-values less than 0.05 were considered statistically significant.
Results
Subject Demographic and Descriptive Data
In Table 1, subject demographic and descriptive data by early life stress and cocaine status are displayed. The median split resulted in equal numbers of males and females in each of the four groups and there were no differences in the median number of early life stressors endorsed by the cocaine-dependent and non-dependent subjects (F=0.009, df=1, 85 p=0.9). As would be expected, a significant difference in the total number of early life stressors reported between ELS and non-ELS groups was observed (F=45.4, df=3, 85, p<0.001). While there were no significant differences in age, marital or smoking status between groups, there were significant group differences in education (p<0.01), employment status (p=0.05), and race (p<0.05). There was no effect of duration of abstinence on peak ACTH (p=0.64) or cortisol (p=0.77) between cocaine-dependent individuals who reported abstinence as compared to those reporting use in the two weeks prior to the study procedures. Although current marijuana abuse/dependence was not a basis for exclusion, only 3 out of 87 subjects met criteria for current marijuana abuse/dependence.
Neuroendocrine Response
CRH Response
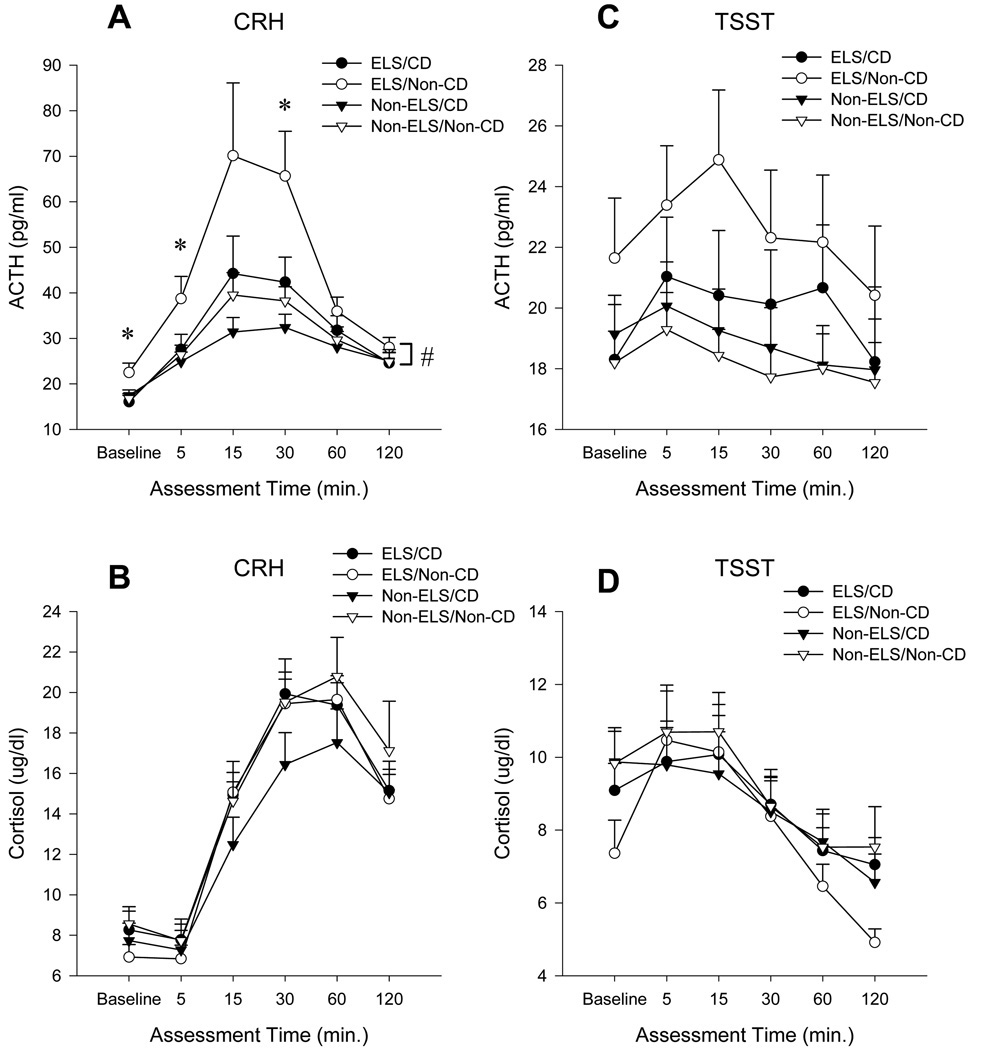
Subjects in the ELS/non-CD group exhibited a significantly higher baseline level of ACTH than the ELS/CD. A significant two-way interaction (time × early life stress; F=2.3, df=5, 180, p<0.05) was found indicating that subjects with a history of early life stress exhibited the greatest increase in ACTH (Figure 1A) regardless of cocaine-dependence status. Post-hoc analysis revealed that the ELS/non-CD group exhibited significantly higher levels of ACTH at baseline, 5 and 30 min post task as compared to the ELS/CD group (Figure 1A). There was also a significant main effect of time on plasma cortisol response (F=7.7, df=5, 275, p<0.001) (Figure 1B). There were no significant between group differences in peak cortisol or ACTH response to the CRH infusion.
Figure 1. Plasma cortisol and ACTH response to CRH and the TSST.
Comparison of mean ± se plasma ACTH (A & C) and Cortisol (B&D) between subjects in the Early Life Stress, Cocaine-Dependent (ELS/CD), Early Life Stress, Non-Cocaine-Dependent (ELS/Non-CD), Non-Early Life Stress, Cocaine-Dependent (Non-ELS/CD), and Non-Early Life Stress, Non-Cocaine-Dependent (Non-ELS/Non-CD) groups at baseline and for 120 minutes post CRH or the TSST. # p < 0.05 (ELS × time interaction); ELS vs. Non-ELS groups. p < 0.05; ELS/Non-CD vs. ELS/CD. p < 0.05.
Trier Response
The TSST produced a significant increase in plasma ACTH in all four groups as evidenced by a significant main effect of time (F=2.9, df=5, 265, p<0.01) (Figure 1C). There were also significant elevations in plasma cortisol in response to the TSST (F=9.2, 5, 260, p<0.001) (Figure 1D). There was no impact of trauma or cocaine status on the neuroendocrine response to the Trier.
Heart Rate (HR)
CRH Response
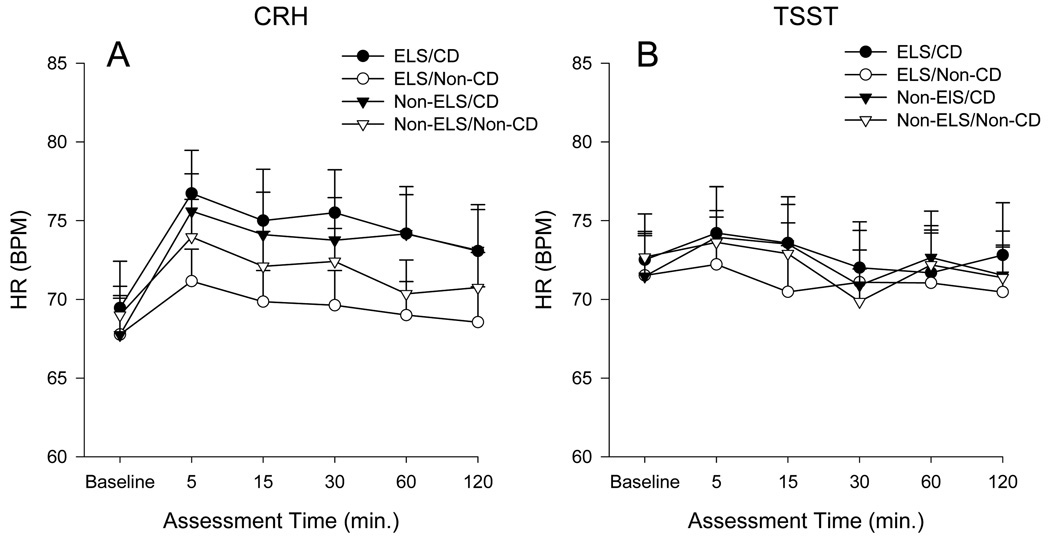
There was a significant main effect of time on the mean HR response to CRH (F=5.0, df=5, 305, p<0.001) (Figure 2A). No significant differences in the peak HR response to CRH were found between any of the four groups.
Figure 2. Heart Rate Response to CRH and the TSST.
Comparison of mean ± se heart rate between subjects in the Early Life Stress, Cocaine-Dependent (ELS/CD), Early Life Stress, Non-Cocaine-Dependent (ELS/Non-CD), Non-Early Life Stress, Cocaine-Dependent (Non-ELS/CD), and Non-Early Life Stress, Non-Cocaine-Dependent (Non-ELS/Non-CD) groups at baseline and for 120 minutes post CRH (A) and the TSST (B).
Trier Response
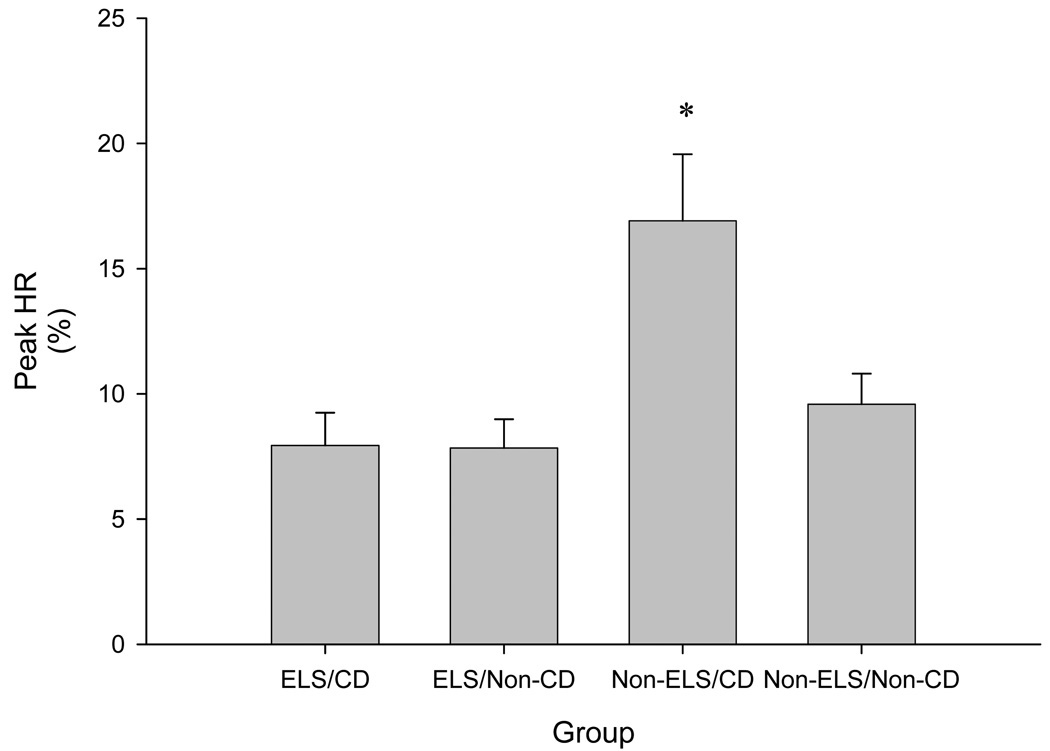
A significant effect of time on the mean HR response to the TSST was found (F=2.5, df=5, 320, p<0.05) (Figure 2B). In addition, a significant effect of the TSST on peak change in HR (F=6.4, df=3, 68, p=0.001) was observed. Post-hoc analysis revealed that the non-ELS/CD group exhibited a significantly greater change in HR to the TSST as compared to the other three groups (Figure 3).
Figure 3. Peak HR response to the TSST.
Comparison of mean change in peak heart rate ± se in response to the Trier between subjects in the Early Life Stress, Cocaine-Dependent (ELS/CD), Early Life Stress, Non-Cocaine-Dependent (ELS/Non-CD), Non-Early Life Stress, Cocaine-Dependent (Non-ELS/CD), and Non-Early Life Stress, Non-Cocaine-Dependent (Non-ELS/Non-CD) groups. Non-ELS/CD vs. ELS/CD, ELS/Non-CD, and Non-ELS/Non-CD. *p < 0.05.
Subjective Measures
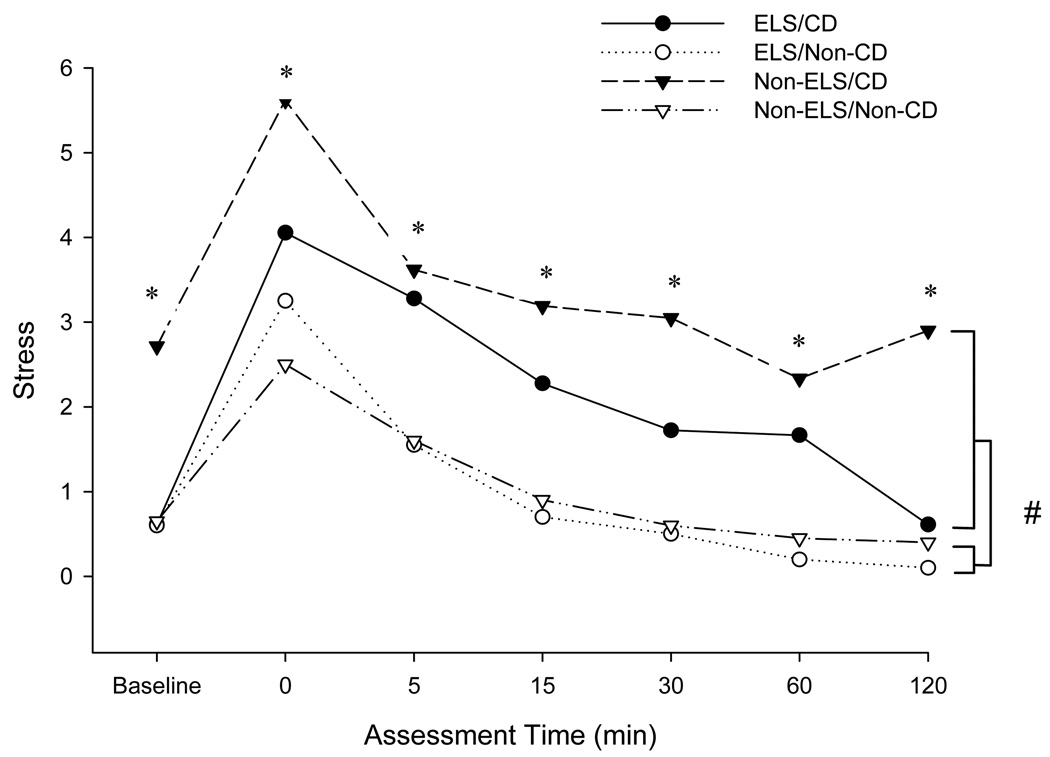
The magnitude of the subjective stress response to the TSST was significantly greater in cocaine-dependent as compared to non-dependent subjects (p<0.001), and was significantly more robust in the non-ELS/CD group across all time points by AUC (p < 0.05) (Figure 4). Subjects without cocaine dependence did not report craving, therefore craving data were only compared between the ELS/CD and non-ELS/CD groups. The number of subjects who reported craving following the TSST was similar between the ELS/CD (8/18) and the non-ELS/CD (10/20) groups. Craving response to CRH was also similar between ELS/CD (8/18) and non-ELS/CD (4/19) groups. The peak craving response to both paradigms was similar in both groups.
Figure 4. Subjective stress response to the TSST.
Comparison of mean subjective stress response to the Trier between subjects in the Early Life Stress, Cocaine-Dependent (ELS/CD), Early Life Stress, Non-Cocaine-Dependent (ELS/Non-CD), Non-Early Life Stress, Cocaine-Dependent (Non-ELS/CD), and Non-Early Life Stress, Non-Cocaine-Dependent (Non-ELS/Non-CD) groups at baseline and for 120 minutes following the TSST. CD vs. Non-CD. #p < 0.001; ELS/CD vs. ELS/Non-CD. *p < 0.05.
Correlation Analyses
Peak stress and craving were correlated in response to CRH in both the ELS/CD (rs=0.49, p<0.05) and the non-ELS/CD (rs=0.52, p<0.05) groups. However, in response to the TSST the correlation between stress and craving was only found in the non-ELS/CD group (rs=0.56, p<0.01).
Discussion
In the present study, the influence of early life stress and cocaine dependence on the physiologic, neuroendocrine, and subjective responses to CRH and the Trier Social Stress Task was examined. Among the most unexpected findings were the equal and high prevalence of early life adversity in the cocaine dependent and non-cocaine dependent groups. A number of studies have reported that cocaine dependent individuals have more early life trauma as compared to the general population (Medrano et al., 2002; Paivio and Cramer 2004). However, in this study both the cocaine dependent and non-dependent subjects reported a similar number of adverse early life events. The fact that study participation required several assessment study visits and a two-day hospital stay may have introduced a bias in terms of the individuals willing to volunteer, making the non-cocaine dependent group less representative of the general population. Of interest, individuals with current psychiatric disorders (e.g., PTSD, major depression, and bipolar disorder) were excluded, so the high early life stress group without cocaine dependence may represent a particularly resilient group of individuals. This finding allowed for examination of stress responding in a group of individuals who had experienced a considerable amount of early life stress, but had no significant psychopathology. Other studies have found that females tend to report more sexual abuse while males report more physical abuse (Thompson, Kingree et al. 2004; Ullman and Filipas 2005) however, equal numbers of males and females were spread among the four groups, therefore it is unlikely that gender had as significant impact on the present findings (Back, Brady et al. 2005; Uhart, Chong et al. 2006). Age and smoking status have also been shown to influence HPA axis responding; however, there were no differences between the four groups in either of these parameters (Kudielka, Buske-Kirschbaum et al. 2004; Back, Waldrop et al. 2008).
Neuroendocrine response to CRH infusion has been examined in women with a history of childhood trauma and abuse. In agreement with the study of Heim and colleagues (Heim et al., 2001) we found elevated ACTH and blunted cortisol levels in response to CRH in non-cocaine dependent subjects with a history of childhood stress as compared to those without substantial early life stress. Surprisingly, the elevated ACTH response was not found in cocaine-dependent subjects with a history of childhood stress. Two previous studies have demonstrated markedly attenuated ACTH levels following CRH administration in cocaine-dependent females and polysubstance-abusing individuals (Brady et. al., 2009; Contoreggi et al., 2003). It may be that cocaine desensitizes the pituitary to CRH, preventing the heightened ACTH response that was found in subjects in the ELS/Non-CD group. Despite the elevated ACTH response to CRH there was no difference in the mean cortisol levels between subjects with a history of early life stress and controls. This was surprising as we expected that increased ACTH drive from the pituitary would be met with a concomitant increase in cortisol release from the adrenal glands. In another study, Heim and colleagues examined HPA axis hormonal response to the TSST, and found that women with trauma histories exhibited augmented ACTH response without a concomitant increase in cortisol levels (Heim et al., 2000). In the current study, the non-dependent subjects with a history of childhood adversity appeared to exhibit an elevated ACTH response to the TSST, however this was not statistically significant. Of note, Heim’s studies were conducted in women only, while the present study includes men. In addition, Heim’s studies included women with a history of “moderate to severe physical or sexual abuse”, and subjects with a history of exposure to general stressors, such as loss of a parent or sibling, natural disaster, or adoption were excluded from the control group. It may be that heterogeneity of the study group in the present study made it difficult to detect a significant difference in ACTH response to the TSST between groups. More importantly, the sensitized pituitary ACTH response and blunted adrenal cortisol response to CRH found in subjects with a history of childhood maltreatment but without current cocaine-dependence supports the extant literature.
Increased sensitivity of the extended amygdala to CRH has been linked to both childhood trauma and stress-induced relapse to substance use in individuals with substance use disorders (Kreek and Koob 1998; Francis et al., 1999). Therefore, one might expect an augmented response to both the laboratory stress paradigms and CRH in cocaine-dependent subjects with a history of childhood adversity. However, we found no increase in cortisol or ACTH in cocaine-dependent individuals with high childhood adversity as compared to those who had not experienced high childhood adversity. In fact, stress and the heart rate response to the TSST were greatest in cocaine-dependent subjects without a history of childhood stress. It may be that blunting of the neuroendocrine and heart rate response to stress is part of the long-term consequence of early life adversity in individuals who develop cocaine dependence as compared to the augmented responses that has been reported in studies of non-cocaine dependent women with substantial early life adversity (Heim, Newport et al. 2000; Heim, Newport et al. 2001). Previous studies of neuroendocrine and physiologic consequences of early life trauma, such as those conducted by Heim and colleagues, have systematically excluded cocaine-dependent individuals, and so we know very little about the impact of early life stress combined with subsequent chronic substance use on the HPA axis.
In addition, a significant correlation between stress and craving in response to the TSST was observed in cocaine dependent subjects without early life stress, but not in the cocaine-dependent group with early life stress. In a similar study, stress reactivity to a cold pressor task was examined in alcohol-dependent subjects with and without co-morbid PTSD. A blunted ACTH and elevated craving response were predictive of follow-up drinking in the alcohol-only group, but not in the alcohol/PTSD group (Brady et al., 2006). While intuitively one might expect that the relationship between stress and craving would be stronger in individuals with anxiety disorders or early life stress and co-occurring substance use disorders, these data suggest differences in the neurobiological changes that occur as a function of early life stress and those underlying stress-induced drug craving.
The present study has several important limitations. For example, there were unanticipated baseline differences in employment and educational status. Given the limited sample size, employment and education status were not included as covariates in the analysis, however we don’t anticipate that these variables impacted the present findings. Menstrual cycle phase was not controlled for, and has been shown to impact drug seeking behavior and stress reactivity (Feltenstein and See 2007; Fuchs et al., 2005(Kirschbaum, Kudielka et al. 1999). In addition, while the ETI-SR is a common measure employed that has been shown to be valid in the assessment of childhood trauma and adversity (Bremner, Bolus et al. 2007), it is a retrospective self-report assessment and thus subject to recall bias. In addition, significant power is lost by using the median split to categorize subjects according to trauma status. However, previous studies have successfully utilized the median split in assessing neuroendocrine response across groups. For example Lee and colleagues found significantly higher levels of CRH in subjects with trauma scores above the median (Lee, Geracioti et al. 2005). In a separate study the median split was used to subgroup subjects with fibromyalgia according to trauma history. Weissbecker and colleagues found significant differences in basal cortisol levels as a function of trauma history (Weissbecker, Floyd et al. 2006). Finally, the period of abstinence prior to study participation varied between participants. However, we performed several analyses examining the impact of duration of abstinence on peak neuroendocrine response to CRH. We failed to find a significant difference in peak ACTH or cortisol between individuals who reported abstinence as compared to those reporting use in the 2 weeks prior to the study procedures. Thus, we do not anticipate that the period of abstinence had a profound effect on the present findings.
Despite these limitations, this is the first study to report on the impact of early life stress on stress responding in individuals with and without cocaine dependence using two distinctly different laboratory stress paradigms. Consistent with the literature, we found an increased ACTH response to CRH in non-dependent subjects with a history of early life stress. In contrast, cocaine dependence appears to modify the impact of childhood adversity on the neuroendocrine, heart rate and subjective response to stress in an unexpected way. Further research focused on understanding the behavioral and biologic response to stress in cocaine-dependent individuals with a history of childhood adversity could provide insight into pharmacological and behavioral therapies aimed at preventing relapse.
References
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Back SE, Waldrop AE, Saladin ME, Yeatts SD, Simpson A, McRae AL, et al. Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinology. 2008;33:560–568. doi: 10.1016/j.psyneuen.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Champoux M, Suomi SJ, Gunnar MR. Salivary cortisol in nursery-reared rhesus monkeys: reactivity to peer interactions and altered circadian activity. Dev. Psychobiol. 1995;28:257–267. doi: 10.1002/dev.420280502. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, et al. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol. Clin. Exp. Res. 2006;30:938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry. 2009;66:422–430. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J. Nerv. Ment. Dis. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress. Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP. Conditioned responses in a methadone population. A comparison of laboratory, clinic, and natural settings. J. Subst. Abuse Treat. 1986;3:173–179. doi: 10.1016/0740-5472(86)90018-8. [DOI] [PubMed] [Google Scholar]
- Contoreggi C, Herning RI, Na P, Gold PW, Chrousos G, Negro PJ, et al. Stress hormone responses to corticotropin-releasing hormone in substance abusers without severe comorbid psychiatric disease. Biol. Psychiatry. 2003;54:873–878. doi: 10.1016/s0006-3223(03)00167-7. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Frances AJ, Pincus HA, Vettorello N, Davis WW. DSM-IV in progress. Changes in substance-related, schizophrenic, and other primarily adult disorders. Hosp. Community Psychiatry. 1994;45:18–20. doi: 10.1176/ps.45.1.18. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor--norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol. Psychiatry. 1999;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Furukawa TA, Ogura A, Hirai T, Fujihara S, Kitamura T, Takahashi K. Early parental separation experiences among patients with bipolar disorder and major depression: a case-control study. J. Affect Disord. 1999;52:85–91. doi: 10.1016/s0165-0327(98)00054-8. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hien D, Cohen L, Campbell A. Is traumatic stress a vulnerability factor for women with substance use disorders? Clin. Psychol. Rev. 2005;25:813–823. doi: 10.1016/j.cpr.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Sinha R. Childhood maltreatment, perceived stress, and stress-related coping in recently abstinent cocaine dependent adults. Psychol. Addict.Behav. 2007;21:233–238. doi: 10.1037/0893-164X.21.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski JL, Williams LM, Siegel J. Childhood physical and sexual abuse as risk factors for heavy drinking among African-American women: a prospective study. Child Abuse Negl. 2000;24:1061–1071. doi: 10.1016/s0145-2134(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Lee R, Geracioti TD, Kasckow JW, Coccaro EF. Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. Am. J. Psychiatry. 2005;162:995–997. doi: 10.1176/appi.ajp.162.5.995. [DOI] [PubMed] [Google Scholar]
- Medrano MA, Hatch JP, Zule WA, Desmond DP. Psychological distress in childhood trauma survivors who abuse drugs. Am. J. Drug Alcohol Abuse. 2002;28:1–13. doi: 10.1081/ada-120001278. [DOI] [PubMed] [Google Scholar]
- Mullen PE, Martin JL, Anderson JC, Romans SE, Herbison GP. The long-term impact of the physical, emotional, and sexual abuse of children: a community study. Child Abuse Negl. 1996;20:7–21. doi: 10.1016/0145-2134(95)00112-3. [DOI] [PubMed] [Google Scholar]
- Paivio SC, Cramer KM. Factor structure and reliability of the Childhood Trauma Questionnaire in a Canadian undergraduate student sample. Child Abuse Negl. 2004;28:889–904. doi: 10.1016/j.chiabu.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res. Mol. Brain Res. 1983;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J. Trauma Stress. 2001;14:843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct. Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MP, Kingree JB, Desai S. Gender differences in long-term health consequences of physical abuse of children: data from a nationally representative survey. Am. J. Public Health. 2004;94:599–604. doi: 10.2105/ajph.94.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Ullman SE, Filipas HH. Gender differences in social reactions to abuse disclosures, post-abuse coping, and PTSD of child sexual abuse survivors. Child Abuse Negl. 2005;29:767–782. doi: 10.1016/j.chiabu.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Waldrop AE, Price KL, Desantis SM, Simpson AN, Back SE, McRae AL, Spratt EG, Kreek MJ, Brady KT. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.11.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, Weiler BL, Cottler LB. Childhood victimization and drug abuse: a comparison of prospective and retrospective findings. J. Consult. Clin. Psychol. 1999;67:867–880. doi: 10.1037//0022-006x.67.6.867. [DOI] [PubMed] [Google Scholar]
- Weissbecker I, A. Floyd A, Dedert E, Salmon P, Sephton S. Childhoodtrauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology. 2006;31:312–324. doi: 10.1016/j.psyneuen.2005.08.009. [DOI] [PubMed] [Google Scholar]