Abstract
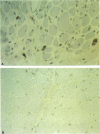
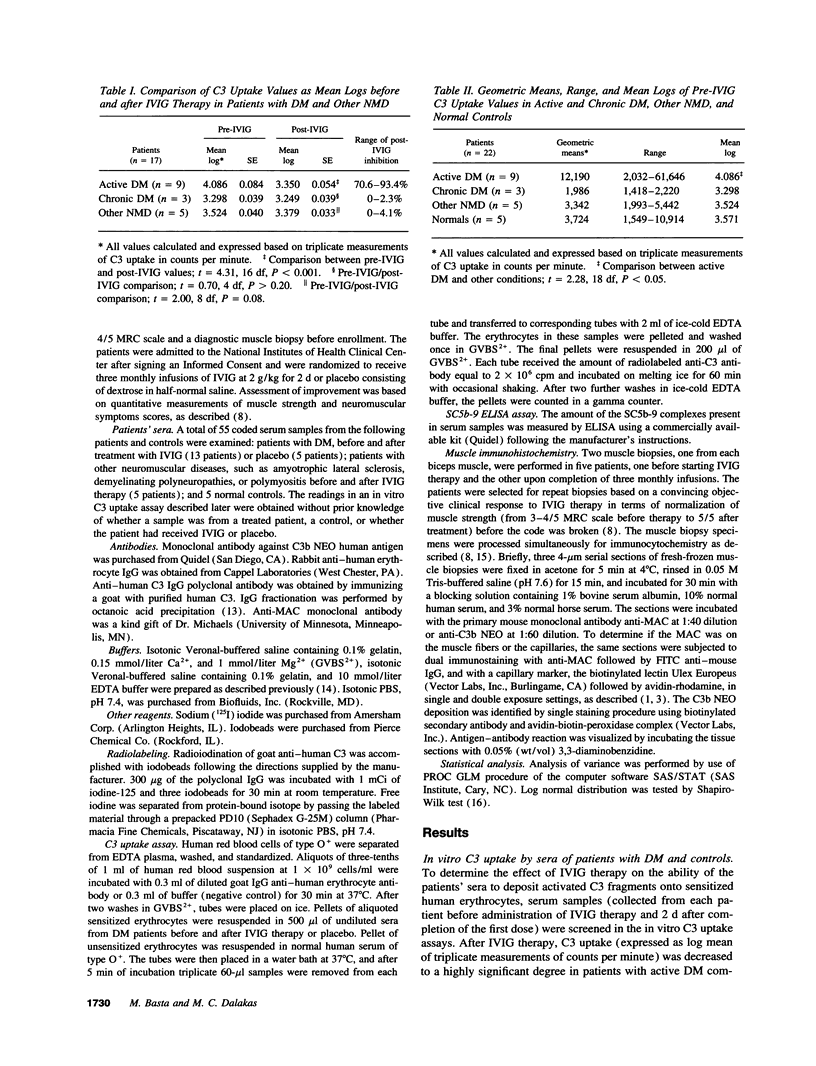
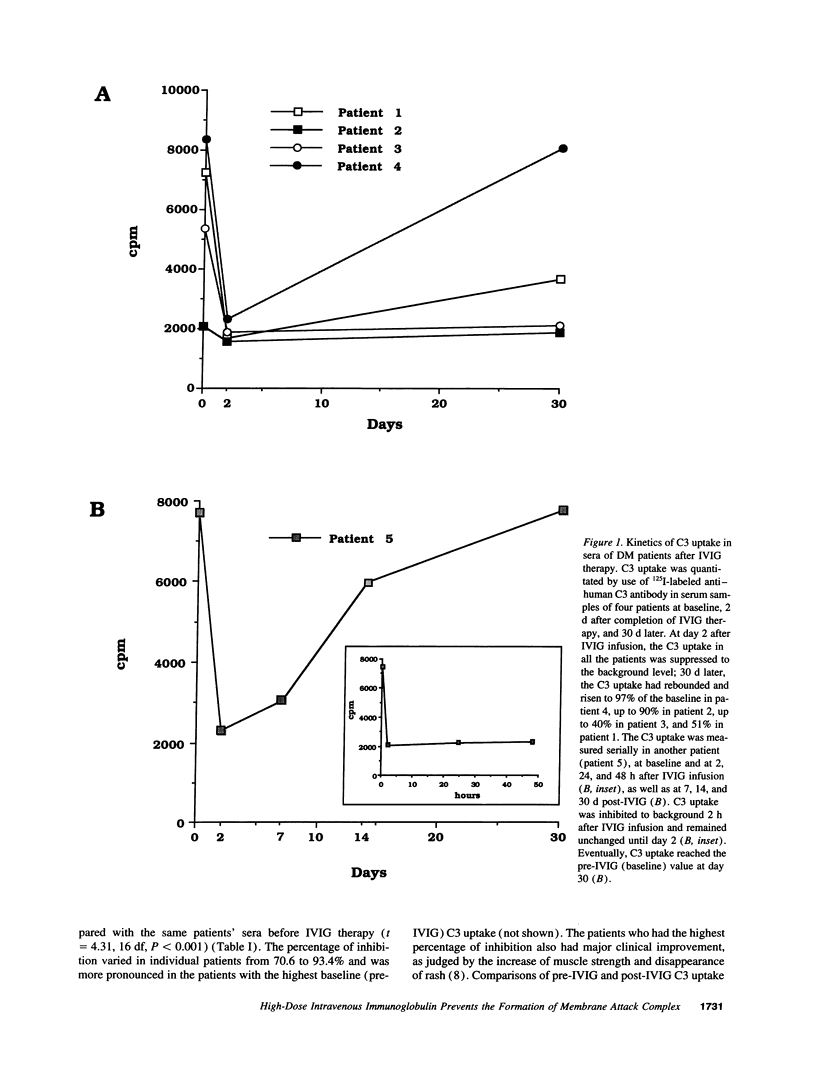
In patients with dermatomyositis (DM) the earliest lesion is microvasculopathy mediated by deposition of C5b-C9 membranolytic attack complex (MAC) on intramuscular capillaries. This leads sequentially to muscle ischemia, necrosis of muscle fibers, and muscle weakness. High-dose intravenous immunoglobulin (IVIG), which can modulate complement-dependent tissue damage in animal models, has been shown to be effective in the treatment of patients with DM. We used an in vitro C3 uptake assay to examine 55 coded sera from 13 patients with DM and 5 patients with other non-complement-mediated neuromuscular diseases, before and after treatment with IVIG or placebo. Patients with active DM had a significantly higher baseline C3 uptake compared with the others (geometric mean 12,190 vs 3,090 cpm). Post-IVIG but not post-placebo sera inhibited the C3 uptake, without depleting the complement components, by 70.6-93.4%. The maximum inhibition of C3 uptake occurred within hours after IVIG infusion, started to rebound 2 d later, and reached pretreatment levels after 30 d. The serum levels of SC5b-9 complex production were high at baseline but normalized after IVIG therapy. Repeat biopsies from muscles of improved patients showed disappearance of C3b NEO and MAC deposits from the endomysial capillaries and restoration of the capillary network. We conclude that IVIG exerts its beneficial clinical effect by intercepting the assembly and deposition of MAC on the endomysial capillaries through the formation of complexes between the infused immunoglobulins and C3b, thereby preventing the incorporation of activated C3 molecules into C5 convertase. These findings provide the first serological and in situ evidence that IVIG modulates complement attack in a human disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguado M. T., Lambris J. D., Tsokos G. C., Burger R., Bitter-Suermann D., Tamerius J. D., Dixon F. J., Theofilopoulos A. N. Monoclonal antibodies against complement 3 neoantigens for detection of immune complexes and complement activation. Relationship between immune complex levels, state of C3, and numbers of receptors for C3b. J Clin Invest. 1985 Oct;76(4):1418–1426. doi: 10.1172/JCI112119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J. P., Andersson U. G. Human intravenous immunoglobulin modulates monokine production in vitro. Immunology. 1990 Nov;71(3):372–376. [PMC free article] [PubMed] [Google Scholar]
- Arsura E. L., Bick A., Brunner N. G., Namba T., Grob D. High-dose intravenous immunoglobulin in the management of myasthenia gravis. Arch Intern Med. 1986 Jul;146(7):1365–1368. [PubMed] [Google Scholar]
- Basta M., Fries L. F., Frank M. M. High doses of intravenous Ig inhibit in vitro uptake of C4 fragments onto sensitized erythrocytes. Blood. 1991 Jan 15;77(2):376–380. [PubMed] [Google Scholar]
- Basta M., Fries L. F., Frank M. M. High doses of intravenous immunoglobulin do not affect the recognition phase of the classical complement pathway. Blood. 1991 Aug 1;78(3):700–702. [PubMed] [Google Scholar]
- Basta M., Kirshbom P., Frank M. M., Fries L. F. Mechanism of therapeutic effect of high-dose intravenous immunoglobulin. Attenuation of acute, complement-dependent immune damage in a guinea pig model. J Clin Invest. 1989 Dec;84(6):1974–1981. doi: 10.1172/JCI114387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta M., Langlois P. F., Marques M., Frank M. M., Fries L. F. High-dose intravenous immunoglobulin modifies complement-mediated in vivo clearance. Blood. 1989 Jul;74(1):326–333. [PubMed] [Google Scholar]
- Blasczyk R., Westhoff U., Grosse-Wilde H. Soluble CD4, CD8, and HLA molecules in commercial immunoglobulin preparations. Lancet. 1993 Mar 27;341(8848):789–790. doi: 10.1016/0140-6736(93)90563-v. [DOI] [PubMed] [Google Scholar]
- Cervera R., Ramírez G., Fernández-Solà J., D'Cruz D., Casademont J., Grau J. M., Asherson R. A., Khamashta M. A., Urbano-Márquez A., Hughes G. R. Antibodies to endothelial cells in dermatomyositis: association with interstitial lung disease. BMJ. 1991 Apr 13;302(6781):880–881. doi: 10.1136/bmj.302.6781.880-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherin P., Herson S., Wechsler B., Piette J. C., Bletry O., Coutellier A., Ziza J. M., Godeau P. Efficacy of intravenous gammaglobulin therapy in chronic refractory polymyositis and dermatomyositis: an open study with 20 adult patients. Am J Med. 1991 Aug;91(2):162–168. doi: 10.1016/0002-9343(91)90009-m. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C. Clinical, immunopathologic, and therapeutic considerations of inflammatory myopathies. Clin Neuropharmacol. 1992 Oct;15(5):327–351. doi: 10.1097/00002826-199210000-00001. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C., Illa I., Dambrosia J. M., Soueidan S. A., Stein D. P., Otero C., Dinsmore S. T., McCrosky S. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993 Dec 30;329(27):1993–2000. doi: 10.1056/NEJM199312303292704. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C. Inflammatory and toxic myopathies. Curr Opin Neurol Neurosurg. 1992 Oct;5(5):645–654. [PubMed] [Google Scholar]
- Dalakas M. C. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991 Nov 21;325(21):1487–1498. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Dysfunctions of pokeweed mitogen-stimulated T and B lymphocyte responses induced by gammaglobulin therapy. J Clin Invest. 1981 Mar;67(3):867–877. doi: 10.1172/JCI110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie-Smith A. M., Engel A. G. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. 1990 Apr;27(4):343–356. doi: 10.1002/ana.410270402. [DOI] [PubMed] [Google Scholar]
- Goldstein R., Blanchette V. S., Huebsch L. B., McKendry R. J. Treatment of gold-induced thrombocytopenia by high-dose intravenous gamma globulin. Arthritis Rheum. 1986 Mar;29(3):426–430. doi: 10.1002/art.1780290319. [DOI] [PubMed] [Google Scholar]
- Illa I., Leon-Monzon M., Dalakas M. C. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol. 1992 Jan;31(1):46–52. doi: 10.1002/ana.410310109. [DOI] [PubMed] [Google Scholar]
- Imbach P., Barandun S., d'Apuzzo V., Baumgartner C., Hirt A., Morell A., Rossi E., Schöni M., Vest M., Wagner H. P. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981 Jun 6;1(8232):1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- Kissel J. T., Mendell J. R., Rammohan K. W. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med. 1986 Feb 6;314(6):329–334. doi: 10.1056/NEJM198602063140601. [DOI] [PubMed] [Google Scholar]
- Koski C. L., Sanders M. E., Swoveland P. T., Lawley T. J., Shin M. L., Frank M. M., Joiner K. A. Activation of terminal components of complement in patients with Guillain-Barré syndrome and other demyelinating neuropathies. J Clin Invest. 1987 Nov;80(5):1492–1497. doi: 10.1172/JCI113231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlander R. J., Ellison D. M., Hall J. The blockade of Fc receptor-mediated clearance of immune complexes in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes. J Immunol. 1984 Aug;133(2):855–862. [PubMed] [Google Scholar]
- Lang B. A., Laxer R. M., Murphy G., Silverman E. D., Roifman C. M. Treatment of dermatomyositis with intravenous gammaglobulin. Am J Med. 1991 Aug;91(2):169–172. doi: 10.1016/0002-9343(91)90010-u. [DOI] [PubMed] [Google Scholar]
- Leung D. Y., Cotran R. S., Kurt-Jones E., Burns J. C., Newburger J. W., Pober J. S. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989 Dec 2;2(8675):1298–1302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- May J. E., Frank M. M. Complement-mediated tissue damage: contribution of the classical and alternate complement pathways in the Forssman reaction. J Immunol. 1972 Jun;108(6):1517–1525. [PubMed] [Google Scholar]
- Newburger J. W., Takahashi M., Burns J. C., Beiser A. S., Chung K. J., Duffy C. E., Glode M. P., Mason W. H., Reddy V., Sanders S. P. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986 Aug 7;315(6):341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- Oda H., Honda A., Sugita K., Nakamura A., Nakajima H. High-dose intravenous intact IgG infusion in refractory autoimmune hemolytic anemia (Evans syndrome). J Pediatr. 1985 Nov;107(5):744–746. doi: 10.1016/s0022-3476(85)80405-4. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]