Abstract
To investigate the effect on the functioning of the vestibular system of a rupture of Reissner’s membrane, artificial endolymph was injected in scala media of ten guinea pigs and vestibular evoked potentials (VsEPs), evoked by vertical acceleration pulses, were measured. Directly after injection of a sufficient volume to cause rupture, all ears showed a complete disappearance of VsEP, followed by partial recovery. To investigate the effect of perilymphatic potassium concentration on the vestibular sensory and neural structures, different concentrations of KCl were injected directly into the vestibule. The KCl injections resulted in a dose-dependent decrease of VsEP, followed by a dose-dependent slow recovery. This animal model clearly shows a disturbing effect of a higher than normal K+ concentration in perilymph on the vestibular and neural structures in the inner ear. Potassium intoxication is the most probable explanation for the observed effects. It is one of the explanations for Menière attacks.
Keywords: VsEP, Vestibular evoked potential, Vestibular function, Endolymph, Perforation, Reissner’s membrane, Guinea pig, Acute attack, Menière, Potassium, Toxic, Vestibular and neural structures, Osmolarity
Introduction
Since 1938 endolymphatic hydrops is generally accepted as the histopathological substrate for Menière’s disease, based on autopsy studies of the ear of Menière patients [1, 2]. However, it is still unclear if endolymphatic hydrops is the cause of the symptoms, or just a histological marker of the disease [3].
The effect on cochlear functioning of an endolymphatic hydrops has been extensively studied in animals during the last half century [4, 5]. Although unpredictable attacks of vertigo are the most severe complaint of Menière patients, much less information is available on the influence of hydrops on vestibular functioning, most probably because this is more difficult to investigate. Reduced or altered vestibular function has been reported after creation of an endolymphatic hydrops in the guinea pig [6] and in the rabbit [7].
The most widely used method for the creation of an endolymphatic hydrops is surgical obliteration or dissection of the endolymphatic sac [8]. With this technique a hydrops develops in a period of days to weeks. An obvious disadvantage of the model is the destruction of an organ that is essential for inner ear homeostasis [9].
An acute endolymphatic hydrops can be created by directly injecting artificial endolymph into scala media [10, 11]. We developed this technique to study inner ear fluid pressure [12, 13] and cochlear functioning during an acute hydrops [14, 15] in the guinea pig.
Recording of evoked potentials is widely used to study cochlear functioning. Böhmer et al. [16] showed in the chinchilla that evoked potentials can also be measured in the vestibular system, if the head of the animal is subject to linear acceleration pulses. Plotnik [17] confirmed this finding and showed that these short latency vestibular evoked potentials (VsEPs) mainly originate from the otolith organs.
In an earlier experiment we combined measurement of VsEPs with injection of artificial endolymph, to study the effect of an acute endolymphatic hydrops in the guinea pig on the functioning of (part of) the vestibular system [18]. Apart from a short lasting hydromechanical effect of fluid injection through the basilar membrane, no significant difference in VsEP deterioration was found between animals in which an artificial hydrops was created and (control) animals in which the injection pipette perforated the basilar membrane and was withdrawn without injecting fluid. This suggested that it was not the (artificial) hydrops that caused the slow decrease of VsEP amplitude during a time course of hours, but possibly a leakage of potassium ions into the perilymphatic space.
Intoxication by potassium ions [19] leaking through a ruptured [20, 21] or micro-lesioned [22] Reissner’s membrane is one possible explanation for acute Menière attacks.
For the present paper the influence of perilymphatic potassium concentration on the VsEP was investigated in the guinea pig in two ways: firstly, artificial endolymph was injected slowly through the basilar membrane until Reissner’s membrane ruptured, causing a massive leakage of high potassium endolymph into the perilymphatic space.
Secondly, to control the effect of potassium concentration on the vestibular sensory and neural structures in the perilymphatic space, different concentrations of KCl were injected directly into the vestibule.
Materials and methods
In this study 20 albino guinea pigs, weighing between 350 and 600 g, with normal Preyer’s reflex were used. The care and use of the animals was in accordance with the principles of the declaration of Helsinki and approved by the Groningen animal experiment committee. (Protocol numbers 4310B and 4463A).
General anaesthesia was induced by intramuscular injection of ketamine/xylazine (60/3.6 mg/kg) and maintained by administering additional anaesthetics every hour (40/2.4 mg/kg). A tracheostoma was created for artificial ventilation (Columbus Instruments, model 7950) and intramuscular suxamethoniumchloride (2.5 mg/kg) was given every hour for muscle relaxation.
The body temperature was maintained at 38°C with a heating blanket. Skin electrodes placed on both sides of the thorax monitored the heart rate.
To stimulate the vestibular system by acceleration pulses and fixate the head during operation and microinjection, a steel bolt was cemented upside down on the skull of the guinea pig with dental cement. After opening the bulla to expose the round window, a platinum electrode was implanted in both ears in the facial nerve canal, up to the first curvature. Only a thin bony wall separates the vestibular nerve from the electrode. A reference electrode was placed in the neck muscles. Acceleration pulses were generated with a Bruel and Kjaer vibration exciter (type 4809) and monitored with an accelerometer, connected to a Bruel and Kjaer amplifier (type 2651). Linear acceleration pulses were applied in the direction of the earth vertical axis perpendicular to the top of the skull. The shaker was driven by computer-generated stimuli that consisted of Gaussian-shaped pulses, amplified by a power amplifier (Bruel and Kjaer type 2712) and computer controlled with a programmable attenuator. The vestibular stimulus consisted of 500 alternating pulses with peak amplitude of 40 m/s² at 0.5 ms after onset, at a rate of 51/s.
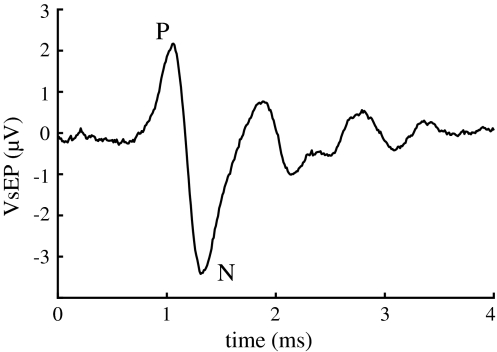
The signals of the active electrodes were amplified (Disa, type 15 c01) and band-pass filtered (100 Hz–5 kHz). The first 10 ms of the electrode and accelerometer signals were recorded, averaged and processed, using a Tucker-Davis BioSig-stimulate/record system, version 2.0. The P–N signal complex in the electrode signal, appearing first between 1 and 2 ms after stimulus onset, is of vestibular origin [18, 23, 24], and is therefore called vestibular evoked potential (VsEP; see Fig. 1).
Fig. 1.
Gross electrode potential evoked by acceleration pulses. The first part of it, appearing between 1 and 2 ms after stimulus onset, is of peripheral vestibular origin
In ten guinea pigs artificial endolymph was injected into scala media until one or more membranes surrounding the endolymphatic compartments ruptured. From earlier experiments we knew that this always occurred after injection of more than 3 μl of fluid [13, 14]. To inject artificial endolymph, the tip of a double barrel micropipette was inserted through the round window and the basilar membrane. The other ear served as control.
In a separate set of experiments we evaluated the effect of injecting 4 μl of a KCl solution directly into the perilymphatic space, through a small hole that was made in the bony wall of the vestibule next to the oval window. The injected concentrations of K+ were 0.250, 0.375, 0.500 and 3.00 M, in respectively 3, 3, 2 and 2 guinea pigs. Again the other ear served as control. Because the perilymphatic space behind the round window is directly connected to the cerebrospinal fluid space through the cochlear aqueduct we did not inject through the round window membrane.
Double barrel micropipettes were drawn from borosilicate glass and the tips were bevelled (Narishige EG-40). Tip diameters were around 60 μm per barrel, which is a compromise between a low enough flow resistance for fluid injection and a small tip size. One of the barrels was used to measure the DC potential (to monitor tip position) and inner ear pressure (WPI 900A Micropressure system). The other barrel was used to inject artificial endolymph (140 mM KCL + 25 mM KHCO3), by applying pneumatic pressure to the fluid interface (WPI PV 830 Pico Pump). Since the inner diameter of a barrel is precisely known (0.84 mm) the injected volume could be measured by displacement of the fluid interface in the barrel. The injection rate was about 50 nl/s. DC potential was monitored with a Kipp chart recorder.
After opening the bulla and positioning the electrodes in the facial canal the experiment started with measuring VsEPs in both ears. The difference in potential between the first positive (P) and negative (N) peak (Fig. 1) was used as a measure of the functionality of the linear acceleration detecting part of the vestibular organ. This initial value was used as a reference for the vestibular system under investigation, because of interindividual differences of VsEP amplitude. Directly after rupturing Reissner’s membrane in the first series of experiments and after injection of KCl in the second series, the VsEP amplitude was measured again and then after 1, 2, 3, 4 and 5 h. Between the measurements the animal recovered from heavy shaking. It was detached from the shaker during these periods.
After the last measurement the animals were terminated by means of intracardial administration of an overdose of pentobarbital. The temporal bones were removed and fixated by immersion in a solution of 2.5% glutaraldehyde in 0.1 M Na-cacodylate buffer and 2 mM calcium chloride (pH 7.4; 320 mOsm) for later study.
After fixation three specimens of the first series of (rupture) experiments were decalcified in 10% EDTA (pH 7.4) for 5 days, carefully rinsed in distilled water, dehydrated in a graded ethanol series and embedded in plastic. From the bullae sections of the cochlea and the vestibule were cut (midmodiolar plane) and stained with toluidine blue for light microscopic examination to check for damage caused by injection of (too much) artificial endolymph.
Time-dependent changes in VsEP between injected ears and control ears were compared using ANCOVA (SPSS 15.0) for repeated measurements.
Results
Reissner’s membrane rupture (RMR) experiments
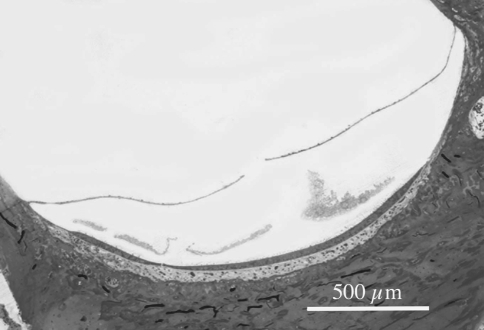
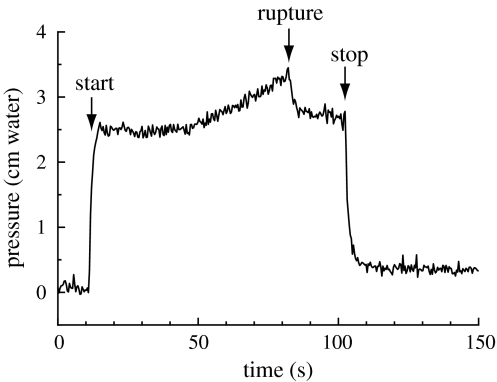
Injection of more than 3 μl of artificial endolymph in scala media did not only cause a fissure in Reissner’s membrane in the cochlea, but also in the membrane in the saccule (Fig. 2) in all three investigated ears, which makes it very likely that not only the hearing system will be directly affected but also the balance system. All ears showed a VsEP before intervention. Each experiment was successful in reaching scala media: DC potential abruptly increased 70–80 mV after the basilar membrane was perforated. Pressure in scala media increased after starting injection of artificial endolymph and decreased to almost initial pressure after stopping injection (Fig. 3). The endocochlear potential (EP) decreased by up to 40% during injection, but recovered almost completely within a few minutes after injection.
Fig. 2.
Light microscopic picture of a rupture in the membrane of the saccule, created by microinjection of 4 μl artificial endolymph into scala media. (From experience we know that the rupture was not caused by preparing it for light microscopic examination)
Fig. 3.
Fluid pressure (cm water) measured at the tip of the micropipette, before, during and after injection of more than 3 μl of artificial endolymph in scala media. This amount of injected fluid ruptures the membrane(s) surrounding the endolymphatic compartment(s), resulting in a sudden small drop of inner ear pressure
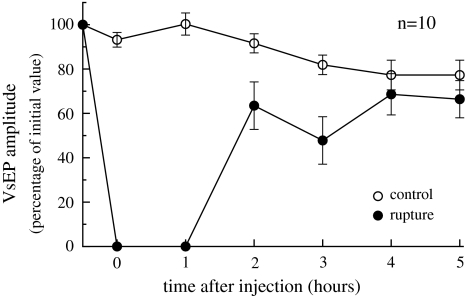
All RMR ears showed a complete disappearance of VsEP after injection with partial recovery subsequently (Fig. 4). In contrast, the contralateral control ears remained nearly stable during the experiment. Differences between VsEP amplitudes over time were statistically significant (p < 0.00).
Fig. 4.
Average VsEP (±1 SE) for the ruptured and control ears before and at different times after rupture of Reissner’s membrane by microinjection of artificial endolymph
KCl injection experiments
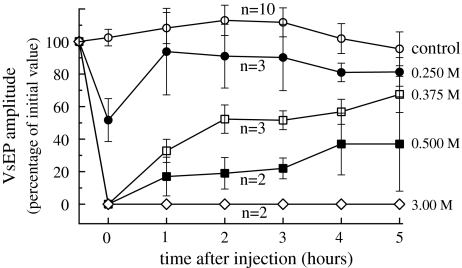
All ears showed a VsEP before intervention. The effect of KCl on the VsEP is depicted in Fig. 5. KCl injection resulted in a dose-dependent decrease and subsequent recovery of the VsEP. The contralateral control ears remained stable during the experiment.
Fig. 5.
Average VsEP (±1 SE) for the different concentrations of injected KCl, measured before and at different times after injection. Injection resulted in a dose-dependent decrease and subsequent partial recovery of the VsEP
Discussion
Multiple studies have demonstrated that potassium has toxic effects on the cochlear function [14, 25–28]. However, studies on this toxic effect on the vestibular function are sparse. A few investigators showed, about 40 years ago, that perfusion of artificial endolymph in the perilymphatic space caused a nystagmus [20, 29]. More recently, Böhmer et al. [16] demonstrated the toxic effect of potassium on the VsEPs by introducing potassium chloride crystals in the perilymphatic space of the inner ear of the chinchilla.
One case report describes a patient who directly applied potassium iodide solution through a tympanostomy tube into his middle ear, which caused a typical episode of Meniere’s disease; vertigo, nausea, vomiting, hearing loss and tinnitus [19]. This shows that potassium intoxication of the inner ear can cause the same clinical symptoms as during an acute Meniere’s attack.
Rupture of one or more membranes enclosing the endolymphatic spaces causes an immediate and complete suppression of the VsEP, as is shown in Fig. 4. During hours after injection the VsEP recovers to about 90% of the value in the control ear, most probably due to dilution of the injected artificial endolymph. A similar slow recovery (from vertigo) is present during an acute episode of Menière’s disease.
In temporal bone preparations of Menière patients hydrops of scala media was sometimes accompanied by a rupture of Reissner’s membrane [3], which can be an explanation for the acute character of vestibular attacks in Menière’s disease. In the first series of experiments (RMR) part of the injected fluid will enter the vestibular part of the inner ear through the ductus reuniens [13] and create a vestibular hydrops also, followed by a distention and rupture of one or more membranes (Fig. 2).
In the second series of experiments (KCl injection) suppression and recovery of the VsEP are dose dependent, as is shown in Fig. 5. After injection of the lowest concentration of KCl (0.25 M) the VsEP recovers almost completely within 1 h. No recovery is observed after injection of the highest concentration of 3 M, in contrast to the result after membrane rupture (Fig. 4), which best resembles the 0.375 M curve in Fig. 5.
Immediately after the injection of fluid into the inner ear fluid pressure inside the inner ear compartment increases. But within a few seconds pressure returns to normal, indicating that excess fluid escapes: perilymph through the cochlear aqueduct and endolymph through the ductus endolymphaticus [12, 13, 30]. So, when a KCl solution is directly injected into the perilymphatic space through a small hole in the bony wall of the vestibule, this solution will mix with perilymph and the total fluid volume in the inner ear will not increase.
The total perilymph volume in the guinea pig is 16 μl [31]. Injection of 4 μl of 0.25 M KCl solution, assuming a homogeneous distribution of K+ in 16 μl shortly after injection, leads to a K+ concentration of 63 mM. This is 7 times the normal concentration of K+ in perilymph in scala vestibuli of the guinea pig, which is 9.0 mM [32].
Not much is known about the mechanism of K+ intoxication of the vestibular system. For the cochlea Zenner et al. [28] found that addition of artificial endolymph to the basolateral surface of outer hair cells resulted in inhibition of the physiological repolarizing K+ efflux from these cells. A similar mechanism is proposed to influence neurotransmitter release from inner hair cells. They also found pathological shortening of outer hair cells; while long-lasting K+ intoxication resulted in chronic and complete loss of outer hair cell motility, and finally in cell death, and suggested this to be a pathophysiological basis in some Menière patients for chronic hearing loss.
Very recently Nakano et al. [33] found elevated perilymphatic K+ concentrations in the nmf329 mutant mouse line. Mice of this line show a widespread loss of sensory hair cells in the hearing organ, most possibly due to a mutation of the claudin-9 gene that encodes a tight junction protein. Claudin-defective tight junctions fail to shield the basolateral side of hair cells from the K+ -rich endolymph.
As a neurotoxic effect of an increased concentration of K+ in the perilymphatic space during a Menière’s attack Meissner [34] suggested a depolarization of the synapse between the vestibular hair cell and the afferent nerve fiber. This suggestion is supported by the conclusion of Kiernan et al. [35] that the toxic effect of K+ on axons, in patients with hyperkalaemia caused by chronic renal failure, is due to membrane depolarization.
Both Böhmer et al. [16] and Plotnik et al. [17] state that the VsEP is most probably a compound action potential of the vestibular nerve and pathways. A toxic effect of K+ on vestibular hair cells will affect the generator potential, leading to less single nerve fiber action potentials, or none at all. A toxic effect on the nerve will affect action potential generation directly.
The (normal) osmolarity of perilymph in the guinea pig is 0.29 and of endolymph it is 0.30 osmol/l [32]. The total perilymph volume is 16 μl [31]. The number of ions in 4 μl of injected 0.250 M KCl is 2 × 10−6 N (N is Avogadro’s number). The number of ions in the remaining 12 μl of perilymph is 3.5 × 10−6 N. So, after injection 5.5 × 10−6 N ions are present in the perilymphatic space, giving an osmolarity of 0.34 osmol/l. This number is slightly higher than the normal value of 0.29 osmol/l. (This is no surprise because the injected fluid is hypertonic with respect to perilymph.) Injection of 0.375, 0.500 and especially 3.00 M KCl will create a still larger difference in osmolarity between perilymph and endolymph, leading to an osmotic pressure difference between the endolymphatic and the perilymphatic compartments. Besides K+ intoxication this could give an additional explanation for the measured deterioration of the VsEP, through hydromechanical effects. However, no hydromechanical effect on the VsEP was observed in an earlier experiment in which artificial endolymph was injected in scala media of the guinea pig to create an acute endolymphatic hydrops [18].
In the first series of experiments (RMR) K+-rich endolymph mixes with perilymph. Because the injected artificial endolymph, natural endolymph and perilymph have almost the same osmolarity [32] this will not cause an osmotic pressure difference between the endolymphatic and the perilymphatic compartments.
Furthermore, if such a pressure difference would occur, pressures would equalize very fast because fluid will flow through the ruptured membrane(s).
So K+ intoxication remains as the only explanation for the VsEP deterioration shown in Fig. 4.
In summary, our study shows that an increase of the K+ concentration in the perilymphatic space of the vestibular system causes a deterioration of the functioning of (part of) the vestibular system, followed by a dose-dependent slow recovery. K+ intoxication is the most likely explanation for this process, which could be an explanation for acute disturbance of vestibular function in Menière patients.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Hallpike CS, Cairns H. Observations on the pathology of Menière’s syndrome. J Laryngol Otol. 1938;53:625–655. doi: 10.1017/S0022215100003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamakawa Y. Über die pathologische Veränderung bei einem Meniere-Kranken. J Otorhinolaryngol Soc Jpn. 1938;44:2310–2312. [Google Scholar]
- 3.Merchant SN, Adams JC, Nadol jr JB. Pathophysiology of Menière’s syndrome: are symptoms caused by endolymphatic hydrops. Otol Neurotol. 2005;26:74–81. doi: 10.1097/00129492-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Kimura RS. An update of our experiences on endolymphatic hydrops. In: Dauman R, Ferrary E, Sauvage JP, Sterkers O, Tran Ba Huy R, editors. Meniere’s disease update. The Hague: Kugler; 2000. pp. 31–36. [Google Scholar]
- 5.Semaan MT, Alagramam KN, Megerian CA. The basic science of Meniere’s disease and endolymphatic hydrops. Curr Opin Otolaryngol Head Neck Surg. 2005;13:301–307. doi: 10.1097/01.moo.0000186335.44206.1c. [DOI] [PubMed] [Google Scholar]
- 6.Horner KC, Erre JP, Cazals Y. Asymmetry of evoked rotatory nystagmus in the guinea pig after experimental induction of endolymphatic hydrops. Acta Otolaryngol Suppl. 1989;468:65–69. doi: 10.3109/00016488909139023. [DOI] [PubMed] [Google Scholar]
- 7.Martin GK, Shaw DWW, Dobie RA, Lonsbury-Martin BL. Endolymphatic hydrops in the rabbit; auditory brainstem responses and cochlear morphology. Hearing Res. 1983;12:65–87. doi: 10.1016/0378-5955(83)90119-3. [DOI] [PubMed] [Google Scholar]
- 8.Kimura RS, Schuknecht HF. Membranous hydrops in the inner ear of the guinea pig after obliteration of the endolymphatic sac. Pract Otorhinolaryngol. 1965;27:343–545. [Google Scholar]
- 9.Peters TA, Tonnaer ELGM, Kuijpers W, Curfs JHAJ. Changes in ultrastructural characteristics of endolymphatic sac ribosome-rich cells of the rat during development. Hear Res. 2003;176:94–104. doi: 10.1016/S0378-5955(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 10.Jin XM, Guo YO, Huangfu MS. Electrocochleography in an experimental animal model of acute endolymphatic hydrops. Acta Otolaryngol. 1990;110:334–341. doi: 10.3109/00016489009107452. [DOI] [PubMed] [Google Scholar]
- 11.Kakigi A, Takeda T. Effect of artificial endolymph injection into the cochlear duct on the endocochlear potential. Hear Res. 1998;116:113–118. doi: 10.1016/S0378-5955(97)00209-8. [DOI] [PubMed] [Google Scholar]
- 12.Wit HP, Thalen EO, Albers FW. Dynamics of inner ear pressure release, measured with a double-barreled micropipette in the guinea pig. Hear Res. 1999;132:131–139. doi: 10.1016/S0378-5955(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 13.Wit HP, Warmerdam TJ, Albers FW. Measurement of the mechanical compliance of the endolymphatic compartments in the guinea pig. Hear Res. 2000;145:82–90. doi: 10.1016/S0378-5955(00)00078-2. [DOI] [PubMed] [Google Scholar]
- 14.Valk WL, Wit HP, Albers FW. Evaluation of cochlear function in an acute endolymphatic hydrops model in the guinea pig by measuring low-level DPOAEs. Hear Res. 2004;192:47–56. doi: 10.1016/j.heares.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Valk WL, Wit HP, Albers FW. Changes in CMPD and DPOAE during acute increased inner ear pressure in the guinea pig. Eur Arch Otorhinolaryngol. 2008;265:287–292. doi: 10.1007/s00405-007-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Böhmer A, Hoffman LF, Honrubia V. Characterization of vestibular potentials evoked by linear acceleration pulses in the chinchilla. Am J Otol. 1995;16:498–504. [PubMed] [Google Scholar]
- 17.Plotnik M, Sichel JY, Elidan J, Honrubia V, Sohmer H. Origins of the short latency vestibular evoked potentials (VsEPs) to linear acceleration impulses. Am J Otol. 1999;20:238–243. [PubMed] [Google Scholar]
- 18.Kingma CM, Wit HP. The effect of acute endolymphatic hydrops on the vestibular evoked potential in the guinea pig. J Vest Res. 2009;19:27–32. doi: 10.3233/VES-2009-0341. [DOI] [PubMed] [Google Scholar]
- 19.Boudewyns A, Claes J. Acute cochleovestibular toxicity due to topical application of potassium iodide. Eur Arch Otorhinolaryngol. 2001;258:109–111. doi: 10.1007/s004050100319. [DOI] [PubMed] [Google Scholar]
- 20.Dohlman G, Johnson WH. Experiments on the mechanism of the Menière attack. Proc Can Otolaryngol. 1965;19:73. [Google Scholar]
- 21.Gates GA (2005) Unresolved clinical issues with Meniere’s disease, Meniere’s disease and inner ear homeostasis disorders. In: Lim DJ (ed) Proceedings of the fifth international symposium. House Ear Institute, Los Angeles, pp 4–8
- 22.Flock A, Flock B. Micro-lesions in Reissner’s membrane evoked by acute hydrops. Audiol Neurotol. 2003;8:59–69. doi: 10.1159/000069002. [DOI] [PubMed] [Google Scholar]
- 23.Oei MLYM, Segenhout JM, Wit HP, Albers FWJ. The vestibular evoked response to linear, alternating, acceleration pulses without acoustic masking as a parameter of vestibular function. Acta Otolaryngol. 2001;121:62–67. doi: 10.1080/000164801300006290. [DOI] [PubMed] [Google Scholar]
- 24.Oei MLYM, Segenhout HM, Dijk F, Stokroos I, Van der Want JJL, Albers FJW. Functional and anatomic alterations in the gentamicin-damaged vestibular system of the guinea pig. Otol Neurotol. 2004;25:57–64. doi: 10.1097/00129492-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence ML, McCabe BF. Inner-ear mechanics and deafness, special consideration of Menière’s syndrome. J Am Med Assoc. 1959;171:1927. doi: 10.1001/jama.1959.03010320017005. [DOI] [PubMed] [Google Scholar]
- 26.Marcon S, Patuzzi R. Changes in cochlear responses in guinea pig with changes in perilymphatic K+. Part I: summating potentials, compound action potentials and DPOAEs. Hear Res. 2008;237:76–89. doi: 10.1016/j.heares.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Tasaki I, Fernandez C. Modification of cochlear microphonics and action potentials by KCL solution and by direct currents. J Neurophysiol. 1952;15:497–512. doi: 10.1152/jn.1952.15.6.497. [DOI] [PubMed] [Google Scholar]
- 28.Zenner HP, Reuter G, Zimmermann U, Gitter AH, Fermin C, LePage EL. Transitory endolymph leakage induced hearing loss and tinnitus, depolarization, biphasic shortening and loss of electromotility of outer hair cells. Eur Arch Otorhinolaryngol. 1994;251:143–153. doi: 10.1007/BF00181826. [DOI] [PubMed] [Google Scholar]
- 29.Silverstein H. The effects of perfusing the perilymphatic space with artificial endolymph. Ann Otol Rhinol Laryngol. 1970;79:754–765. doi: 10.1177/000348947007900408. [DOI] [PubMed] [Google Scholar]
- 30.Wit HP, Feijen RA, Albers FWJ. Cochlear aqueduct flow resistance is not constant during evoked inner ear pressure change in the guinea pig. Hear Res. 2003;175:190–199. doi: 10.1016/S0378-5955(02)00738-4. [DOI] [PubMed] [Google Scholar]
- 31.Shinomori Y, Spack DS, Jones DD, Kimura RS. Volumetric and dimensional analysis of the guinea pig inner ear. Ann Otol Rhinol Laryngol. 2001;110:91–98. doi: 10.1177/000348940111000117. [DOI] [PubMed] [Google Scholar]
- 32.Konishi T, Hamrick PE, Mori H. Water permeability of the endolymph–perilymph barrier in the guinea pig cochlea. Hear Res. 1984;15:51–58. doi: 10.1016/0378-5955(84)90224-7. [DOI] [PubMed] [Google Scholar]
- 33.Nakano Y, Kim SH, Kim H-M, Sanneman JD, Zhang Y, Smith RJH, Marcus DC, Wangemann P, Nessler RA, Bánfi B (2009) A claudin-9-based ion permeability barrier is essential for hearing. PLoS Genet 5(8):e1000610. doi:10.1371/journal.pgen.1000610 [DOI] [PMC free article] [PubMed]
- 34.Meissner R. Behavior of the nystagmus in Menière’s attack. Arch Otorhinolaryngol. 1981;233:173–177. doi: 10.1007/BF00453641. [DOI] [PubMed] [Google Scholar]
- 35.Kiernan MC, Walters RJL, Andersen KV, Taube D, Murray NMF, Bostock H. Nerve excitability changes in chronic renal failure indicate membrane depolarization due to hyperkalaemia. Brain. 2002;125:1366–1378. doi: 10.1093/brain/awf123. [DOI] [PubMed] [Google Scholar]