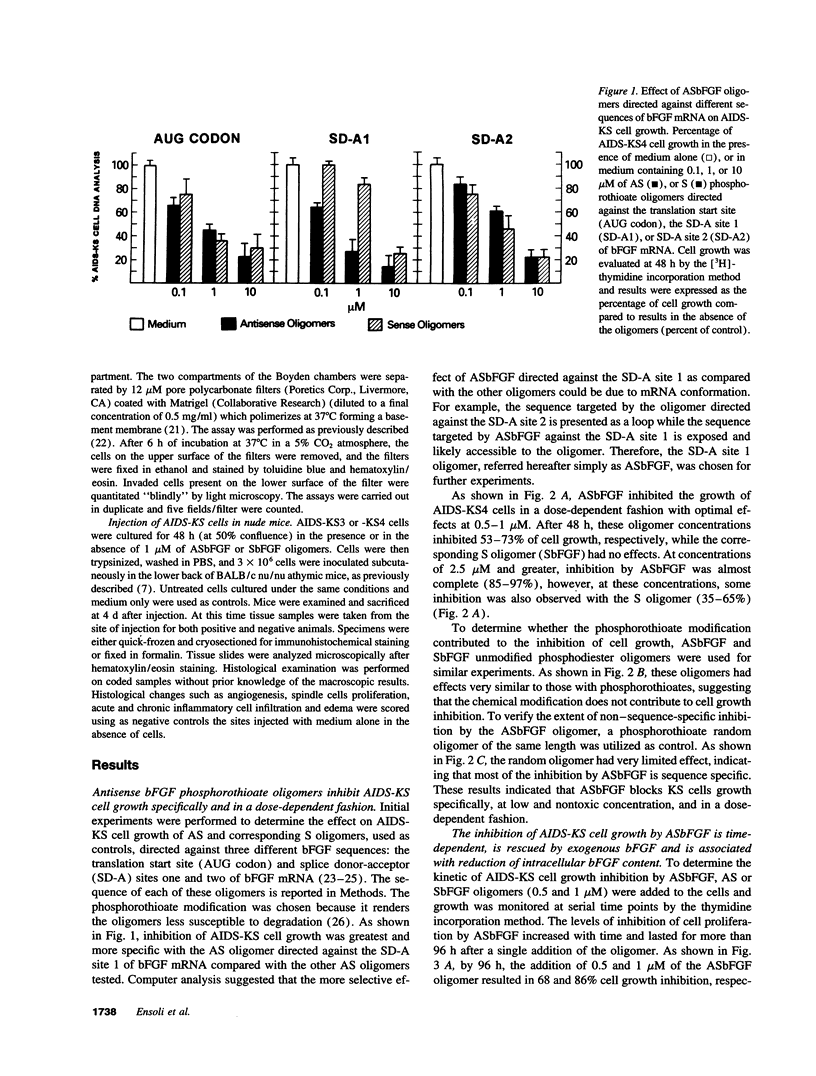
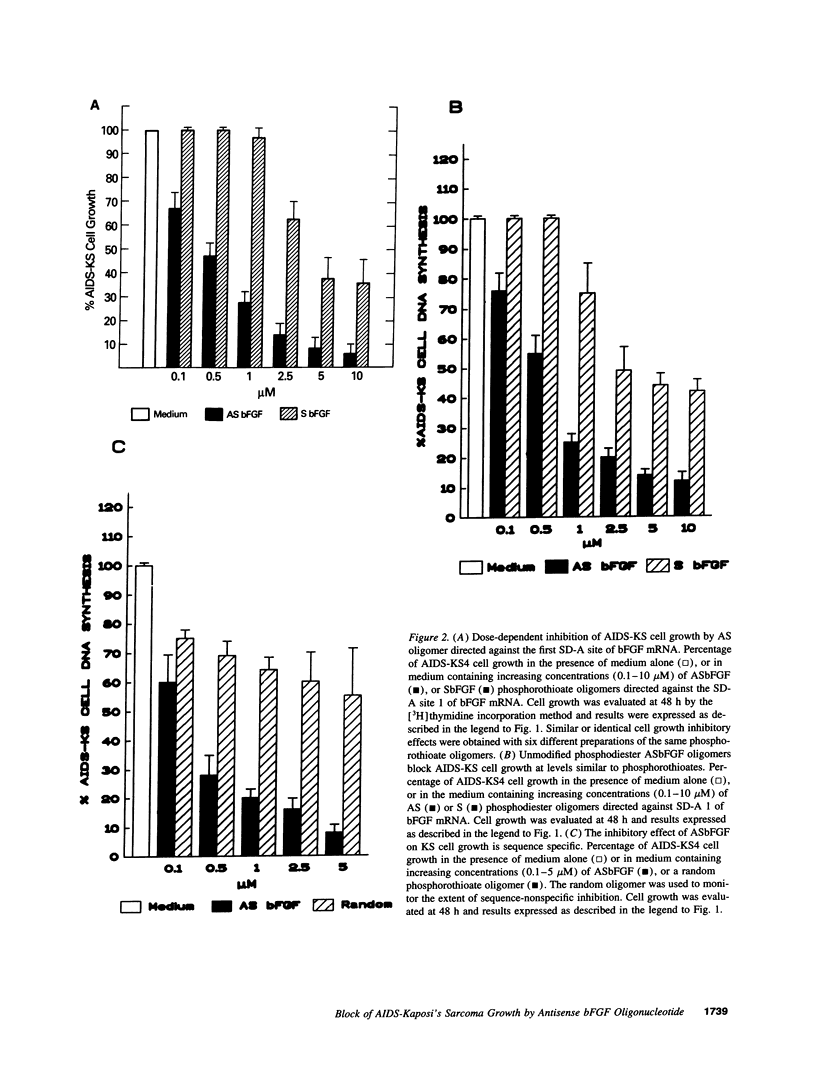
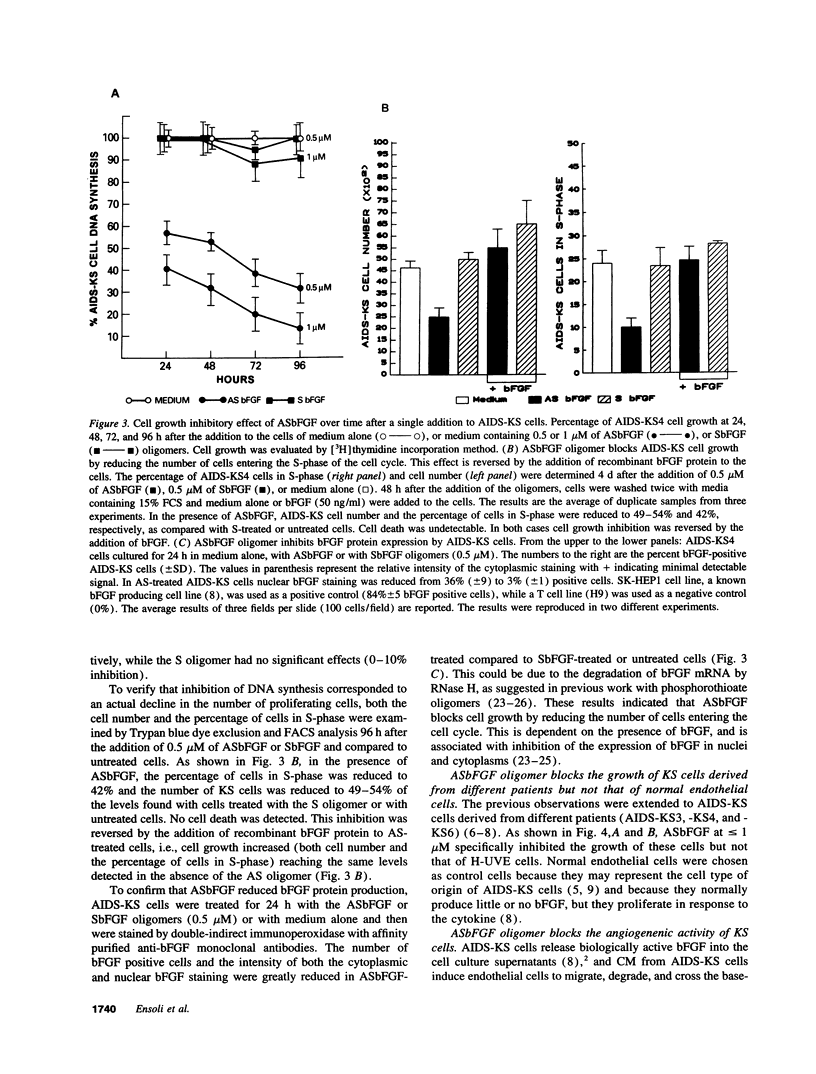
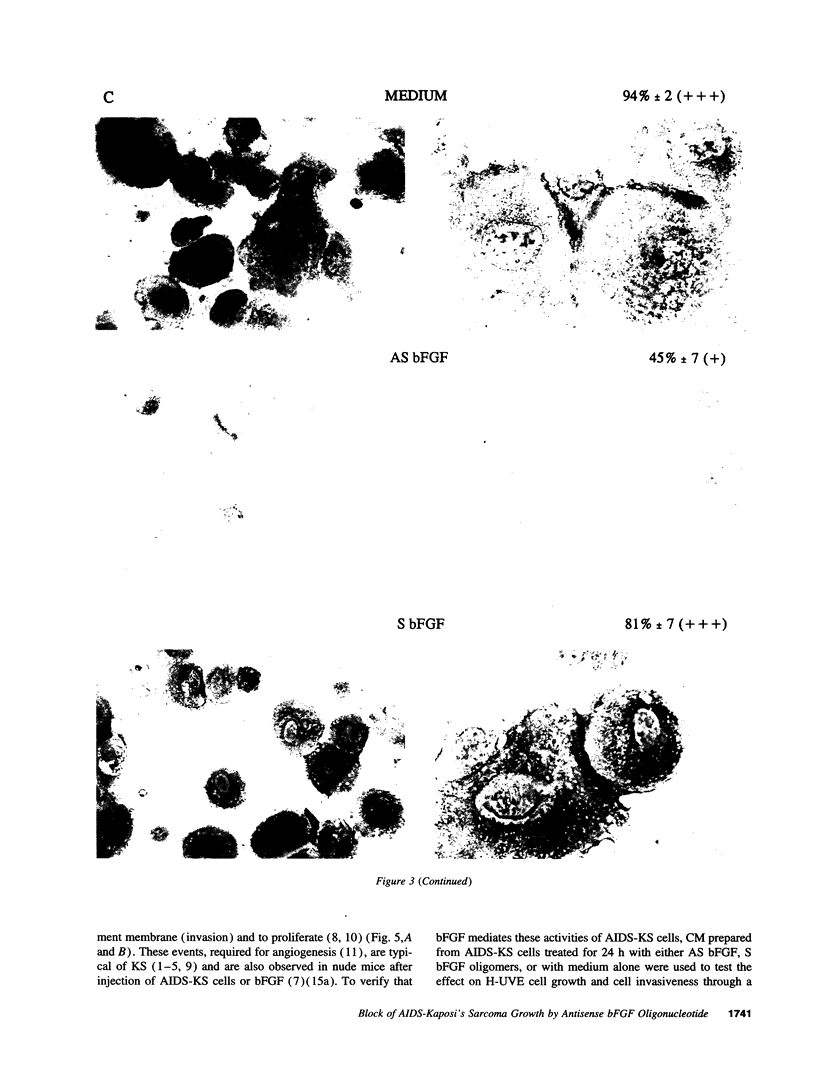
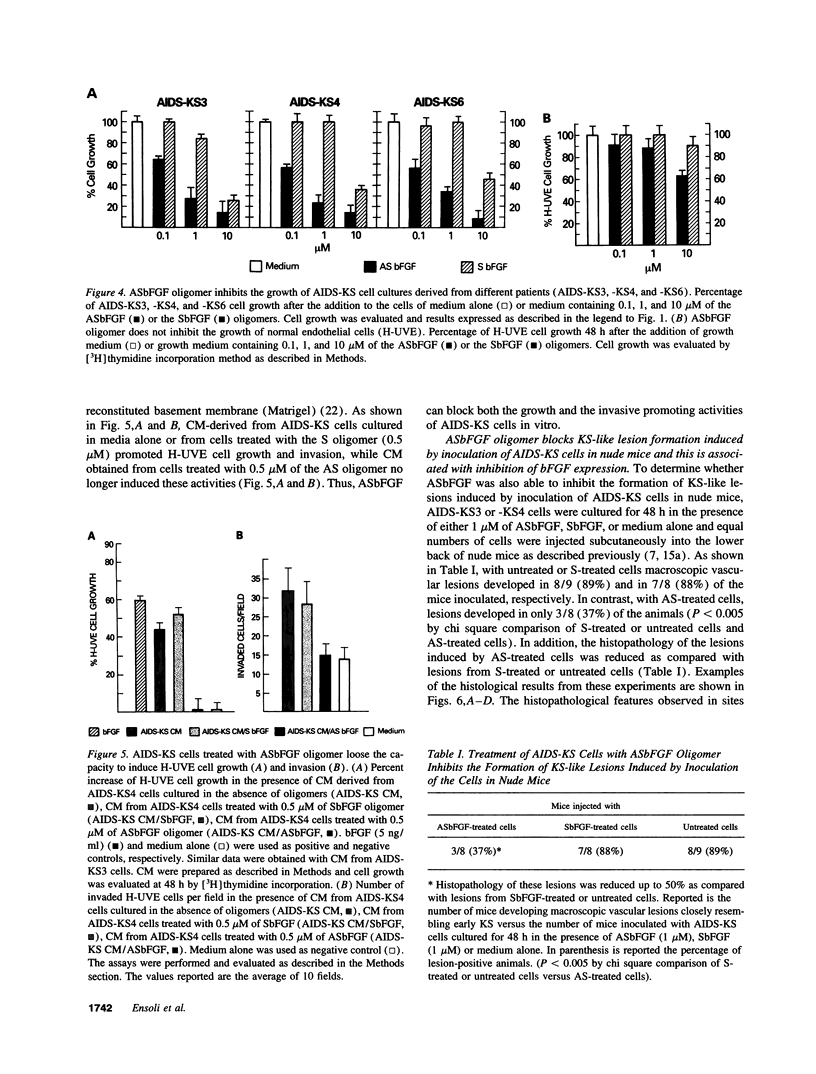
Abstract
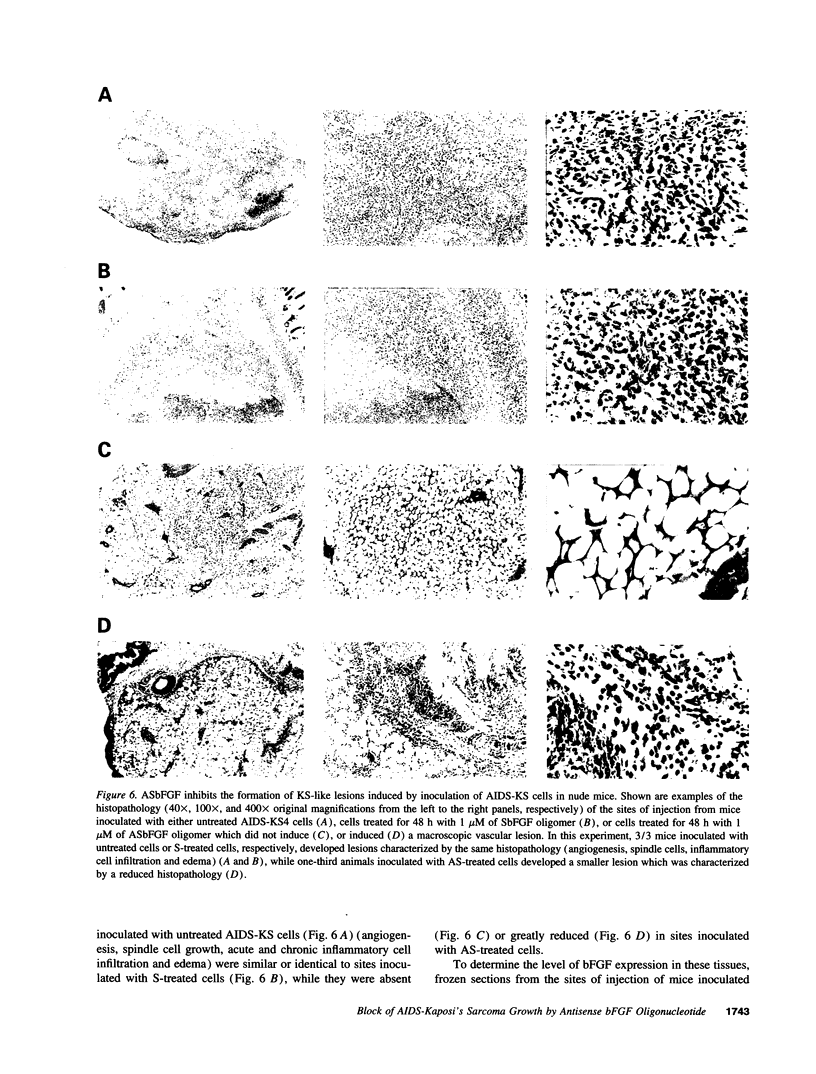
Kaposi's sarcoma (KS) is the most frequent tumor of HIV-1-infected individuals (AIDS-KS). Typical features of KS are proliferating spindle-shaped cells, considered to be the tumor cells of KS, and endothelial cells forming blood vessels. Basic fibroblast growth factor (bFGF), a potent angiogenic factor, is highly expressed by KS spindle cells in vivo and after injection in nude mice it induces vascular lesions closely resembling early KS in humans. Similar lesions are induced by inoculating nude mice with cultured spindle cells from AIDS-KS lesions (AIDS-KS cells) which produce and release bFGF. Here we show that phosphorothioate antisense (AS) oligonucleotides directed against bFGF mRNA (ASbFGF) inhibit both the growth of AIDS-KS cells derived from different patients and the angiogenic activity associated with these cells, including the induction of KS-like lesions in nude mice. These effects are due to the block of the production of bFGF which is required by AIDS-KS cells to enter the cell cycle and which, after release, mediates angiogenesis. The effects of ASbFGF are specific, dose dependent, achieved at low (0.1-1 microM), nontoxic, oligomer concentrations, and are reversed by the addition of bFGF to the cells, suggesting that ASbFGF oligomers are promising drug candidates for KS therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Barillari G., Buonaguro L., Fiorelli V., Hoffman J., Michaels F., Gallo R. C., Ensoli B. Effects of cytokines from activated immune cells on vascular cell growth and HIV-1 gene expression. Implications for AIDS-Kaposi's sarcoma pathogenesis. J Immunol. 1992 Dec 1;149(11):3727–3734. [PubMed] [Google Scholar]
- Becker D., Meier C. B., Herlyn M. Proliferation of human malignant melanomas is inhibited by antisense oligodeoxynucleotides targeted against basic fibroblast growth factor. EMBO J. 1989 Dec 1;8(12):3685–3691. doi: 10.1002/j.1460-2075.1989.tb08543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Buonaguro L., Barillari G., Fiorelli V., Gendelman R., Morgan R. A., Wingfield P., Gallo R. C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993 Jan;67(1):277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Nakamura S., Salahuddin S. Z., Biberfeld P., Larsson L., Beaver B., Wong-Staal F., Gallo R. C. AIDS-Kaposi's sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989 Jan 13;243(4888):223–226. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- Fan J., Bass H. Z., Fahey J. L. Elevated IFN-gamma and decreased IL-2 gene expression are associated with HIV infection. J Immunol. 1993 Nov 1;151(9):5031–5040. [PubMed] [Google Scholar]
- Folkman J., Ingber D. Inhibition of angiogenesis. Semin Cancer Biol. 1992 Apr;3(2):89–96. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Friedman-Kien A. E. Disseminated Kaposi's sarcoma syndrome in young homosexual men. J Am Acad Dermatol. 1981 Oct;5(4):468–471. doi: 10.1016/s0190-9622(81)80010-2. [DOI] [PubMed] [Google Scholar]
- Gay C. G., Winkles J. A. Interleukin 1 regulates heparin-binding growth factor 2 gene expression in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):296–300. doi: 10.1073/pnas.88.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkos H. W., Drotman D. P. Prevalence of Kaposi's sarcoma among patients with AIDS. N Engl J Med. 1985 Jun 6;312(23):1518–1518. [PubMed] [Google Scholar]
- Ingber D., Fujita T., Kishimoto S., Sudo K., Kanamaru T., Brem H., Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990 Dec 6;348(6301):555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- Krown S. E. The role of interferon in the therapy of epidemic Kaposi's sarcoma. Semin Oncol. 1987 Jun;14(2 Suppl 3):27–33. [PubMed] [Google Scholar]
- Kubota Y., Kleinman H. K., Martin G. R., Lawley T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988 Oct;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Huang Y. Q., Moscatelli D., Nicolaides A., Zhang W. C., Friedman-Kien A. E. Expression of fibroblast growth factors and their receptors in acquired immunodeficiency syndrome-associated Kaposi sarcoma tissue and derived cells. Cancer. 1993 Oct 1;72(7):2253–2259. doi: 10.1002/1097-0142(19931001)72:7<2253::aid-cncr2820720732>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Mitsuyasu R. T. Interferon alpha in the treatment of AIDS-related Kaposi's sarcoma. Br J Haematol. 1991 Oct;79 (Suppl 1):69–73. doi: 10.1111/j.1365-2141.1991.tb08124.x. [DOI] [PubMed] [Google Scholar]
- Morrison R. S. Suppression of basic fibroblast growth factor expression by antisense oligodeoxynucleotides inhibits the growth of transformed human astrocytes. J Biol Chem. 1991 Jan 15;266(2):728–734. [PubMed] [Google Scholar]
- Murphy P. R., Sato Y., Knee R. S. Phosphorothioate antisense oligonucleotides against basic fibroblast growth factor inhibit anchorage-dependent and anchorage-independent growth of a malignant glioblastoma cell line. Mol Endocrinol. 1992 Jun;6(6):877–884. doi: 10.1210/mend.6.6.1323055. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Sakurada S., Salahuddin S. Z., Osada Y., Tanaka N. G., Sakamoto N., Sekiguchi M., Gallo R. C. Inhibition of development of Kaposi's sarcoma-related lesions by a bacterial cell wall complex. Science. 1992 Mar 13;255(5050):1437–1440. doi: 10.1126/science.1371891. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Salahuddin S. Z., Biberfeld P., Ensoli B., Markham P. D., Wong-Staal F., Gallo R. C. Kaposi's sarcoma cells: long-term culture with growth factor from retrovirus-infected CD4+ T cells. Science. 1988 Oct 21;242(4877):426–430. doi: 10.1126/science.3262925. [DOI] [PubMed] [Google Scholar]
- Neckers L., Whitesell L., Rosolen A., Geselowitz D. A. Antisense inhibition of oncogene expression. Crit Rev Oncog. 1992;3(1-2):175–231. [PubMed] [Google Scholar]
- Okamura K., Sato Y., Matsuda T., Hamanaka R., Ono M., Kohno K., Kuwano M. Endogenous basic fibroblast growth factor-dependent induction of collagenase and interleukin-6 in tumor necrosis factor-treated human microvascular endothelial cells. J Biol Chem. 1991 Oct 15;266(29):19162–19165. [PubMed] [Google Scholar]
- Ratajczak M. Z., Luger S. M., DeRiel K., Abrahm J., Calabretta B., Gewirtz A. M. Role of the KIT protooncogene in normal and malignant human hematopoiesis. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1710–1714. doi: 10.1073/pnas.89.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regezi J. A., MacPhail L. A., Daniels T. E., DeSouza Y. G., Greenspan J. S., Greenspan D. Human immunodeficiency virus-associated oral Kaposi's sarcoma. A heterogeneous cell population dominated by spindle-shaped endothelial cells. Am J Pathol. 1993 Jul;143(1):240–249. [PMC free article] [PubMed] [Google Scholar]
- Ruszczak Z., Mayer-Da Silva A., Orfanos C. E. Kaposi's sarcoma in AIDS. Multicentric angioneoplasia in early skin lesions. Am J Dermatopathol. 1987 Oct;9(5):388–398. [PubMed] [Google Scholar]
- Safai B., Johnson K. G., Myskowski P. L., Koziner B., Yang S. Y., Cunningham-Rundles S., Godbold J. H., Dupont B. The natural history of Kaposi's sarcoma in the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):744–750. doi: 10.7326/0003-4819-103-5-744. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Nakamura S., Biberfeld P., Kaplan M. H., Markham P. D., Larsson L., Gallo R. C. Angiogenic properties of Kaposi's sarcoma-derived cells after long-term culture in vitro. Science. 1988 Oct 21;242(4877):430–433. doi: 10.1126/science.2459779. [DOI] [PubMed] [Google Scholar]
- Schaart F. M., Bratzke B., Ruszczak Z., Stadler R., Ehlers G., Orfanos C. E. Long-term therapy of HIV-associated Kaposi's sarcoma with recombinant interferon alpha-2a. Br J Dermatol. 1991 Jan;124(1):62–68. doi: 10.1111/j.1365-2133.1991.tb03283.x. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Thompson E. W., Nakamura S., Shima T. B., Melchiori A., Martin G. R., Salahuddin S. Z., Gallo R. C., Albini A. Supernatants of acquired immunodeficiency syndrome-related Kaposi's sarcoma cells induce endothelial cell chemotaxis and invasiveness. Cancer Res. 1991 May 15;51(10):2670–2676. [PubMed] [Google Scholar]
- Wahlestedt C., Golanov E., Yamamoto S., Yee F., Ericson H., Yoo H., Inturrisi C. E., Reis D. J. Antisense oligodeoxynucleotides to NMDA-R1 receptor channel protect cortical neurons from excitotoxicity and reduce focal ischaemic infarctions. Nature. 1993 May 20;363(6426):260–263. doi: 10.1038/363260a0. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Geselowitz D., Chavany C., Fahmy B., Walbridge S., Alger J. R., Neckers L. M. Stability, clearance, and disposition of intraventricularly administered oligodeoxynucleotides: implications for therapeutic application within the central nervous system. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4665–4669. doi: 10.1073/pnas.90.10.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri L., Hassoun J., Planche J., Guigou V., Grob J. J., Parc P., Birnbaum D., deLapeyriere O. Fibroblast growth factor gene expression in AIDS-Kaposi's sarcoma detected by in situ hybridization. Am J Pathol. 1991 Jan;138(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Zon G., Geiser T. G. Phosphorothioate oligonucleotides: chemistry, purification, analysis, scale-up and future directions. Anticancer Drug Des. 1991 Dec;6(6):539–568. [PubMed] [Google Scholar]