Abstract
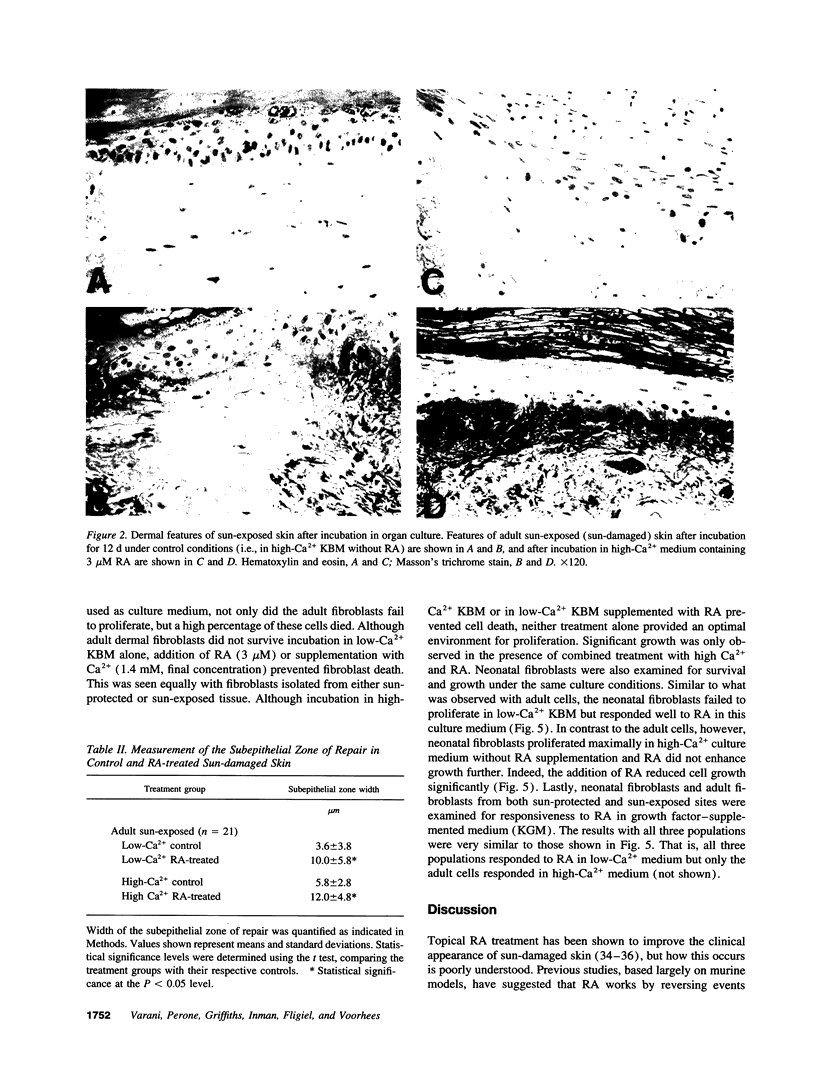
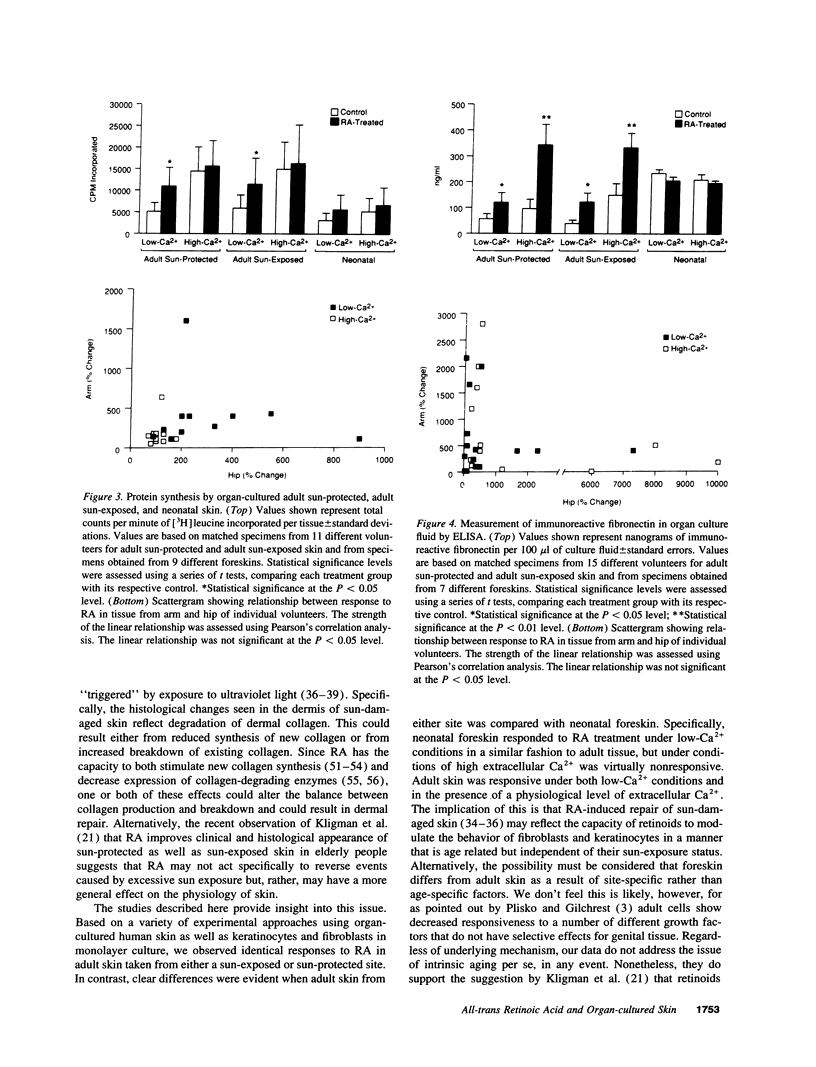
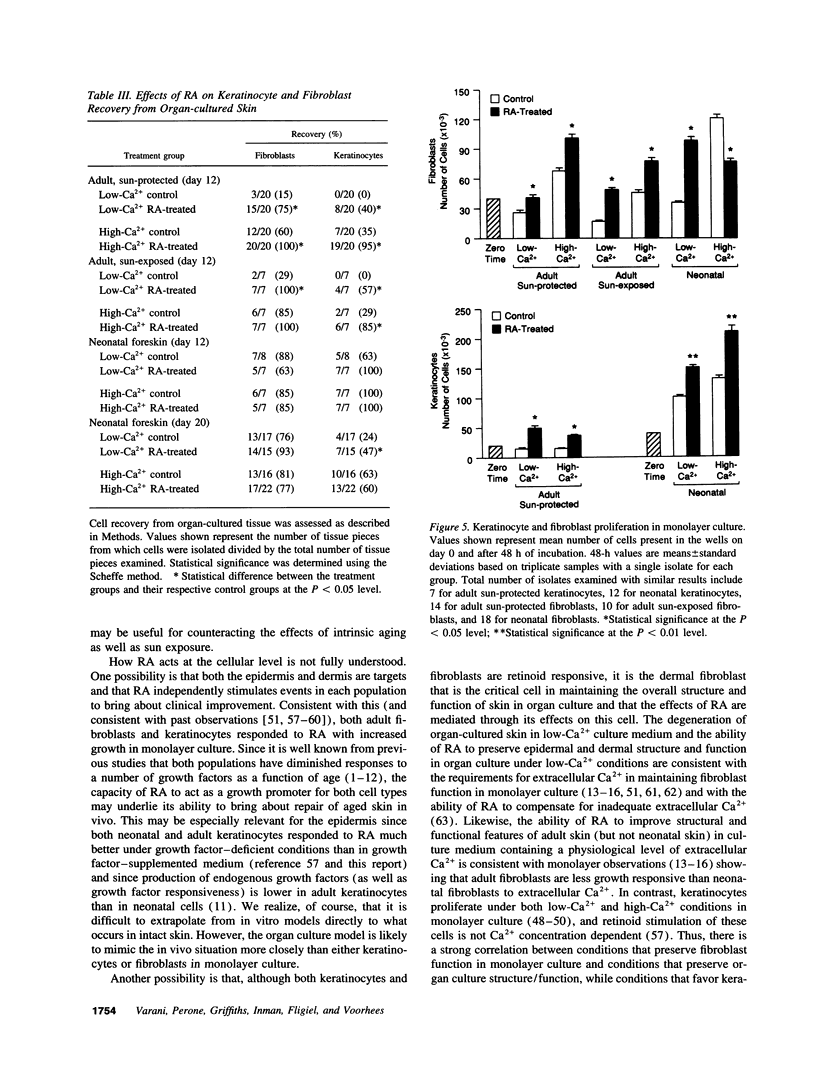
Adult human skin from a sun-protected site (hip) and from a sun-exposed site (forearm) was maintained in organ culture for 12 d in the presence of a serum-free, growth factor-free basal medium. Cultures were incubated under conditions optimized for keratinocyte growth (i.e., in 0.15 mM extracellular Ca2+) or for fibroblast growth (i.e., in 1.4 mM extracellular Ca2+). Treatment with all-trans retinoic acid (RA) induced histological changes in the organ-cultured skin under both conditions which were similar to the changes seen in intact skin after topical application. These included expansion of the viable portion of the epidermis and activation of cells in the dermis. In sun-damaged skin samples, which were characterized by destruction of normal connective tissue elements and presence of thick, dark-staining elastotic fibers, a zone of healthy connective tissue could be seen immediately below the dermo-epidermal junction. This zone was more prominent in RA-treated organ cultures than in matched controls. Associated with these histological changes was an increase in overall protein and extracellular matrix synthesis. In concomitant studies, it was found that RA treatment enhanced survival and proliferation of adult keratinocytes and adult dermal fibroblasts under both low- and high-Ca2+ conditions. In all of these assays, responses of sun-protected and sun-exposed skin were identical. In contrast, responses of neonatal foreskin to RA were similar to those of adult skin in the presence of low-Ca2+ culture medium, but under conditions of high extracellular Ca2+ RA provided little or no additional stimulus. Together these studies suggest that the ability of RA to enhance repair of sun-damaged skin (documented in previous studies) may reflect its ability to influence the behavior of skin in a manner that is age dependent but independent of sun-exposure status.
Full text
PDF


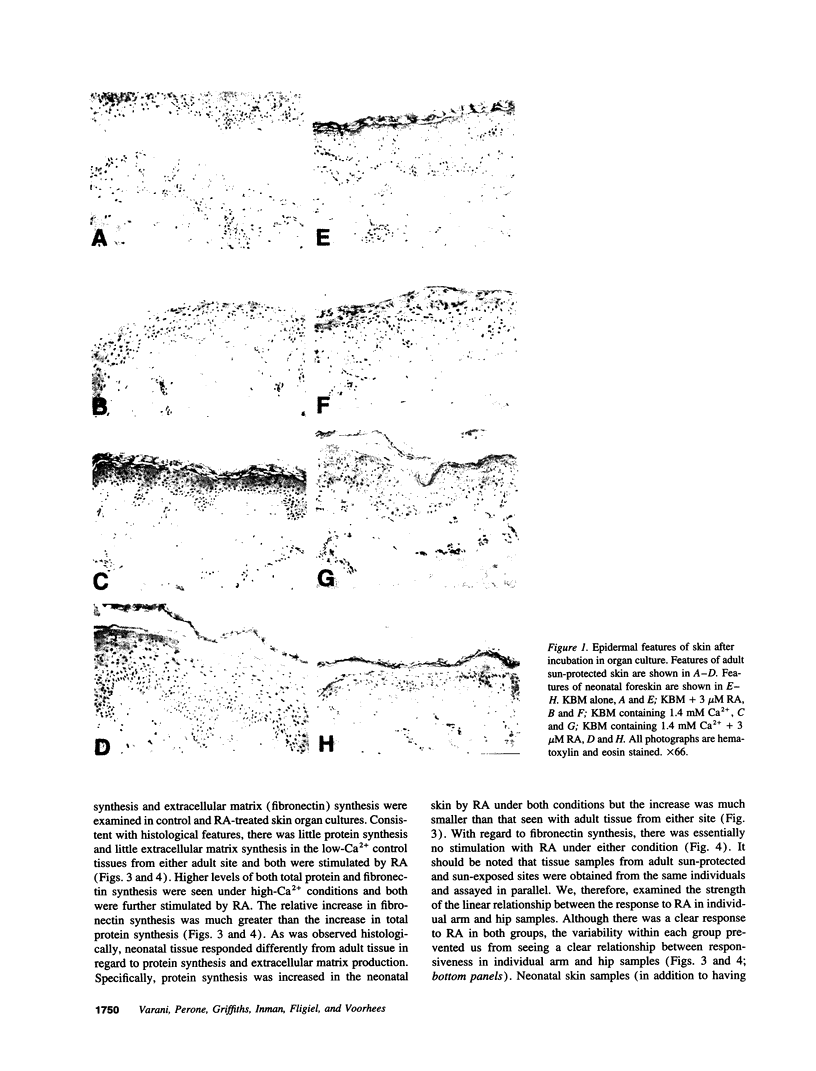
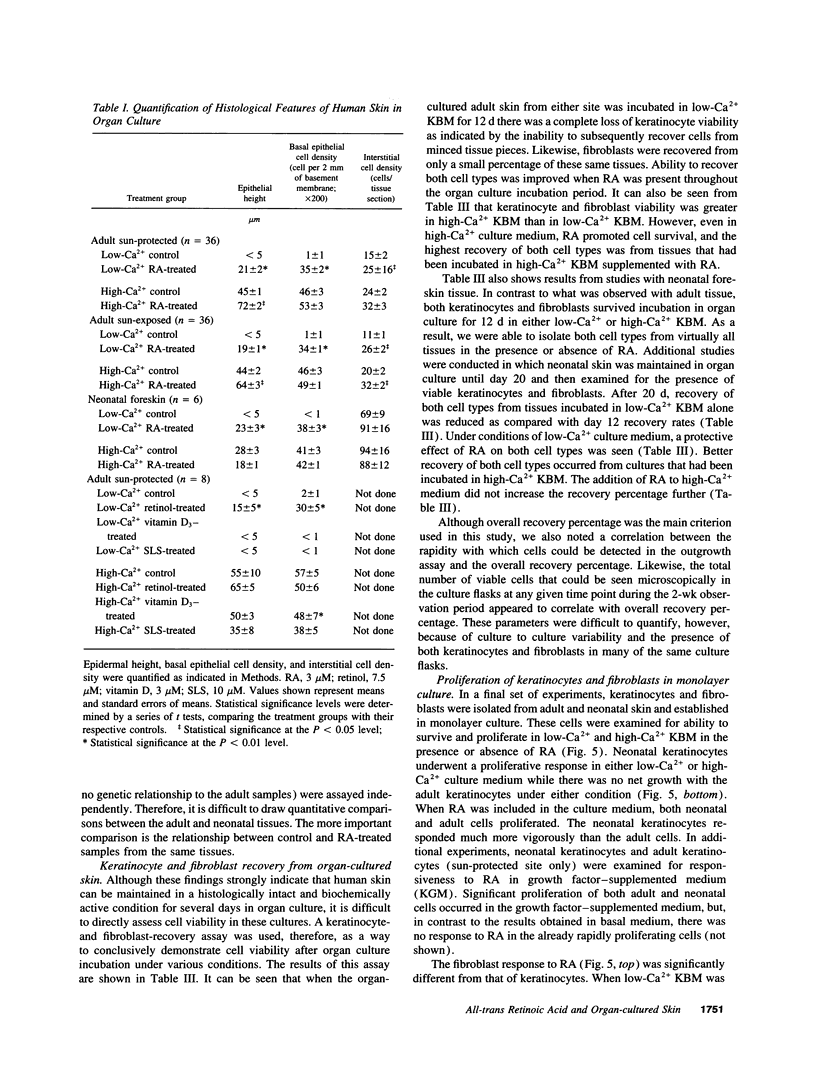


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer E. A., Seltzer J. L., Eisen A. Z. Inhibition of collagen degradative enzymes by retinoic acid in vitro. J Am Acad Dermatol. 1982 Apr;6(4 Pt 2 Suppl):603–607. doi: 10.1016/s0190-9622(82)70049-0. [DOI] [PubMed] [Google Scholar]
- Berger H., Tsambaos D., Mahrle G. Experimental elastosis induced by chronic ultraviolet exposure. Light- and electron-microscopic study. Arch Dermatol Res. 1980;269(1):39–49. doi: 10.1007/BF00404456. [DOI] [PubMed] [Google Scholar]
- Bissett D. L., Hannon D. P., Orr T. V. An animal model of solar-aged skin: histological, physical, and visible changes in UV-irradiated hairless mouse skin. Photochem Photobiol. 1987 Sep;46(3):367–378. doi: 10.1111/j.1751-1097.1987.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Morton H. J. Control of 3T3 cell proliferation by calcium. In Vitro. 1974 Jul-Aug;10:12–17. doi: 10.1007/BF02615333. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Tremblay R. The control of human WI-38 cell proliferation by extracellular calcium and its elimination by SV-40 virus-induced proliferative transformation. J Cell Physiol. 1977 Aug;92(2):241–247. doi: 10.1002/jcp.1040920212. [DOI] [PubMed] [Google Scholar]
- Chen S., Kiss I., Tramposch K. M. The priming effect of ultraviolet B radiation on retinoic acid-stimulated collagen synthesis in the mouse photodamage model. Photodermatol Photoimmunol Photomed. 1992 Jun;9(3):104–108. [PubMed] [Google Scholar]
- Clark S. D., Kobayashi D. K., Welgus H. G. Regulation of the expression of tissue inhibitor of metalloproteinases and collagenase by retinoids and glucocorticoids in human fibroblasts. J Clin Invest. 1987 Nov;80(5):1280–1288. doi: 10.1172/JCI113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. A., Davies R. E., Forbes P. D., D'Aloisio L. C. Comparison of action spectra for acute cutaneous responses to ultraviolet radiation: man and albino hairless mouse. Photochem Photobiol. 1983 Jun;37(6):623–631. doi: 10.1111/j.1751-1097.1983.tb04531.x. [DOI] [PubMed] [Google Scholar]
- Colige A., Nusgens B., Lapiere C. M. Response to epidermal growth factor of skin fibroblasts from donors of varying age is modulated by the extracellular matrix. J Cell Physiol. 1990 Dec;145(3):450–457. doi: 10.1002/jcp.1041450309. [DOI] [PubMed] [Google Scholar]
- Constantine V. S., Hartley M. W. Collagen and elastic fibers in normal dermis and severe actinic (senile) elastosis. A light and electron microscopic study. Ala J Med Sci. 1966 Jul;3(3):329–342. [PubMed] [Google Scholar]
- Covant H. A., Hardy M. H. Excess retinoid acts through the stroma to produce mucous glands from newborn hamster cheek pouch in vitro. J Exp Zool. 1990 Mar;253(3):271–279. doi: 10.1002/jez.1402530306. [DOI] [PubMed] [Google Scholar]
- Duell E. A., Aström A., Griffiths C. E., Chambon P., Voorhees J. J. Human skin levels of retinoic acid and cytochrome P-450-derived 4-hydroxyretinoic acid after topical application of retinoic acid in vivo compared to concentrations required to stimulate retinoic acid receptor-mediated transcription in vitro. J Clin Invest. 1992 Oct;90(4):1269–1274. doi: 10.1172/JCI115990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Bryce G. F., Shapiro S. S. Ultrastructural effects of UVB radiation and subsequent retinoic acid treatment on the skin of hairless mice. J Cutan Pathol. 1991 Feb;18(1):46–55. doi: 10.1111/j.1600-0560.1991.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A. In vitro assessment of keratinocyte aging. J Invest Dermatol. 1983 Jul;81(1 Suppl):184s–189s. doi: 10.1111/1523-1747.ep12541084. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A. Prior chronic sun exposure decreases the lifespan of human skin fibroblasts in vitro. J Gerontol. 1980 Jul;35(4):537–541. doi: 10.1093/geronj/35.4.537. [DOI] [PubMed] [Google Scholar]
- Gilchrest B. A. Relationship between actinic damage and chronologic aging in keratinocyte cultures of human skin. J Invest Dermatol. 1979 May;72(5):219–223. doi: 10.1111/1523-1747.ep12530769. [DOI] [PubMed] [Google Scholar]
- Griffiths C. E., Russman A. N., Majmudar G., Singer R. S., Hamilton T. A., Voorhees J. J. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid) N Engl J Med. 1993 Aug 19;329(8):530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- Grove G. L. Physiologic changes in older skin. Dermatol Clin. 1986 Jul;4(3):425–432. [PubMed] [Google Scholar]
- Hardy M. H., Dhouailly D., Törmä H., Vahlquist A. Either chick embryo dermis or retinoid-treated mouse dermis can initiate glandular morphogenesis from mammalian epidermal tissue. J Exp Zool. 1990 Dec;256(3):279–289. doi: 10.1002/jez.1402560307. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Goldstein S., Posner B. I., Guyda H. Decreased sensitivity of old and progeric human fibroblasts to a preparation of factors with insulinlike activity. J Clin Invest. 1981 Oct;68(4):988–994. doi: 10.1172/JCI110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper R. A., Burgoon T. Differential effects of retinoic acid on the growth of normal fibroblast-like cells in vitro from human, swine and rabbit skin. Cell Biol Int Rep. 1982 Feb;6(2):163–170. doi: 10.1016/0309-1651(82)90093-5. [DOI] [PubMed] [Google Scholar]
- Harper R. A., Savage C. R., Jr Vitamin A potentiates the mitogenic effect of epidermal growth factor in cultures of normal adult human skin fibroblasts. Endocrinology. 1980 Dec;107(6):2113–2114. doi: 10.1210/endo-107-6-2113. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Kaji K., Matsuo M. Responsiveness of human lung diploid fibroblast ageing in vitro to epidermal growth factor: saturation density and lifespan. Mech Ageing Dev. 1983 Jun;22(2):129–133. doi: 10.1016/0047-6374(83)90106-9. [DOI] [PubMed] [Google Scholar]
- Karasek M. A. Dermal factors affecting epidermal cells in vitro. J Invest Dermatol. 1972 Jul;59(1):99–101. doi: 10.1111/1523-1747.ep12625861. [DOI] [PubMed] [Google Scholar]
- Kligman A. M., Dogadkina D., Lavker R. M. Effects of topical tretinoin on non-sun-exposed protected skin of the elderly. J Am Acad Dermatol. 1993 Jul;29(1):25–33. doi: 10.1016/0190-9622(93)70147-l. [DOI] [PubMed] [Google Scholar]
- Kligman A. M., Grove G. L., Hirose R., Leyden J. J. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):836–859. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Akin F. J., Kligman A. M. Prevention of ultraviolet damage to the dermis of hairless mice by sunscreens. J Invest Dermatol. 1982 Feb;78(2):181–189. doi: 10.1111/1523-1747.ep12506359. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Akin F. J., Kligman A. M. Sunscreens promote repair of ultraviolet radiation-induced dermal damage. J Invest Dermatol. 1983 Aug;81(2):98–102. doi: 10.1111/1523-1747.ep12542169. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Duo C. H., Kligman A. M. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12(2):139–150. doi: 10.3109/03008208408992779. [DOI] [PubMed] [Google Scholar]
- Kligman L. H. Effects of all-trans-retinoic acid on the dermis of hairless mice. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):779-85, 884-7. doi: 10.1016/s0190-9622(86)70234-x. [DOI] [PubMed] [Google Scholar]
- Lavker R. M. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol. 1979 Jul;73(1):59–66. doi: 10.1111/1523-1747.ep12532763. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Karasek M. Isolation and growth of adult human epidermal keratinocytes in cell culture. J Invest Dermatol. 1978 Aug;71(2):157–162. doi: 10.1111/1523-1747.ep12546943. [DOI] [PubMed] [Google Scholar]
- Obinata A., Kawada M., Endo H. Induction of epidermal mucous metaplasia by culture of recombinants of undifferentiated epidermis and retinol-treated dermis in a chemically defined medium. Dev Biol. 1987 Sep;123(1):59–62. doi: 10.1016/0012-1606(87)90427-1. [DOI] [PubMed] [Google Scholar]
- Ohno T. Strict relationship between dialyzed serum concentration and cellular life span in vitro. Mech Ageing Dev. 1979 Oct;11(3):179–183. doi: 10.1016/0047-6374(79)90053-8. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Varani J., Smith D. Rat lung fibroblast collagen metabolism in bleomycin-induced pulmonary fibrosis. J Clin Invest. 1985 Jul;76(1):241–247. doi: 10.1172/JCI111953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. D., Kaji K., Cristofalo V. J. Progressive loss of the proliferative response of senescing WI-38 cells to platelet-derived growth factor, epidermal growth factor, insulin, transferrin, and dexamethasone. J Gerontol. 1984 Jan;39(1):11–17. doi: 10.1093/geronj/39.1.11. [DOI] [PubMed] [Google Scholar]
- Pieraggi M. T., Julian M., Bouissou H. Fibroblast changes in cutaneous ageing. Virchows Arch A Pathol Anat Histopathol. 1984;402(3):275–287. doi: 10.1007/BF00695081. [DOI] [PubMed] [Google Scholar]
- Plastow S. R., Lovell C. R., Young A. R. UVB-induced collagen changes in the skin of the hairless albino mouse. J Invest Dermatol. 1987 Feb;88(2):145–148. doi: 10.1111/1523-1747.ep12525293. [DOI] [PubMed] [Google Scholar]
- Plisko A., Gilchrest B. A. Growth factor responsiveness of cultured human fibroblasts declines with age. J Gerontol. 1983 Sep;38(5):513–518. doi: 10.1093/geronj/38.5.513. [DOI] [PubMed] [Google Scholar]
- Poulsen J. T., Staberg B., Wulf H. C., Brodthagen H. Dermal elastosis in hairless mice after UV-B and UV-A applied simultaneously, separately or sequentially. Br J Dermatol. 1984 May;110(5):531–538. doi: 10.1111/j.1365-2133.1984.tb04675.x. [DOI] [PubMed] [Google Scholar]
- Praeger F. C., Cristofalo V. J. Modulation of WI-38 cell proliferation by elevated levels of CaCl2. J Cell Physiol. 1986 Oct;129(1):27–35. doi: 10.1002/jcp.1041290105. [DOI] [PubMed] [Google Scholar]
- Praeger F. C., Gilchrest B. A. Influence of increased extracellular calcium concentration and donor age on density-dependent growth inhibition of human fibroblasts. Proc Soc Exp Biol Med. 1986 Jul;182(3):315–321. doi: 10.3181/00379727-182-42345. [DOI] [PubMed] [Google Scholar]
- SMITH J. G., Jr, DAVIDSON E. A., SAMS W. M., Jr, CLARK R. D. Alterations in human dermal connective tissue with age and chronic sun damage. J Invest Dermatol. 1962 Oct;39:347–350. doi: 10.1038/jid.1962.122. [DOI] [PubMed] [Google Scholar]
- Saiag P., Coulomb B., Lebreton C., Bell E., Dubertret L. Psoriatic fibroblasts induce hyperproliferation of normal keratinocytes in a skin equivalent model in vitro. Science. 1985 Nov 8;230(4726):669–672. doi: 10.1126/science.2413549. [DOI] [PubMed] [Google Scholar]
- Sanquer S., Coulomb B., Lebreton C., Dubertret L. Human dermal fibroblasts modulate the effects of retinoids on epidermal growth. J Invest Dermatol. 1990 Dec;95(6):700–704. doi: 10.1111/1523-1747.ep12514500. [DOI] [PubMed] [Google Scholar]
- Sauder D. N., Stanulis-Praeger B. M., Gilchrest B. A. Autocrine growth stimulation of human keratinocytes by epidermal cell-derived thymocyte-activating factor: implications for skin aging. Arch Dermatol Res. 1988;280(2):71–76. doi: 10.1007/BF00417707. [DOI] [PubMed] [Google Scholar]
- Schuger L., Varani J., Mitra R., Jr, Gilbride K. Retinoic acid stimulates mouse lung development by a mechanism involving epithelial-mesenchymal interaction and regulation of epidermal growth factor receptors. Dev Biol. 1993 Oct;159(2):462–473. doi: 10.1006/dbio.1993.1256. [DOI] [PubMed] [Google Scholar]
- Schwartz E. Connective tissue alterations in the skin of ultraviolet irradiated hairless mice. J Invest Dermatol. 1988 Aug;91(2):158–161. doi: 10.1111/1523-1747.ep12464405. [DOI] [PubMed] [Google Scholar]
- Schwartz E., Cruickshank F. A., Mezick J. A., Kligman L. H. Topical all-trans retinoic acid stimulates collagen synthesis in vivo. J Invest Dermatol. 1991 Jun;96(6):975–978. doi: 10.1111/1523-1747.ep12476385. [DOI] [PubMed] [Google Scholar]
- Stanulis-Praeger B. M., Gilchrest B. A. Effect of donor age and prior sun exposure on growth inhibition of cultured human dermal fibroblasts by all trans-retinoic acid. J Cell Physiol. 1989 Apr;139(1):116–124. doi: 10.1002/jcp.1041390117. [DOI] [PubMed] [Google Scholar]
- Stanulis-Praeger B. M., Gilchrest B. A. Growth factor responsiveness declines during adulthood for human skin-derived cells. Mech Ageing Dev. 1986 Jul;35(2):185–198. doi: 10.1016/0047-6374(86)90009-6. [DOI] [PubMed] [Google Scholar]
- Stanulis-Praeger B. M., Yaar M., Redziniak G., Meybeck A., Gilchrest B. A. An extract of bovine thymus stimulates human keratinocyte growth in vitro. J Invest Dermatol. 1988 May;90(5):749–754. doi: 10.1111/1523-1747.ep12560950. [DOI] [PubMed] [Google Scholar]
- Tamm I., Kikuchi T., Wang E., Pfeffer L. M. Growth rate of control and beta-interferon-treated human fibroblast populations over the course of their in vitro life span. Cancer Res. 1984 Jun;44(6):2291–2296. [PubMed] [Google Scholar]
- Tsuji Y., Ide T., Ishibashi S., Nishikawa K. Loss of responsiveness in senescent human TIG-1 cells to the DNA synthesis-inducing effect of various growth factors. Mech Ageing Dev. 1984 Oct 15;27(2):219–232. doi: 10.1016/0047-6374(84)90047-2. [DOI] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Perone P., Inman D. R., Voorhees J. J. Effects of sodium lauryl sulfate on human skin in organ culture: comparison with all-trans-retinoic acid and epidermal growth factor. Dermatology. 1993;187(1):19–25. doi: 10.1159/000247191. [DOI] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Schuger L., Perone P., Inman D., Griffiths C. E., Voorhees J. J. Effects of all-trans retinoic acid and Ca++ on human skin in organ culture. Am J Pathol. 1993 Jan;142(1):189–198. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Larson B. K., Perone P., Inman D. R., Fligiel S. E., Voorhees J. J. All-trans retinoic acid and extracellular Ca2+ differentially influence extracellular matrix production by human skin in organ culture. Am J Pathol. 1993 Jun;142(6):1813–1822. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Lovett E. J., 3rd, McCoy J. P., Jr, Shibata S., Maddox D. E., Goldstein I. J., Wicha M. Differential expression of a lamininlike substance by high- and low-metastatic tumor cells. Am J Pathol. 1983 Apr;111(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Mitra R. S., Gibbs D., Phan S. H., Dixit V. M., Mitra R., Jr, Wang T., Siebert K. J., Nickoloff B. J., Voorhees J. J. All-trans retinoic acid stimulates growth and extracellular matrix production in growth-inhibited cultured human skin fibroblasts. J Invest Dermatol. 1990 May;94(5):717–723. doi: 10.1111/1523-1747.ep12876294. [DOI] [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Dixit V. M., Mitra R. S., Voorhees J. J. All-trans retinoic acid stimulates growth of adult human keratinocytes cultured in growth factor-deficient medium, inhibits production of thrombospondin and fibronectin, and reduces adhesion. J Invest Dermatol. 1989 Oct;93(4):449–454. doi: 10.1111/1523-1747.ep12284020. [DOI] [PubMed] [Google Scholar]
- Varani J., Shayevitz J., Perry D., Mitra R. S., Nickoloff B. J., Voorhees J. J. Retinoic acid stimulation of human dermal fibroblast proliferation is dependent on suboptimal extracellular Ca2+ concentration. Am J Pathol. 1990 Jun;136(6):1275–1281. [PMC free article] [PubMed] [Google Scholar]
- Viallet J. P., Ruberte E., du Manoir S., Krust A., Zelent A., Dhouailly D. Retinoic acid-induced glandular metaplasia in mouse skin is linked to the dermal expression of retinoic acid receptor beta mRNA. Dev Biol. 1991 Apr;144(2):424–428. doi: 10.1016/0012-1606(91)90434-5. [DOI] [PubMed] [Google Scholar]
- Weiss J. S., Ellis C. N., Headington J. T., Tincoff T., Hamilton T. A., Voorhees J. J. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA. 1988 Jan 22;259(4):527–532. [PubMed] [Google Scholar]
- Yaeger P. C., Stiles C. D., Rollins B. J. Human keratinocyte growth-promoting activity on the surface of fibroblasts. J Cell Physiol. 1991 Oct;149(1):110–116. doi: 10.1002/jcp.1041490114. [DOI] [PubMed] [Google Scholar]