SUMMARY
The human tubercle bacillus Mycobacterium tuberculosis can synthesize NAD+ using the de novo biosynthesis pathway or the salvage pathway. The salvage pathway of the bovine tubercle bacillus M. bovis was reported defective due to a mutation in the nicotinamidase PncA. This defect prevents nicotinic acid secretion, which is the basis for the niacin test that clinically distinguishes M. bovis from M. tuberculosis. Surprisingly, we found that the NAD+ de novo biosynthesis pathway (nadABC) can be deleted from M. bovis, demonstrating a functioning salvage pathway. M. bovis ΔnadABC fails to grow in mice, whereas M. tuberculosis ΔnadABC grows normally in mice, suggesting that M. tuberculosis can acquire nicotinamide from its host. The introduction of M. tuberculosis pncA into M. bovis ΔnadABC is sufficient to fully restore growth in a mouse, proving that the functional salvage pathway enables nicotinamide acquisition by the tubercle bacilli. This study demonstrates that NAD+ starvation is a cidal event in the tubercle bacilli and confirms that enzymes common to the de novo and salvage pathways may be good drug targets.
Keywords: bovis, tuberculosis, NAD+, starvation, pncA
INTRODUCTION
The rapid spread of tuberculosis (TB) strains that are multi-drug- and extensively drug-resistant has rendered crucial the need to develop new drugs to fight TB, a disease caused by the bacillus Mycobacterium tuberculosis. Novel drug targets have to be identified in order to discover drugs that will be effective against these drug-resistant TB strains.
Nicotinamide adenine dinucleotide (NAD+) is an essential molecule implicated in hundreds of biological reactions. NAD+ is a hydride acceptor in redox reactions and a substrate in reactions involving ADP-ribose (Khan et al., 2007). The enzymes implicated in the eukaryotic NAD+ biosynthesis have been shown to be good drug targets for several diseases, such as autoimmune diseases, cancer, cardiac diseases, and multiple sclerosis (Khan et al., 2007; Sauve, 2008). The de novo NAD+ biosynthesis pathway differs between eukaryotes and prokaryotes. The eukaryotes synthesize NAD+ using the kyurenate pathway, which requires eight enzymes to obtain NAD+ from tryptophan. In prokaryotes, the de novo biosynthesis pathway synthesizes NAD+ from aspartate using five enzymes (Fig. 1A). The first step is the oxidation of the amino group of L-aspartate to an imine by the aspartate oxidase NadB. The quinolinic acid synthase NadA then condenses the resulting iminoaspartic acid with dihydroxyacetone phosphate to form quinolinic acid. Monodecarboxylation of quinolinic acid and conversion to the nucleotide form by the quinolinate phosphoribosyl transferase NadC yields nicotinic acid mononucleotide (NAMN). The NAMN adenyl transferase NadD catalyzes the adenylation of NAMN to nicotinic acid adenine dinucleotide (NAAD), which is amidated by the deamido-NAD ligase NadE to form NAD+. The last 2 enzymes of the de novo pathway, NadD and NadE, are also common to the NAD+ salvage or recycling pathway. The salvage of NAD+ can occur via the degradation of NAD+ to nicotinamide (NAm) by the NAD+ glycohydrolase (Gholson, 1966), followed by the hydrolysis of NAm to nicotinic acid (NAc) by the nicotinamidase PncA, and then the transfer of a phosphoribosyl group to NAc by PncB to give NAMN, the product of NadC. NAMN is the convergence point of the salvage and the de novo pathways (Fig. 1A). The synthesis of NAD+ from NAc is also referred to as the Preiss-Handler pathway. Another branch of the salvage pathway found in bacteria is the formation of nicotinamide mononucleotide (NMN), either by cleavage of the adenyl monophosphate (AMP) group of NAD+ or by the transfer of a phosphoribosyl unit to NAm. NMN can then be either degraded to NAm via glycohydrolase or retransformed into NAD+ by transfer of an adenyl group. Mycobacteria can synthesize NAD+ by using either the de novo biosynthesis pathway or the salvage pathway (Begley et al., 2001; Foster and Moat, 1980).
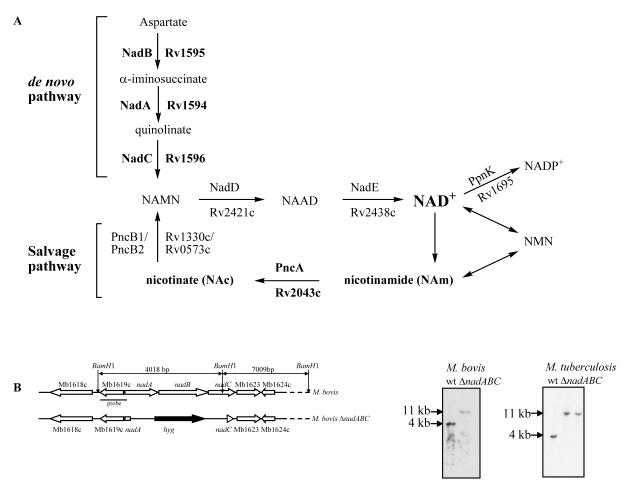
Fig. 1.
Deletion of the NAD+ de novo pathway genes, nadABC, in M. bovis and M. tuberculosis. A. NAD+ biosynthetic and salvage pathways. NadA, NadB, and NadC enzymes are unique to the NAD+ de novo biosynthesis pathway, while the two last enzymes, NadD and NadE, are common to both the de novo and the salvage NAD+ pathways. The salvage pathway decomposes NAD+ into nicotinamide (NAm) or NMN, or converts NAm from the media to nicotinic acid mononucleotide (NAMN) using 2 enzymes, PncA and PncB. The enzymes and intermediates involved in this study are indicated in bold. B.Southern analysis of M. bovis ΔnadABC and M. tuberculosis ΔnadABC transductants. The diagram shows the replacement of the nadABC operon in M. bovis by a hygromycin resistance gene (hyg) using specialized transduction. Southern analysis of the genomic DNA from wt and ΔnadABC mutants, digested with BamH1, confirmed the replacement of the nadABC genes by hyg in M. bovis and M. tuberculosis.
Due to the presence of the NAD+ salvage pathway, the only enzymes in the NAD+ biosynthesis pathway that could be considered as drug targets are NadD and NadE (Gerdes et al., 2002). Inhibitors of the NadD enzyme of Escherichia coli and Bacillus anthracis have been identified that have no activity against the corresponding human enzyme (Sorci et al., 2009). NadE inhibitors with good antibacterial activities have been developed against Bacillus subtilis and Staphylococcus aureus (Velu et al., 2003; Velu et al., 2007). Some of these NadE inhibitors were tested against M. tuberculosis and exhibited bacteriocidal activity but also high cytotoxicity (Boshoff et al., 2008). In this study, Boshoff et al. demonstrated that deletion of the first two genes of the NAD+ biosynthesis pathway, nadA and nadB, had no effect on the growth of M. tuberculosis in vivo (Boshoff et al., 2008). This result was attributed to the ability of M. tuberculosis to scavenge NAm or NAc from the host and use the salvage pathway to maintain its redox balance. Indeed, it has been reported that mammalian tissues contain large amounts of NAm (Qin et al., 2006; Yang and Sauve, 2006). Interestingly, the pathogen responsible for the bovine TB, M. bovis, is known to have a defective salvage pathway due to a mutation in the pncA gene, a single-base pair change (c169g) resulting in the amino acid change His57Asp (Scorpio and Zhang, 1996). This defect is the basis of the resistance of M. bovis to the TB drug pyrazinamide (PZA) as well as of the negative niacin test, which is one of the tests employed clinically to differentiate M. tuberculosis from M. bovis infections in patients (Konno, 1956; Konno et al., 1957; Konno et al., 1958). The niacin production test is based on the accumulation of niacin in M. tuberculosis culture fluids, which does not occur with M. bovis. This was first observed by Pope and Smith in 1946 (Pope and Smith, 1946), and later was attributed to a higher level of NAD+ glycohydrolase activity in M. tuberculosis than in M. bovis (Kasarov and Moat, 1972). Kasarov and Moat also demonstrated that the source of NAc in M. tuberculosis was NAD+ (Kasarov and Moat, 1972). To test the role of the salvage pathway in the survival in vivo of mycobacteria lacking the ability to synthesize NAD+ de novo, we attempted the deletion of the NAD+ de novo pathway (nadABC) in M. bovis as well as in M. tuberculosis.
Here we report the surprising result that deletion of the de novo NAD+ biosynthetic genes is possible in M. bovis despite the presumed lack of a functioning salvage pathway and that, in contrast to M. tuberculosis, NAm starvation is a cidal event in M. bovis ΔnadABC. This leads to the conclusion that a combination of drugs targeting both the de novo and the salvage pathways could also efficiently kill M. tuberculosis.
RESULTS
NAD+ de novo genes can be deleted in M. bovis, demonstrating a functional salvage pathway
The three genes specific to the de novo NAD+ biosynthesis pathway, nadA, nadB, and nadC, are in an operon in M. tuberculosis and in M. bovis. This 3490 bp operon is quite similar in M. tuberculosis and in M. bovis, with only a 1 bp difference in the nadC gene (a190g), which causes an N64D amino acid change. The specialized transduction system (Bardarov et al., 2002) was used to replace the nadABC operon in both M. tuberculosis CDC1551 and M. bovis Ravenel by a hygromycin resistance gene (hyg) (Fig. 1B). As the left- and right-flanking regions of the nadABC operon are quite similar with 2 bp differences that do not cause any amino acid changes, the same knockout phage was used to perform the transductions in both M. tuberculosis and M. bovis.
Since the salvage pathway is functional in M. tuberculosis (Boshoff et al., 2008), we plated our transductions on NAm-containing plates to allow for growth of the M. tuberculosis ΔnadABC transductants. The transductions with M. bovis were plated on plates containing either NAm or NAc. We did not expect to obtain transductants on NAm-containing plates since the nicotinamidase PncA has been shown to be defective in M. bovis (Scorpio and Zhang, 1996). Surprisingly, M. bovis ΔnadABC transductants were obtained on NAm-containing plates at a low frequency (<10−8). Southern analyses on two M. tuberculosis ΔnadABC transductants and two M. bovis ΔnadABC transductants isolated on NAm-containing plates confirmed that the nadABC genes had been replaced with the hyg gene (Fig. 1B). These transductants were used for the rest of the study. To ensure that the M. bovis ΔnadABC transductants had not acquired compensatory mutations in pncA, the gene and promoter region of pncA in M. bovis ΔnadABC were sequenced and compared to the sequence of wt M. bovis. We found no other mutation than the described pncA mutation (c169g) in M. bovis ΔnadABC. The M. bovis ΔnadABC deletion mutants, like the M. tuberculosis ΔnadABC deletion mutants, were found to be NAm auxotrophs, suggesting that the nicotinamidase and the Preiss-Handler pathway are active in M. bovis. To confirm that M. bovis ΔnadABC (mc24957) can utilize NAm to synthesize NAD+, we added radioactive NAm to a culture of M. bovis ΔnadABC and analyzed the radioactive metabolites by HPLC. M. bovis ΔnadABC was able to synthesize NAD+ from NAm. The predominant metabolites obtained were NAD+, NADH, and NADP+, with no trace of radioactive NAm (Fig. 2). We therefore conclude that the salvage pathway is indeed functional in M. bovis.
Fig. 2.
Metabolization of NAm by M. bovis. M. bovis strains were labeled with 14C-NAm for 72 hr. The cell pellets were washed intensively and lyzed. The 14C-labeled metabolites extracted from the cell pellets were separated by reverse-phase HPLC and detected with a beta-gamma radiation detector. The metabolites were identified by comparison with chromatograms of standards. The position of the signal for NAm is indicated by an arrow. Comp stands for complemented.
M. bovis ΔnadABC requires more NAm for growth than M. tuberculosis ΔnadABC
Mycobacteria have genes for both the de novo pathway and the salvage pathway to synthesize NAD+ (Fig. 1A). Following deletion of the nadABC genes from the de novo pathway, we observed that M. bovis ΔnadABC could synthesize NAD+ from NAm. To establish whether M. bovis ΔnadABC was as efficient as M. tuberculosis ΔnadABC in using NAm, we determined the minimum amount of NAm required for the growth of M. bovis ΔnadABC in liquid cultures. The strains were grown in media containing 20 mg/l of NAm, spun down, and washed extensively with PBS-tyloxapol to remove any trace of NAm. The strains were then inoculated in media containing different amounts of NAm (from 0.01 mg/l to 20 mg/l). No significant difference in growth rate was observed when the ΔnadABC mutants were grown in the presence of NAm at concentrations ranging from 1 - 20 mg/l but the M. tuberculosis ΔnadABC and M. bovis ΔnadABC strains showed a difference in their ability to utilize low concentrations of NAm (<1 mg/l) to grow (Fig. 3A). M. bovis ΔnadABC needed more than 10 times the amount of NAm to maintain growth than did M. tuberculosis ΔnadABC. This reflects the known mutation in the M. bovis pncA gene (Scorpio and Zhang, 1996). Recently, this mutation was shown to result in a 99% loss of pyrazinamidase/nicotinamidase activity (Zhang et al., 2008). In contrast, no growth differences between M. bovis ΔnadABC and M. tuberculosis ΔnadABC were observed when the mutants were supplemented with NAc. The smallest amount of NAc that allowed for the growth of the ΔnadABC strains was 0.1 mg/l (0.8 μM). Complementation of M. bovis ΔnadABC with the M. bovis nadABC operon cloned downstream of the hsp60 promoter of an integrative E. coli mycobacterial shuttle plasmid (Stover et al., 1991) resulted in a strain that grew without any supplement and at a rate similar to that of wild-type M. bovis Ravenel (Fig. 3A).
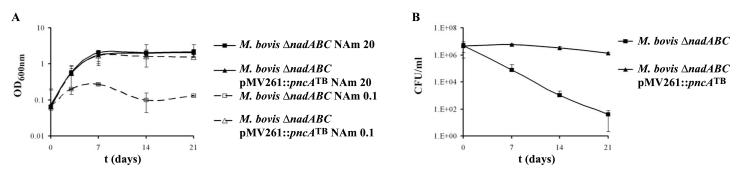
Fig. 3.
Growth of M. bovis ΔnadABC and M. tuberculosis ΔnadABC in vitro. A. The strains were grown with NAm (20 mg/l) to log phase, spun down, washed 5 X with PBS, resuspended in media without NAm, and inoculated into media containing either NAm at different concentrations or NAc at 20 mg/l. The complemented strains were grown without any supplement. The cultures were incubated with shaking at 37°C for 3 weeks and growth was followed by measuring OD600nm. B. The strains were grown, washed, diluted, and inoculated in media containing or not containing NAm (20 mg/l). At each time point, samples were taken, diluted, and plated onto Middlebrook 7H10 plates containing OADC, glycerol, and NAm (20 mg/l). The plates were incubated at 37°C for 4-5 weeks and colonies were counted. The concentrations are in mg/l.
NAD+ starvation is a cidal event in M. bovis ΔnadABC
Starvation for different nutrients can be bacteriostatic (such as the leucine auxotroph, F. C. Bange and W. R. Jacobs, Jr., unpublished) or bacteriocidal (Gordhan et al., 2002). To determine if NAD+ starvation was a static or a cidal event in the ΔnadABC strains, we measured colony forming units (CFUs) over a period of 3 weeks while the strains were starved for NAm (Fig. 3B). NAD+ starvation of the M. bovis ΔnadABC strain was a cidal event, resulting in a 5 - 6 log drop in CFUs after 3 weeks. In contrast, no significant loss in viability was recorded for M. tuberculosis ΔnadABC for the first 2 weeks of starvation. Only in the following week did we observe a slight decrease in CFUs (1 log). The difference in the death kinetics between M. tuberculosis ΔnadABC and M. bovis ΔnadABC during NAm starvation could be due to the ability of M. tuberculosis to uptake secreted NAc from the media, which could then be re-used to synthesize NAD+ via the Preiss-Handler pathway (Fig. 1A). The niacin test for M. bovis ΔnadABC is negative, indicating that the strain might not produce and secrete NAc at a level sufficient for re-utilization. To test this hypothesis, we mixed cultures of M. tuberculosis ΔnadABC and M. bovis ΔnadABC pMV361 and starved them for NAm. The starved cultures were plated on plates containing or not kanamycin (the marker in the empty vector pMV361) to allow for the distinction between M. tuberculosis ΔnadABC and M. bovis ΔnadABC. As observed with M. tuberculosis ΔnadABC, the starvation of M. bovis ΔnadABC pMV361 mixed with M. tuberculosis ΔnadABC was bacteriostatic (no loss or gain in CFUs) for the first 2 weeks while starvation of M. bovis ΔnadABC pMV361 was cidal. This result suggests that the difference in the death rates between the two mutants arises from the processing of internal NAD+ pools.
Transformation with the pncA gene from M. tuberculosis reverses the bacteriocidal phenotype of M. bovis ΔnadABC upon NAm starvation
The M. bovis ΔnadABC strain displays two phenotypes different from M. tuberculosis ΔnadABC: increased requirements for NAm and bacteriocidal killing in its absence. To test if these phenotypes were solely caused by the known mutation in M. bovis pncA, the M. tuberculosis pncA gene was cloned into the replicative plasmid pMV261 under the hsp60 promoter and transformed into M. bovis ΔnadABC.
The introduction of M. tuberculosis pncA into M. bovis ΔnadABC resulted in a strain that was able to grow on a lower amount of NAm than was the parent strain (Fig. 4A). The M. bovis ΔnadABC pMV261::pncATB requirement for NAm was similar to that of M. tuberculosis ΔnadABC. Addition of radioactive NAm to M. bovis ΔnadABC pMV261::pncATB culture led to the same formation of radioactive NAD+ and NADP+, with no trace of NAm or NAc as M. bovis ΔnadABC, M. bovis ΔnadABC complemented, and M. tuberculosis ΔnadABC (Fig. 2). As expected, the M. bovis ΔnadABC pMV261::pncATB strain also regained one of the features that differentiate M. tuberculosis and M. bovis: a positive niacin test. Furthermore, in contrast with the parent strain M. bovis ΔnadABC, NAm starvation of M. bovis ΔnadABC overexpressing M. tuberculosis pncA was not a bacteriocidal event (Fig. 4B). We observed a slight increase in CFUs after 7 days of NAm starvation followed by a very slow decrease in CFUs in the subsequent 2 weeks, comparable to the NAm starvation of M. tuberculosis ΔnadABC. These results suggest that the defect in pncA is the key component in the bacteriocidal phenotype of M. bovis ΔnadABC upon NAm starvation. Thus, inhibition of both the de novo and the salvage pathways has a dramatic effect on the viability of the tubercle bacilli.
Fig. 4.
Growth of M. bovis ΔnadABC pMV261::pncATB in vitro. M. bovis ΔnadABC was transformed with M. tuberculosis pncA cloned into the replicative plasmid pMV261. A. M. bovis ΔnadABC pMV261::pncATB and its parent strain M. bovis ΔnadABC were grown in NAm-containing media, washed 5 X in PBS, diluted, and inoculated in media containing NAm (20 and 0.1 mg/l). Growth was followed by measuring OD600nm at diverse time points. B. M. bovis ΔnadABC pMV261::pncATB and M. bovis ΔnadABC were grown and washed as described above, diluted 1-50, and inoculated in media without NAm (20 mg/l). At each time, samples were taken, diluted, and plated onto Middlebrook 7H10 plates containing NAm (20 mg/l). The plates were incubated at 37°C for 4-5 weeks and colonies were counted.
M. bovis generates more NAD+ than M. tuberculosis
Previous studies have shown that the inhibition of either the de novo NAD+ biosynthesis pathway or the NAD+ salvage pathway has no remarkable effect on the viability of M. tuberculosis (Boshoff et al., 2008) while we have shown that simultaneous inhibition of both together is a cidal event. To test the contribution of each pathway to the NAD+ pool, the concentration of NAD+ and NADH in both wild-type and NAm-starved ΔnadABC strains was measured.
The cellular concentrations of NAD+ and NADH nucleotides were first determined in the ΔnadABC strains grown with 20 mg/l of NAm. Both the M. tuberculosis ΔnadABC and M. bovis ΔnadABC strains had NAD+ and NADH concentrations 60% lower than wild-type when they relied solely on the salvage pathway to produce NAD+ (Fig. 5A). Complementation of the ΔnadABC strains with the integrative plasmid pMV361::nadABC restored NAD+ concentration to the wild-type levels in both strains (Fig. 5A). Interestingly, the NAD+ and NADH concentrations were also very different between wild type M. tuberculosis and M. bovis, with M. bovis containing about 65% more NAD+ than M. tuberculosis. The same ratio was also observed in the M. tuberculosis ΔnadABC and M. bovis ΔnadABC strains grown on NAm. In this case, these strains are using only the salvage pathway, yet the M. bovis ΔnadABC strain contains twice as much NAD+ as M. tuberculosis ΔnadABC, despite an impaired salvage pathway. The difference in NAD+ cellular levels between M. bovis and M. tuberculosis could be the result of the degradation by M. tuberculosis of “excess” NAD+ to NAc, which is then secreted. The NAD+ level in the M. bovis ΔnadABC pMV261::pncATB strain is similar to the M. bovis ΔnadABC strain (Fig. 5A), yet the strain is capable of secreting NAc, as seen by its positive niacin test. Therefore, the difference in NAD+ levels between M. bovis and M. tuberculosis might reflect the defect of M. bovis in the degradation of NAD+ to NAm or NMN, as previously reported (Kasarov and Moat, 1972), rather than the defect in the nicotinamidase activity or be the result of other physiological differences between M. tuberculosis and M. bovis.
Fig. 5.
NAD+ and NADH concentrations in ΔnadABC strains. A. The strains were grown to log-phase (OD600nm ≈ 1) before NAD+ and NADH were extracted, as described in Experimental Procedures. M. bovis ΔnadABC, M. bovis ΔnadABC pMV261::pncATB and M. tuberculosis ΔnadABC were grown in media containing NAm (20 mg/l). B. M. tuberculosis ΔnadABC, M. bovis ΔnadABC, and M. bovis ΔnadABC pMV261::pncATB were grown to early log phase (OD600nm ≈ 0.5), spun down, washed 5 X with PBS, and resuspended in media without NAm. The resulting OD600nm after washes were 0.45 for M. tuberculosis ΔnadABC, 0.3 for M. bovis ΔnadABC, and 0.2 for M. bovis ΔnadABC pMV261::pncATB. NAD+ and NADH concentrations were measured after 2, 7, and 14 days of NAm starvation. The experiments were done in triplicate and the average is plotted with the standard deviation.
As expected, upon NAm starvation, the levels of NAD+ in the M. bovis ΔnadABC strain decreased much more rapidly than did the NAD+ levels in M. tuberculosis ΔnadABC. On average, after 2 days of NAm starvation, the M. bovis ΔnadABC and M. tuberculosis ΔnadABC strains had lost 60% and 15% of their NAD+ levels, respectively (Fig. 5B). The decrease in the M. bovis ΔnadABC NAD+ levels became more severe as the starvation period increased, reaching 90% and 98% loss after 7 and 14 days, respectively. In contrast, NAm starvation had a much less severe effect on the NAD+ levels of the M. bovis ΔnadABC strain overexpressing M. tuberculosis pncA. After 2 days of NAm starvation, a slight increase in the NAD+ levels was measured followed by a slow decrease over time, correlating with the bacteriostasis observed in this strain upon NAm starvation. We conclude that the recycling of NAD+ by the salvage pathway prevents the cidal phenotype of M. tuberculosis ΔnadABC starvation.
Transformation with the pncA gene from M. tuberculosis reverses the defect of M. bovis ΔnadABC in vivo growth
An M. tuberculosis mutant defective for the NAD+ de novo pathway was recently shown to have no growth defect in vivo due to the ability of M. tuberculosis to salvage NAm or NAc from the host (Boshoff et al., 2008). With the dramatic difference in death curves observed during NAD+ starvation between the M. bovis ΔnadABC and M. tuberculosis ΔnadABC strains, we hypothesized that M. bovis ΔnadABC would be unable to grow in mice. To test this hypothesis, low-dose aerosol infection of C57Bl/6 mice was performed with the M. bovis ΔnadABC and M. tuberculosis ΔnadABC strains, their parental strains, and the complemented strains. The growth of the organisms in the lungs, livers, and spleens was followed by plating for CFU at different days post infection.
In the lungs of C57Bl/6 mice, the M. tuberculosis ΔnadABC mutant grew as well as did the wild-type M. tuberculosis and the complemented mutant (Fig. 6B). In striking contrast, the growth of M. bovis ΔnadABC was highly attenuated, compared to wild-type M. bovis (Fig. 6A). Notably, after 3 weeks post-infection, there was more than a 3-log difference between the growth of the wild-type M. bovis strain and the growth of the M. bovis ΔnadABC mutant. Although the strain kept on growing slowly, even after 17 weeks there was still a 2-log difference between the mutant and the wild-type. Consistent with a lack of growth in the lungs, M. bovis ΔnadABC never grew in either the spleen or the liver, even after 17 weeks, suggesting that there was no hematogenous spread of organisms.
Fig. 6.
Growth in the lungs of immunocompetent C57Bl/6 mice, following low-dose aerosol infection, of: A. M. bovis, M. bovis ΔnadABC and M. bovis ΔnadABC complemented; B. M. tuberculosis, M. tuberculosis ΔnadABC and M. tuberculosis ΔnadABC complemented; and C. M. bovis ΔnadABC, M. bovis ΔnadABC pMV261::pncATB, and M. bovis ΔnadABC complemented
Puzzled by the growth of M. bovis ΔnadABC in the lungs of the C56Bl/6 mice, we hypothesized that the colonies growing were mutants of M. bovis ΔnadABC that had perhaps acquired another mutation in the pncA gene that allowed them to salvage and utilize NAm from the host, like M. tuberculosis is able to. Sequencing of the pncA gene and the promoter region of the only three colonies isolated from the lungs at 3 weeks post-infection revealed no other mutations in the pncA region other than the parent strain mutation in pncA (c169g). Since there are at least ten putative amidases in the TB genome, we wondered whether the M. bovis ΔnadABC strains isolated in vivo had turned on another amidase to allow for the hydrolysis of NAm taken from the host. To test this hypothesis, the niacin test was performed on the colonies isolated from the lungs at 3 weeks, and we observed that all the colonies tested were positive for this test, while the initial M. bovis ΔnadABC strain was not.
To assess the role of the salvage pathway in the survival of M. tuberculosis NAD+ de novo mutants in vivo, we tested the in vivo growth of M. bovis ΔnadABC pMV261::pncATB (Fig. 6C). Low-dose aerosol infection of C57Bl/6 mice was performed with M. bovis ΔnadABC pMV261::pncATB, M. bovis ΔnadABC, and M. bovis ΔnadABC complemented. CFUs were measured at days 1, 7, 21, and 56 following infection. Growth in the lungs (Fig. 6C), livers, and spleens of mice infected by M. bovis ΔnadABC pMV261::pncATB and by M. bovis ΔnadABC complemented followed a similar kinetics, while the livers and spleens of mice infected with M. bovis ΔnadABC were culture-negative and only one organism was isolated from the lung of an M. bovis ΔnadABC-infected mouse at day 63.
DISCUSSION
The impaired salvage pathway in M. bovis is one of the factors differentiating M. tuberculosis from M. bovis infection in patients. The known allele in M. bovis pncA (c169g, His57Asp), the gene encoding the pyrazinamidase/nicotinamidase that hydrolyzes the NAD+ degradation product NAm into NAc, is the basis for the negative niacin test and for the resistance to PZA (Scorpio and Zhang, 1996) observed in M. bovis. Given these well-established facts, we were rather surprised to obtain an M. bovis mutant in which the three genes involved in the de novo NAD+ biosynthesis, nadA, nadB, and nadC, had been deleted. This successful deletion of the de novo NAD+ biosynthesis pathway in M. bovis highlighted interesting differences from the previously reported deletion of nadA and nadB in M. tuberculosis (Boshoff et al., 2008) and from our own M. tuberculosis ΔnadABC knockout. M. bovis ΔnadABC requires 10 times more NAm to grow than does M. tuberculosis ΔnadABC. This increased NAm requirement in M. bovis was a direct result of the pncA mutation since introducing the M. tuberculosis pncA gene into the M. bovis ΔnadABC auxotroph restored the same NAm need for growth as did the M. tuberculosis auxotroph. We conclude that M. bovis PncA must have low-level nicotinamidase activity. This is consistent with previous observations that demonstrated a greatly reduced activity for the M. bovis nicotinamidase compared to M. tuberculosis (Konno et al., 1960; Zhang et al., 2008). Based on our mouse studies, we can conclude that M. bovis does not typically utilize the salvage pathway for growth in mice since a deletion of the de novo pathway impairs growth. It is intriguing that numerous independent M. bovis strains have the defective pncA allele, which we and others have shown correlates with an impairment of the salvage pathway. It is noteworthy that Shigella species, but not the related Escherichia coli species, all appear to have mutations in the de novo NAD+ pathway (Prunier et al., 2007). While the selective basis for the maintenance of an allele that causes an impairment of the salvage pathway remains unknown, we can speculate that it might play a role in the host specificity difference between M. tuberculosis and M. bovis. Further studies need to be performed to elucidate the basis of host specificities, and the use of these mutants in the de novo pathways of M. tuberculosis and M. bovis should aid in such studies.
Another interesting question that arises is how to explain the resistance of M. bovis to PZA. Since M. bovis ΔnadABC can use NAm to grow, indicating that the hydrolysis of NAm to NAc does occur in M. bovis, we would predict that a similar hydrolysis of PZA to pyrazinoic acid (POA), the active compound of PZA, could also happen in M. bovis, based on the high degree of chemical similarity between NAm and PZA. Thus, one possible explanation is that the level of POA generated by M. bovis PncA is directly proportional to the killing activity of PZA. Alternatively, the PZA resistance in M. bovis has also been attributed to a lack of PZA uptake. However, since Raynaud et al. demonstrated that PZA and NAm use the same transport system (Raynaud et al., 1999), and since we have shown that M. bovis ΔnadABC can use NAm to grow, we can reasonably hypothesize that there is no defect in the transport of NAm and that there is likely no defect in the transport of PZA in M. bovis. To distinguish between these two possibilities, the minimum inhibitory concentration (MIC) of PZA for the M. tuberculosis :pncA-overexpressing M. bovis ΔnadABC strain and its parent strain was measured. The M. bovis ΔnadABC strain overexpressing M. tuberculosis pncA was 100 times more sensitive to PZA (MIC = 3.1 mg/L) than its parent strain (MIC = 400 mg/L). Therefore, we conclude that the resistance of M. bovis to PZA is more likely due to a lower level of PncA activity than a transport problem.
Another notable difference between M. tuberculosis and M. bovis is the inability of the M. bovis auxotroph to utilize the NAm available in a mouse, the way M. tuberculosis ΔnadABC does. Most auxotrophs of M. tuberculosis and M. bovis fail to grow in mice (Hondalus et al., 2000; Jackson et al., 1999; Pavelka and Jacobs, 1999), indicating that the in vivo environment of the tubercle bacillus does not provide adequate levels of specific metabolites or biosynthetic precursors. Other auxotrophs of M. tuberculosis, such as argF (Gordhan et al., 2002), proC, or trpD (Smith et al., 2001), failed to grow in the lungs of infected immunocompetent mice initially, but a few organisms could be isolated in these organs after a few months, suggesting that the availability of specific metabolites could be also dependent on the stage of infection. The growth of M. tuberculosis ΔnadABC in vivo can be attributed to the ability of M. tuberculosis to scavenge precursors for NAD+ biosynthesis from the host, as previously suggested (Bekierkunst and Artman, 1962; Boshoff et al., 2008; Windman and Bekierkunst, 1967). This suggests that the synthesizing of drugs that link bacteriocidal moieties to NAm may be an effective drug development strategy by making poorly-absorbed compounds competent for uptake into M. tuberculosis cells in vivo. Previous studies had demonstrated that the NAD+ glycohydrolase was also impaired in M. bovis (Kasarov and Moat, 1972). The survival in vivo and the positive niacin test of the M. bovis ΔnadABC pMV261::pncATB strain suggest that: 1) only the defect in pncA is responsible for the cidal effect of NAD+ starvation in M. bovis, and 2) the ability of this strain to secrete NAc does not correlate with a deficient NAD+ glycohydrolase.
The striking killing differences between M. bovis and M. tuberculosis upon NAD+ starvation led us to test whether M. bovis ΔnadABC infected and grew differently in macrophages than did M. tuberculosis ΔnadABC or wild-type M. bovis. We found no significant differences between these strains in their capacity to infect macrophages, and measured only a slight loss in CFU for M. bovis ΔnadABC after 3 days in macrophages. To identify differences in gene expression that might be responsible for the divergent phenotypes, we examined the transcriptional response of M. bovis ΔnadABC and M. tuberculosis ΔnadABC to 7 days of NAm starvation (Tables S1-S4). In a two-class SAM test to identify genes that are significantly differentially regulated by NAm starvation between M. tuberculosis and M. bovis, 983 were deemed significant, with many more positively significant (848 up-regulated in M. bovis, and down-regulated, absent, or not strongly induced in M. tuberculosis) than negatively significant (135 up-regulated in M. tuberculosis, down-regulated, absent, or not strongly induced in M. bovis). The preponderance of positively significant genes is likely reflective of the severity of the phenotypic change in M. bovis. The transcriptional state of the dying M. bovis cells showed very limited similarity with various drug treatments or stress conditions (Boshoff et al., 2004). An interesting finding was the broad similarity of the response of M. tuberculosis starved for NAm to that of M. tuberculosis starved for all nutrients, as reported (Betts et al., 2002). This similarity was absent from the M. bovis response to NAm starvation, suggesting that M. bovis is unable to mount an adaptive response that may protect M. tuberculosis. Part of this potential adaptive response is the down-regulation of the nuo operon encoding the NADH dehydrogenase type I, which oxidizes NADH to NAD+ and leads to the replenishment of the NAD+ pool. M. bovis up-regulates the nuo operon in an apparently futile attempt to restore NAD+ levels. Interestingly, the up-regulation of the nuo operon had also been observed in Escherichia coli treated with bacteriocidal drugs, leading to the hypothesis that cell death is ultimately caused by the rapid increase in NADH consumption leading to high levels of superoxide (Kohanski et al., 2007).
The role of pncA in the cidal death of M. bovis ΔnadABC is crucial, since repairing the M. bovis salvage pathway by introducing the M. tuberculosis pncA gene results in loss of the bacteriocidal phenotype upon NAm starvation. In addition, the transformation of the M. bovis ΔnadABC with M. tuberculosis pncA also restores 3 other independent phenotypes simultaneously: 1) growth on lower concentration of NAm, 2) niacin secretion, and 3) reduction of NAD+ loss upon NAm starvation. Our study demonstrates that inhibiting both the de novo and the salvage pathways simultaneously will lead to mycobacterial cell death. Another option is to design inhibitors that target the enzymes common to both pathways: NadD and NadE. Boshoff et al. recently showed that inhibitors of the NAD+ synthetase NadE killed mycobacteria but were also cytotoxic (Boshoff et al., 2008). The mycobacterial NadE enzyme is more similar to the eukaryotic NadE enzyme than to other bacterial NadE enzymes. The amidation of NAAD to NAD+ performed by NadE occurs in two steps: 1) activation of the carboxylic group of NAAD with ATP by the addition of AMP and the release of PPi followed by 2) amidation and the release of AMP. Two classes of NAD+ synthetases have been defined depending on the donor for the amidation reaction: ammonia-dependent NadE and glutamine-dependent NadE. Most bacteria have ammonia-dependent NadE but M. tuberculosis has a glutamine-dependent NadE, like the one found in eukaryotes (Magni et al., 2009). Designing NadE inhibitors that are specific for the mycobacterial NadE with no activity against eukaryotic NadE might, therefore, be challenging.
Our data suggest that targeting the de novo pathway could also lead to new drug candidates for treating M. bovis infections. While zoonotic TB is extremely rare in humans in economically developed nations as a result of the pasteurization of dairy products and the systematic elimination of TB-infected cattle, this is not the case in the developing world (Grange, 2001). Inhibiting the NAD+ de novo pathway would also be valuable for the treatment of patients infected with PZA-resistant M. tuberculosis, since 70-93% of PZA-resistant M. tuberculosis clinical strains carry mutations in the pncA gene or in its upstream region, leading to the loss of pyrazinamidase/nicotinamidase activity (Cheng et al., 2000; Louw et al., 2006; Singh et al., 2006). Resistance to PZA is especially high in patients with multidrug-resistant and extensively drug-resistant TB, which renders their treatment unlikely to succeed (Eker et al., 2008). Since none of the current anti-tuberculosis drugs, either first-, second-, or third-line, target NAD+ biosynthesis, developing new compounds that inhibit NAD+ biosynthesis might be a valuable endeavor to combat multidrug-resistant and extensively drug-resistant TB. We propose that simultaneously targeting both the salvage and the de novo NAD+ biosynthesis pathways represents a potentially effective way to treat infection with tubercle bacilli.
EXPERIMENTAL PROCEDURES
Bacterial strains, plasmids, phages, and media
The M. bovis Ravenel and M. tuberculosis CDC1551 strains were obtained from laboratory stocks. The strains were grown in Middlebrook 7H9 medium (Difco, Sparks, MD) supplemented with 10% (v/v) OADC enrichment (Difco), 0.2% (v/v) glycerol, and 0.05% (v/v) tyloxapol. The solid media used was the same as that described above, with the addition of 1.5% (w/v) agar. The plasmids pMV261 (Stover et al., 1991), pMV361 (Stover et al., 1991), p004S (Hsu, K. & Jacobs Jr., W.R., unpublished), the shuttle cosmid pYUB412 (Bardarov, S. & Jacobs Jr., W.R., unpublished), and the shuttle phasmid phAE159 (Kriakov, J. & Jacobs Jr., W.R., unpublished) were obtained from laboratory stocks. Hygromycin was used at concentrations of 50 mg/l for mycobacteria and 150 mg/l for Escherichia coli. Kanamycin was used at concentrations of 20 mg/l for mycobacteria and 40 mg/l for Escherichia coli. Nicotinic acid (Sigma, St. Louis, MO) and nicotinamide (Sigma) stocks were prepared in sterile water and used at a concentration of 20 mg/l, unless otherwise stated. 14C-NAm was obtained from American Radiolabeled Chemicals (St. Louis, MO). All other chemicals used in this study were obtained from Sigma.
Construction of the ΔnadABC strains
The nadABC operons of M. tuberculosis and M. bovis were replaced by a hygromycin cassette, using the specialized transduction system previously described (Bardarov et al., 2002). Briefly, a 1 kb region flanking the left and right sides of the nadABC operon were PCR-amplified from M. tuberculosis genomic DNA using the following primers (the cloning sites are underlined): nadAL1 (Van91I) TTTTTTTTCCATAAATTGGCGTGGCGCCTATCACAGGTTG, nadAL2 (Van91I) TTTTTTTTCCATTTCTTGGGTCGGCAGTCAGTTCATCCAC, nadCR1 (BstAP1) TTTTTTTTGCATAGATTGCCACACTCAGTGCGCGTGCTCG, nadCR2 (BstAP1) TTTTTTTTGCATCTTTTGCGCGTACTGATCGGCCCCGTTG. The PCR fragments were cut with the appropriate enzyme (site indicated above) and cloned into Van91I-cut p004S. The resulting cosmid was sequenced before digesting with pacI. The linearized cosmid was ligated to the pacI-cut shuttle phasmid phAE159 and the resulting phasmid was packaged in vitro (GigapackII, Stratagene, La Jolla, CA). High-titer phage lysates were used to transduce M. tuberculosis CDC1551 and M. bovis Ravenel (Bardarov et al., 2002). The transductants were checked for the deletion of the nadABC operon by Southern analysis (the genomic DNA of the transductants was cut with BamHI and probed with the left flank of the nadABC region). The ΔnadABC strains were grown in Middlebrook 7H9 medium (see above) containing NAm (20 mg/l). For all the growth experiments (in vitro, in macrophages, and in vivo) and the starvation experiments, the strains were washed 3-5 X with phosphate buffer saline (PBS) prior to the experiment.
Complementation of knockout strains
The complementing plasmids were obtained by PCR amplification of the nadABC region from M. tuberculosis H37Rv and M. bovis Ravenel genomic DNA, using the following primers: nadAF (EcoR1) TTGAATTCGCAATGACTGTGCTGAATCG, nadCR (HindIII) TTTAAGCTTCAGATCG GGTGCAGCATTTC. The PCR products were cloned downstream of the hsp60 promoter of the E. coli-mycobacteria shuttle plasmid vector pMV261 (replicative vector) and the integrative vector pMV361, using the cloning sites indicated above, and sequenced. The plasmids were introduced into the knockout strains by electroporation. The cultures (50 ml) were grown at 37°C in 500 ml roller bottles to log phase and spun down. The cell pellets were washed twice with a 10% glycerol aqueous solution (50 ml) and resuspended in 0.5 ml of 10% glycerol aqueous solution. To the cell suspension (0.17 ml) was added the appropriate complementing plasmid (2 μl). After electroporation (2.5 V, 25 μFd, 1000 Ω), the cell suspension was added to 1 ml of Middlebrook media and incubated at 37°C for 24 hr before plating on kanamycin- and hygromycin-containing plates.
Analysis of 14C-NAm metabolites
Cultures (10 ml), grown to early log phase (OD600nm ≈ 0.3), were washed 3 X with PBS, resuspended in media without NAm, and labeled with 14C-NAm (5 μCi) for 72 hr at 37°C. The cultures were spun down and washed three times with PBS. The cell pellets were resuspended in 0.3 ml of water. Chloroform (0.3 ml) and glass beads (0.1 g) were added. The suspensions were lyzed using the Thermo Scientific FastPrep machine (45 sec, speed 6), spun down and the aqueous phase was filtered through a 0.22 μm filter. The aqueous fraction (50 μl) was injected in a Hewlett-Packard model HP1100 gradient chromatograph equipped with an IN/US β-RAM model 2B flow-through radioisotope beta-gamma radiation detector. Separation was achieved using an Alltech all-guard column (part no. 77082 and 96080) coupled to a reverse-phase C18 column (4.6 by 150 mm; 3-mm column diameter; Alltima C18 [Alltech]). The flow rate was set at 1 ml/min and the wavelength at 260 nm. The mobile phase was 0.1M KH2PO4 - 6 mM tetrabutylammonium phosphate monobasic (pH = 5.75)/methanol. The samples were run using the elution system described previously (Sestini et al., 2000).
Determination of NADH and NAD+ cellular concentrations
Cultures (30 ml) were grown at 37°C to log phase (OD600nm ≈ 1.0) in Middlebrook 7H9 medium (see above) with NAm (20 mg/l) when necessary, prior to NAD+ and NADH extractions. For the starvation experiments, the strains (150 ml) were grown to early log-phase (OD600nm ≈ 0.5), spun down, washed 5 X with PBS, resuspended in Middlebrook 7H9 medium (see above) without NAm, and incubated at 37°C for 2, 7, and 14 days, prior to NAD+ and NADH extractions. A sample (12 ml) of each culture was spun down, treated with 0.2M HCl (1 ml, NAD+ extraction) or 0.2M NaOH (1 ml, NADH extraction), and heated at 50°C for 10 min. The samples were cooled to 0°C and neutralized with 0.1M NaOH (1 ml, NAD+ extraction) or 0.1M HCl (1 ml, NADH extraction). After centrifugation, the supernatant was filtered twice through a 0.2 μm filter and analyzed. The concentration of NAD+ (or NADH) was obtained by measuring spectrophotometrically the rate of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide reduction by the yeast type II alcohol dehydrogenase in the presence of phenazine ethosulfate at 570 nm (Leonardo et al., 1996; San et al., 2002).
Aerosol infection of C57Bl/6 mice
C57Bl/6 mice (National Cancer Institute, Bethesda, MD), 20 mice per group, were infected via the aerosol route using a 106 CFU/ml mycobacterial suspension in PBS containing 0.05% tween 80 and 0.04% antifoam. Three mice from each group were sacrificed after 24 hr of infection and lung homogenates were plated on Middlebrook 7H10 plates containing the appropriate antibiotic to determine the initial infection dose. At 1, 3, 8, and 17 (for M. bovis strains only) weeks post-infection, 3 mice were sacrificed from each group to determine the bacterial burden in the lung, spleen, and liver.
Determination of the minimum inhibitory concentration (MIC) of PZA
M. bovis ΔnadABC and M. bovis ΔnadABC pMV261::pncATB strains were grown to an OD600nm ≈ 1.0 and diluted to 1 × 107 CFU/ml. 0.05 ml of the dilutions were used to inoculate 5 ml of Middlebrook 7H9 media supplemented as described above, acidified to pH = 6.0, and containing various concentrations of PZA (0, 1.56, 3.12, 6.25, 12.5, 25, 50, 100, 200, 400, and 800 mg/L). The cultures were incubated while shaking at 37°C for 2 weeks. MIC was determined as the concentration of PZA that inhibited growth.
Macrophage infection and phagocytosis assay
Bone marrow-derived macrophages from C57Bl/6 were differentiated in 10% conditioned medium from L cell for 7 days on glass coverslips. Before the infection, the M. bovis strains (wild-type, M. bovis ΔnadABC, and M. bovis ΔnadABC complemented) were washed 3 X with PBS. Differentiated macrophages were infected with the M. bovis strains for 4 hr at a multiplicity of infection (MOI) of 1. After the infection, cells were fixed in 2% paraformaldehyde and blocked in 10% goat serum. Extracellular mycobacteria were stained by a rabbit polyclonal antibody against Mycobacterium species (Cygnus Technologies, Inc., Southport, NC). Extracellular and intracellular mycobacteria were visualized using an anti-Mycobacterium antibody and their ability to auto-fluoresce after excitation at 390-410 nm. Cells were counted on a fluorescent microscope (100 X magnification). Typically, infected cells had 20-40 bacteria. Infected cells were scored positive for phagocytosis when they had 5 or fewer extracellular bacteria. The percentage of infected cells positive for phagocytosis was determined from 50 cells in each of four coverslips for each strain. Growth in macrophage was determined by infecting C57Bl/6 bone marrow-derived macrophages with M. bovis strains, as described above. Gentamycin (0.05mg/ml) was added to the DMEM media. After 4hr, 1, 2 and 3 days, infected macrophages were washed twice with PBS and lyzed with 0.05% SDS (0.2 ml). After a 5 min wait, the cells were pipetted up and down 10 times. The lysates were diluted and plated on Middlebrook 7H10 plates (see above) containing NAm if necessary. The plates were incubated at 37°C for 4-5 weeks.
Microarray analysis
Triplicate cultures (50 ml) of M. tuberculosis and M. bovis ΔnadABC mutants were grown in 7H9 OADC glycerol 0.05% tween broth in 500 ml roller bottles to an OD600nm= 0.1 in a roller incubator at 37°C. The cells were washed 3 X in PBS and resuspended in 50 ml 7H9 OADC glycerol 0.05% tween broth with or without 20mg/l NAm and returned to the incubator. After 7 days, cultures were harvested and the cell pellet was resuspended in 1 ml Qiagen RNA Protect reagent (Qiagen, Germantown, MD) and incubated 4 hr at room temperature before storage at − 80°C. Cells were collected by centrifugation and resuspended in 1 ml buffer RLT from a Qiagen RNeasy kit. The suspension was transferred to Fast-Prep Blue Cap tubes and processed for 45 sec at speed 6 in a Fast-Prep apparatus (MP Bio, Irvine, CA). After a brief incubation on ice, the debris was spun down and the supernatant (~750 μL) was removed to a fresh microcentrifuge tube. 500 μl absolute ethanol was added and the samples were applied to RNeasy columns in two applications. RNA was purified, as recommended by the Qiagen protocol. RNA yield, purity, and integrity were checked on a Nanodrop spectrophotometer (Nanodrop Products, Wilmington, DE) and an Agilent Bioanalyzer 2100 microfluidic system (Agilent Technologies, Santa Clara, CA). Contaminating DNA was removed with Ambion TURBO DNA-free (Ambion, Austin, TX), according to the manufacturer’s instructions. cDNA probes were prepared as per PFGRC protocol SOP#M007 (http://pfgrc.jcvi.org/index.php/microarray/protocols.html). Briefly, 2 μg total RNA was used as template for reverse transcription with random hexamer primers and aminoallyl-dUTP in a 2:3 ratio to dTTP in the nucleotide mix. After overnight incubation at 42°C, the reaction was stopped, RNA was hydrolyzed, and unincorporated nucleotides and free amines were removed with the Qiagen MinElute kit as per protocol, with amine-free phosphate wash and elution buffers. cDNA concentration and purity were measured by Nanodrop. Cy3 and Cy5 dyes were coupled to the aminoallyl-cDNA, which was then purified using a MinElute kit. Probe concentration, purity, and dye incorporation were measured by Nanodrop before probes were combined, speed-vac dried, and dissolved in hybridization buffer. Probes were hybridized according to the PFGRC protocol SOP#M008 to 70-mer oligo DNA microarrays representing the complete M. tuberculosis genome with 4 in-slide replicates (JCVI PFGRC M. tuberculosis v. 4), which had been prehybridized. One of the replicates was dye-flipped. Slides were washed and dried, then scanned on a GenePix 4000A scanner (Molecular Devices, Sunnyvale, CA). Images were processed with the TM4 software suite (www.TM4.org). TIGR Spotfinder was used to grid and quantitate spots. TIGR MIDAS was used for Lowess normalization, standard deviation regularization, and in-slide replicate analysis, with all quality control flags on and one bad channel tolerance policy set to generous. Results were analyzed in MeV with Significance Analysis of Microarrays (SAM) and hierarchical clustering algorithms. The array data have been deposited in the Gene Expression Omnibus at NCBI (www.ncbi.nlm.nih.gov/geo) with accession number GSE18909.
Quantitative real-time PCR (qRT-PCR)
The differential expression of a pair of genes from each set (Tables S1-S4) was verified by quantitative real-time PCR. The DNA-free RNA samples used for the microarray experiments (3 biological replicates) were reverse transcribed in 20 μL reactions containing ~400 ng RNA, 3 μg random hexamers, and 500 μM final dNTPs. Reactions were incubated for 5 min at 65°C, and for 1 min on ice. The first strand buffer, 5 mM DTT, 1 μl RNaseOUT, and 1 μl Superscript III RT, was then added and left for 5 min at room temperature, followed by a 1 hr incubation at 50°C. For the real-time reaction, each primer (250 nM final concentration) and 7.5 μl of template reaction (1:20 dilution) in 25 μl volume with Applied Biosystems Power SYBR Green PCR master mix (Applied Biosystems, Foster City, CA) were used. Samples (8 μl) were loaded in triplicate in 384-well plates and run on an ABI 7900 HT quantitative thermocycler with the following program: 2 min at 50°C, 10 min at 95°C, 40 cycles of 10 sec at 95°C, 20 sec at 60°C, and 30sec at 72°C, followed by a dissociation stage of 15 sec at 95°C, 15 sec at 60°C, and 15 sec at 95°C to check specificity of the products. Threshold cycles were normalized to those for 16S rRNA.
qRT-PCR Primers: ctpF F: CAACACCTTGGCGGTGGTAA; ctpF R: GGCCTCTGCCTTGGATTCTTl; atpD F: GGTGTTCTCCAAGGGCATCT; atpD R: CCTCCGACAACTCGTTCGATA; lat F: ACGAGTTTGATGCACTGCTG; lat R: TAAGATTGCCACCCCATGTCl; rrs F: GCCGTAAACGGTGGGTACTAl; rrs R: TGCATGTCAAACCCAGGTAA; pknD F: CCGTTGATCTCGCCGTAGTCA; pknD R: CCGAGGACACCCGCAAGC; nuoH F: CGCTCAACGGCTTTCGCAACA; nuoH R: GGCGCTGGGCTGGAAGTTATT; katG F: GAAGCCGAACCCGAACGTCTT; katG R: AACAGCTGGCCCGACAAC; hspX F: TCCGCGCGTACCTCGTAG; hspX R: GGCCACCACCCTTCCCGTTCA; esxI F: CGTTGGCCTGCTCGTAGATCA; esxI R: TGTTGACCGCGAGTGAC.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Mei Chen, John Kim, Weijun Liu, and Regy Lukose for technical assistance. We acknowledge support for this work from NIH grants AI43268 and AI026170.
Footnotes
Online supplemental material. Tables S1 to S4 represent the genes up- or down-regulated 2-fold or more by NAm starvation in NAm auxotrophs of M. tuberculosis and M. bovis.
REFERENCES
- Bardarov S, Bardarov S, Jr, Pavelka MS, Jr, Sambandamurthy V, Larsen M, Tufariello J, Chan J, Hatfull G, Jacobs WR., Jr Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148:3007–3017. doi: 10.1099/00221287-148-10-3007. Jr. Jr. Jr. [DOI] [PubMed] [Google Scholar]
- Begley TP, Kinsland C, Mehl RA, Osterman A, Dorrestein P. The biosynthesis of nicotinamide adenine dinucleotides in bacteria. Vitam. Horm. 2001;61:103–119. doi: 10.1016/s0083-6729(01)61003-3. [DOI] [PubMed] [Google Scholar]
- Bekierkunst A, Artman M. Tissue metabolism in infection. DPNase activity, DPN levels, and DPN-linked dehydrogenases in tissues from normal and tuberculous mice. Am. Rev. Respir. Dis. 1962;86:832–838. doi: 10.1164/arrd.1962.86.6.832. [DOI] [PubMed] [Google Scholar]
- Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–731. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE., 3rd The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem. 2004;279:40174–40184. doi: 10.1074/jbc.M406796200. [DOI] [PubMed] [Google Scholar]
- Boshoff HI, Xu X, Tahlan K, Dowd CS, Pethe K, Camacho LR, Park TH, Yun CS, Schnappinger D, Ehrt S, Williams KJ, Barry CE., 3rd Biosynthesis and recycling of nicotinamide cofactors in Mycobacterium tuberculosis. An essential role for NAD in nonreplicating bacilli. J. Biol. Chem. 2008;283:19329–19341. doi: 10.1074/jbc.M800694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SJ, Thibert L, Sanchez T, Heifets L, Zhang Y. pncA mutations as a major mechanism of pyrazinamide resistance in Mycobacterium tuberculosis: spread of a monoresistant strain in Quebec, Canada. Antimicrob Agents Chemother. 2000;44:528–532. doi: 10.1128/aac.44.3.528-532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eker B, Ortmann J, Migliori GB, Sotgiu G, Muetterlein R, Centis R, Hoffmann H, Kirsten D, Schaberg T, Ruesch-Gerdes S, Lange C. Multidrug- and extensively drug-resistant tuberculosis, Germany. Emerg. Infect. Dis. 2008;14:1700–1706. doi: 10.3201/eid1411.080729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW, Moat AG. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol. Rev. 1980;44:83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes SY, Scholle MD, D’Souza M, Bernal A, Baev MV, Farrell M, Kurnasov OV, Daugherty MD, Mseeh F, Polanuyer BM, Campbell JW, Anantha S, Shatalin KY, Chowdhury SA, Fonstein MY, Osterman AL. From genetic footprinting to antimicrobial drug targets: examples in cofactor biosynthetic pathways. J Bacteriol. 2002;184:4555–4572. doi: 10.1128/JB.184.16.4555-4572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholson RK. The pyridine nucleotide cycle. Nature. 1966;212:933–935. doi: 10.1038/212933a0. [DOI] [PubMed] [Google Scholar]
- Gordhan BG, Smith DA, Alderton H, McAdam RA, Bancroft GJ, Mizrahi V. Construction and phenotypic characterization of an auxotrophic mutant of Mycobacterium tuberculosis defective in L-arginine biosynthesis. Infect. Immun. 2002;70:3080–3084. doi: 10.1128/IAI.70.6.3080-3084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange JM. Mycobacterium bovis infection in human beings. Tuberculosis (Edinb) 2001;81:71–77. doi: 10.1054/tube.2000.0263. [DOI] [PubMed] [Google Scholar]
- Hondalus MK, Bardarov S, Russell R, Chan J, Jacobs WR, Jr., Bloom BR. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 2000;68:2888–2898. doi: 10.1128/iai.68.5.2888-2898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Phalen SW, Lagranderie M, Ensergueix D, Chavarot P, Marchal G, McMurray DN, Gicquel B, Guilhot C. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 1999;67:2867–2873. doi: 10.1128/iai.67.6.2867-2873.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasarov LB, Moat AG. Metabolism of nicotinamide adenine dinucleotide in human and bovine strains of Mycobacterium tuberculosis. J. Bacteriol. 1972;110:600–603. doi: 10.1128/jb.110.2.600-603.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JA, Forouhar F, Tao X, Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin Ther Targets. 2007;11:695–705. doi: 10.1517/14728222.11.5.695. [DOI] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Konno K. New chemical method to differentiate human-type tubercle bacilli from other mycobacteria. Science. 1956;124:985. doi: 10.1126/science.124.3229.985. [DOI] [PubMed] [Google Scholar]
- Konno K, Kurzmann R, Bird KT. The metabolism of nicotinic acid in Mycobacteria: a method for differentiating tubercle bacilli of human origin from other Mycobacteria. Am. Rev. Tuberc. 1957;75:529–537. doi: 10.1164/artpd.1957.75.4.529. [DOI] [PubMed] [Google Scholar]
- Konno K, Kurzmann R, Bird KT, Sbarra A. Differentiation of human tubercle bacilli from atypical acid-fast bacilli. II. Clinical application. Am. Rev. Tuberc. 1958;77:675–680. doi: 10.1164/artpd.1958.77.4.675. [DOI] [PubMed] [Google Scholar]
- Konno K, Nagayama H, Oka S. Differentiation of bovine tubercle bacilli from other mycobacteria by the determination of nicotinamidase activity. Am Rev Respir Dis. 1960;81:550–554. doi: 10.1164/arrd.1960.81.4.550. [DOI] [PubMed] [Google Scholar]
- Leonardo MR, Dailly Y, Clark DP. Role of NAD in regulating the adhE gene of Escherichia coli. J. Bacteriol. 1996;178:6013–6018. doi: 10.1128/jb.178.20.6013-6018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw GE, Warren RM, Donald PR, Murray MB, Bosman M, Van Helden PD, Young DB, Victor TC. Frequency and implications of pyrazinamide resistance in managing previously treated tuberculosis patients. Int J Tuberc Lung Dis. 2006;10:802–807. [PubMed] [Google Scholar]
- Magni G, Di Stefano M, Orsomando G, Raffaelli N, Ruggieri S. NAD(P) biosynthesis enzymes as potential targets for selective drug design. Curr Med Chem. 2009;16:1372–1390. doi: 10.2174/092986709787846505. [DOI] [PubMed] [Google Scholar]
- Pavelka MS, Jr., Jacobs WR., Jr. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 1999;181:4780–4789. doi: 10.1128/jb.181.16.4780-4789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope H, Smith DT. Synthesis of B-complex vitamins by tubercle bacilli when grown in synthetic media. Amer. Rev. Tuberc. 1946;54:559–563. doi: 10.1164/art.1946.54.6.559. [DOI] [PubMed] [Google Scholar]
- Prunier AL, Schuch R, Fernandez RE, Mumy KL, Kohler H, McCormick BA, Maurelli AT. nadA and nadB of Shigella flexneri 5a are antivirulence loci responsible for the synthesis of quinolinate, a small molecule inhibitor of Shigella pathogenicity. Microbiology. 2007;153:2363–2372. doi: 10.1099/mic.0.2007/006916-0. [DOI] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Raynaud C, Laneelle MA, Senaratne RH, Draper P, Laneelle G, Daffe M. Mechanisms of pyrazinamide resistance in mycobacteria: importance of lack of uptake in addition to lack of pyrazinamidase activity. Microbiology. 1999;145:1359–1367. doi: 10.1099/13500872-145-6-1359. [DOI] [PubMed] [Google Scholar]
- San KY, Bennett GN, Berrios-Rivera SJ, Vadali RV, Yang YT, Horton E, Rudolph FB, Sariyar B, Blackwood K. Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab. Eng. 2002;4:182–192. doi: 10.1006/mben.2001.0220. [DOI] [PubMed] [Google Scholar]
- Sauve A. NAD+ and vitamin B3: from metabolism to therapies. J. Pharmacol. Exp. Ther. 2008;324:883–893. doi: 10.1124/jpet.107.120758. [DOI] [PubMed] [Google Scholar]
- Scorpio A, Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 1996;2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- Sestini S, Jacomelli G, Pescaglini M, Micheli V, Pompucci G. Enzyme activities leading to NAD synthesis in human lymphocytes. Arch Biochem Biophys. 2000;379:277–282. doi: 10.1006/abbi.2000.1888. [DOI] [PubMed] [Google Scholar]
- Singh P, Mishra AK, Malonia SK, Chauhan DS, Sharma VD, Venkatesan K, Katoch VM. The paradox of pyrazinamide: an update on the molecular mechanisms of pyrazinamide resistance in Mycobacteria. J Commun Dis. 2006;38:288–298. [PubMed] [Google Scholar]
- Smith DA, Parish T, Stoker NG, Bancroft GJ. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect. Immun. 2001;69:1142–1150. doi: 10.1128/IAI.69.2.1142-1150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci L, Pan Y, Eyobo Y, Rodionova I, Huang N, Kurnasov O, Zhong S, MacKerell AD, Jr., Zhang H, Osterman AL. Targeting NAD biosynthesis in bacterial pathogens: Structure-based development of inhibitors of nicotinate mononucleotide adenylyltransferase NadD. Chem Biol. 2009;16:849–861. doi: 10.1016/j.chembiol.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, de la Cruz VF, Fuerst TR, Burlein JE, Benson LA, Bennett LT, Bansal GP, Young JF, Lee MH, Hatfull GF, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Velu SE, Cristofoli WA, Garcia GJ, Brouillette CG, Pierson MC, Luan CH, DeLucas LJ, Brouillette WJ. Tethered dimers as NAD synthetase inhibitors with antibacterial activity. J Med Chem. 2003;46:3371–3381. doi: 10.1021/jm030003x. [DOI] [PubMed] [Google Scholar]
- Velu SE, Mou L, Luan CH, Yang ZW, DeLucas LJ, Brouillette CG, Brouillette WJ. Antibacterial nicotinamide adenine dinucleotide synthetase inhibitors: amide- and ether-linked tethered dimers with alpha-amino acid end groups. J Med Chem. 2007;50:2612–2621. doi: 10.1021/jm061349l. [DOI] [PubMed] [Google Scholar]
- Windman I, Bekierkunst A. Oxidized and reduced forms of nicotinamide nucleotides in livers of tuberculous mice. Am. Rev. Respir. Dis. 1967;96:296–298. doi: 10.1164/arrd.1967.96.2.296. [DOI] [PubMed] [Google Scholar]
- Yang T, Sauve AA. NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 2006;8:E632–643. doi: 10.1208/aapsj080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deng JY, Bi LJ, Zhou YF, Zhang ZP, Zhang CG, Zhang Y, Zhang XE. Characterization of Mycobacterium tuberculosis nicotinamidase/pyrazinamidase. FEBS J. 2008;275:753–762. doi: 10.1111/j.1742-4658.2007.06241.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.