Abstract
Objective
Schizophrenia affects men more than women, but this may not be true at all ages. This study examines the incidence of first hospitalization for treatment of schizophrenia in each sex over different ages.
Methods
We compared the incidence of first admission for treatment in a cohort a cohort of 46,388 males and 43,680 females followed from birth until ages 29-41, using life tables and proportional hazards methods.
Results
Life table estimates of cumulative incidence by age 40 were 1.44% in males) and .86% in females. Over all ages the relative risk (RR) in males was 1.6 (95% confidence limits=1.4-1.8) compared with females. Before age 17 there was no significant difference between the sexes (RR=.86, .56-1.3). Excess risk in males was observed only from age 17 (RR=1.7, 1.4-1.9). There was no evidence of the incidence in females catching up with that in males, during the 30s.
Conclusion
In this population, there was a significant change, over age, in the relative incidence of first hospitalization for schizophrenia between the sexes; the excess incidence in males first developed at age 17.
Keywords: schizophrenia, sex, age factors, cohort
Objectives of the study
Analyze data from a large birth cohort to determine the incidence of first treatment of schizophrenia in each sex over different ages in a population. Use prospectively collected data to provide the first description, to our knowledge, of sex-specific incidence in patients younger than age 17.
Background
Men, overall, have a higher incidence of schizophrenia (Jablensky et al., 1992; Kirkbride et al., 2006; McGrath et al., 2004; Thorup, Waltoft, Pedersen, Mortensen, & Nordentoft, 2007). A recent systematic review of over 100 studies from around the world reported the male to female rate ratio for the incidence of schizophrenia to be 1.4 (McGrath, Saha, Chant, & Welham, 2008). However, there has been some suggestion that the ratio of male to female incidence might vary across the lifespan. Men are thought to have an earlier age of onset than women (Hafner, 2003; Leung & Chue, 2000), but there is disagreement as to whether childhood onset schizophrenia is equally common in the two sexes (Kyriakopoulos & Frangou, 2007). Various age limits have been studied for childhood onset of schizophrenia (Leung et al., 2000; Usall, Haro, Ochoa, Marquez, & Araya, 2002) making it difficult to identify which cutoff is meaningful and which should be used in research. There is little if any population-based prospectively collected data about male and female incidence of schizophrenia in childhood or early adolescence. Some researchers have found that in late middle age the incidence in women catches up to that in men (Hafner, 2003), and that women experience a second peak of onset at that time (Leung et al., 2000).
Reported differences in incidence and age of onset between the sexes (Leung et al., 2000), together with differences in symptomatology (Grossman, Harrow, Rosen, & Faull, 2006; Harlap et al., 2007; Leung et al., 2000), hint that men and women may have different variants of schizophrenia. A better understanding of how the incidence of schizophrenia varies over the life span in each sex might help with identification of possible sex-specific subtypes, risk factors or responses to treatments. We analyzed data from a large birth cohort to determine the incidence of first hospitalization for schizophrenia in each sex over different ages in that population.
Methods
Subjects
We used data from the Jerusalem Perinatal Study, a population-based cohort derived from all 92,408 births in 1964–76 to mothers resident in western Jerusalem. The cohort includes linkages within nuclear families and a 29–41 year follow-up as of December 31, 2004. Core information from the notification of birth was supplemented with other data from multiple sources including maternal interviews. The offspring, mothers and fathers have been traced recently, and their vital status assessed; the cohort was linked with Israel's population registry to trace and verify identity (ID) numbers, dates of birth and basic demographic characteristics and to ascertain vital status and dates of death. These data were then linked to Israel's Psychiatric Registry (Harlap et al., 2007).
For information on psychiatric morbidity we relied on Israel's Psychiatric Case Registry. Run by the Ministry of Health since 1950 (Lichtenberg, Kaplan, Grinshpoon, Feldman, & Nahon, 1999), it contains a record of all admissions to psychiatric hospitals or psychiatric wards within general hospitals, as well as admissions to day-facilities for psychiatric treatment; it includes dates of admission and discharge and a single discharge diagnosis for each episode, assigned by a board-certified psychiatrist. These diagnoses are coded with the International Classification of Diseases (ICD); codes from earlier years have been update to 10th Revision and those for psychotic disorders have been validated recently (Weiser et al., 2005).
Personnel at the Ministry of Health matched the files from the Jerusalem cohort to the Psychiatric Registry, using only the ID number. By substituting false ID numbers and removing all other identifying data they created an anonymous file containing a record (if any) of each hospital event in offspring and their parents. The research was approved by institutional review boards at Israel's Ministries of the Interior and Health, Hebrew University of Jerusalem, and New York University Medical Center and certified as exempt from the requirement for informed consent.
Data analysis
We used SAS 9.1 (SAS Institute Inc, Cary, North Carolina) to analyze the data. We assigned a diagnosis of schizophrenia to any person with at least one hospital episode with a discharge diagnosis of any of the schizophrenia spectrum disorders, coded F20-29 in the International Classification of Diseases-10th Revision (ICD-10). We also applied a more stringent definition restricted to those with at least one episode of schizophrenia, coded in the ICD-10 as F20. Cases were reviewed by the first author (KK) and a senior research psychiatrist (DM); nine were excluded who suffered a single episode coded ICD-10=F20-F29 but also showed numerous episodes with mood disorders. The date of onset was taken as the first episode in the psychiatric registry, regardless of the discharge diagnosis assigned to that episode. Both diagnoses and sex (male) were coded as 1 (if present) or 0 (absent).
Tabulations of annual incidence rates of first hospitalization based on person-years, life tables plots and Cox proportional hazards methods were used to compare males and females. Time to event was handled as completed years since birth, i.e. age, until the first hospital admission or death; survivors were censored on 31 December 2004. Time to first hospitalization was used as a proxy for onset of disease because Israel has a very high rate of hospital admission for mental health conditions (Siegel et al., 1993), and because a large study showed that in Israel approximately 90% of those meeting Research Diagnostic Criteria for schizophrenia had been hospitalized for that disorder (Levav et al., 1993). Ties were handled by Efron's method. Because the proportional hazards assumptions were not met, we developed models that incorporated time, using the product of sex (male) and time, either as a continuous variable or as a dichotomy before or after a hypothetical cut-off point. Results are presented as Relative Risks (RR), i.e. Hazard Ratios, with 95% confidence intervals. To take into account correlation between siblings, we used a robust sandwich estimate of the covariance matrix (Liang, 1989), analyzing the subset of 85,393 offspring with traced mothers and fathers.
Results
There were 90,068 offspring available for this study from the original cohort of 92,408 after excluding 949 (1.0%) stillbirths, 1,380 (1.5%) whose ID numbers could not be traced in the Population Registry and 11 (0.001%) with unknown sex. Over the 29-41 years between the births in 1964-76 and the cutoff date (December 31, 2004), there were 2,917,830 million person years observed. Linkage with the Psychiatric Registry identified 860 people admitted to psychiatric hospitals at least once before the cutoff date, and diagnosed with schizophrenia spectrum disorders (ICD-10=F20-F29); of these, 568 included admissions coded with the narrow definition (F20) and 292 included F21-F29 only.
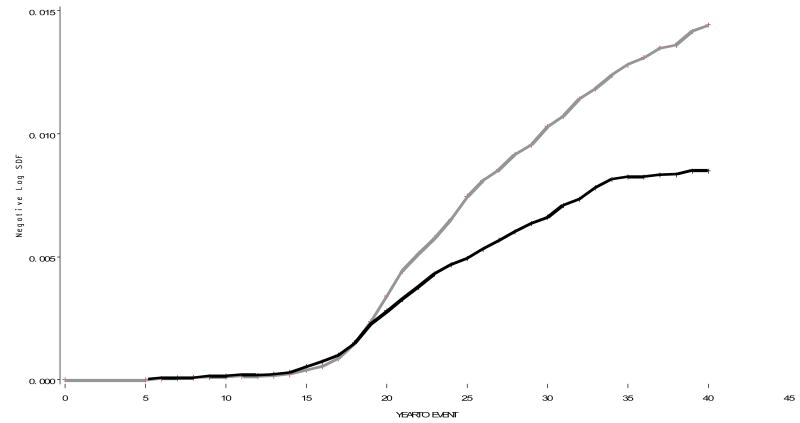
Table 1 shows the numbers of cases, the population at risk and the average annual incidence rates of first admission for psychiatric treatment in five year age groups. With the sexes combined, the incidence rose steadily through childhood and adolescence, reaching a maximum at age 20-24 and declining thereafter. Comparing the sexes, there was a slightly higher incidence in females at ages 5-9 and 10-14; thereafter there was a marked excess in males. Figure 1 compares the life-table cumulative incidence in males and females year by year (i.e. by age); it suggests that there was little difference between the sexes until the mid-teens, whereas from the 20s on there was an escalating excess cumulative incidence in males. The life table estimates of cumulative incidence before ages 15, 20, 25, 30, 35 and 40 were .011, .038, .340, .744, 1.04, and 1.29 % in males, and .014, .054, .279, .493, .672 and .841 % in females. With the sexes combined, corresponding cumulative life table estimates of incidence of first psychiatric hospitalization were .013, .046, .310, .622, .861 and 1.07 %.
Table 1. Numbers of person years observed, cases of schizophrenia spectrum disorders, and average annual incidence of first admission for treatment, by age and sex.
| Age | 0-4 | 5-9 | 10-14 | 15-19 | 20-24 | 25-29 | 30-34 | 35-39 | |
|---|---|---|---|---|---|---|---|---|---|
| Person-years observed | |||||||||
| Males | 227431 | 225757 | 225440 | 224849 | 223061 | 221552 | 220488 | 219979 | |
| Females | 214879 | 213588 | 213354 | 212925 | 212026 | 211300 | 210701 | 210459 | |
| Total | 442310 | 439345 | 438794 | 437774 | 435087 | 432852 | 431189 | 430438 | |
| Schizophrenia cases | |||||||||
| Males | 0 | 5 | 12 | 136 | 181 | 120 | 66 | 16 | |
| Females | 0 | 6 | 17 | 96 | 91 | 69 | 43 | 2 | |
| Total | 0 | 11 | 29 | 232 | 272 | 189 | 109 | 18 | |
| Incidence/100,000 | |||||||||
| Males | 0 | 2.2 | 5.3 | 60.6 | 81.2 | 59.9 | 49.8 | 32.0 | |
| Females | 0 | 2.8 | 8.0 | 45.1 | 42.9 | 36.1 | 33.9 | 4.2 | |
| Total | 0 | 2.5 | 6.6 | 53.0 | 62.5 | 48.3 | 42.0 | 18.4 | |
Figure 1. The life table estimates of cumulative incidence of first hospitalization for schizophrenia by five year age category.
X axis: incidence. Y axis: Year to event. Males=Grey, Females=Black
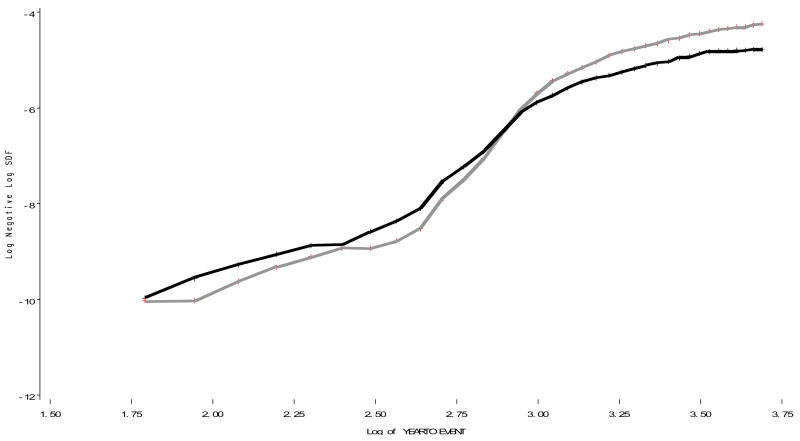
Figure 2 presents the same data as in figure 1 but uses log scales for both age (the x-axis) and incidence (y-axis); this plot reveals the cross-over point reversing the sex-specific cumulative incidences to be at ln (age) ∼2.8 corresponding to age 16-17. Figures 1 and 2 both suggest that the males continue to hold their excess incidence into the late 30s.
Figure 2. The life table estimates of cumulative incidence of first hospitalization for schizophrenia by five year age category.
graphed on natural log scale. Males=Grey, Females=Black
Next, we used Cox proportional hazards models to explore further the sex differences and their change over the time variable, i.e. age. Table 2 compares the relative risk (RR) estimates for males versus females under different models and reports the overall Wald Chi squared value, an indicator of the overall goodness-of-fit of the model. Model 1 ignores any change with age and estimates a 57% excess incidence of first admission in males compared with females. Model 2 confirms a change over time in this overall male excess. In model 3, effects of sex are estimated in two segments as suggested by the plot shown in figure 2: ages up to and including 16 and 17+. This model shows a highly significant 68% excess incidence in males from age 17 and no difference at younger ages. The confidence limits for the two estimates in model 3 show no overlap, indicating that the differing relationships of sex to schizophrenia before and after age 17 are unlikely to be due to chance. Model 4 explores whether, beyond age 17, there is any further divergence or convergence between males and females; the results suggest a slight divergence, but it is trivial. Comparing the overall Wald chi squares, there is no significant advantage between models 2 or 3; however, either one describes the data significantly better than model 1. We also repeated the models using the narrower definition of schizophrenia [ICD-10 code F20]. Relative risk and 95% confidence interval for model 1, using the narrow definition, were 1.62 [95% CI 1.37-1.92]. For model 3, males less than 17 years old had a relative risk of .87 [95% CI .54-1.40] and males 17 and older showed a relative risk of 1.77 [95% CI [1.48-2.13].
Table 2. Relative risk (RR) and 95% confidence interval (CI) for schizophrenia in males versus females in four different proportional hazards models with varied assumptions about changes with age.
| Model | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Male | RR 95% CI p |
1.57 1.37-1.81 P<.0001 |
.72 .41-1.28 .3 |
- | - |
| Male * age (continuous) | RR 95% CI p |
- | 1.04 1.01-1.06 .006 |
- | - |
| Male <16 years old | RR 95%CI p |
- | - |
0.86 0.56-1.32 .5 |
- |
| Male 17+ years old | RR 95% CI P |
- | - |
1.68 1.45-1.95 <.0001 |
1.04 .51-.2.1 .8 |
| Male * age (continuous from age 17) | RR 95%CI p |
- | - | - | 1.02 .99-1.05 .22 |
| Overall Wald X2 for each model d.f. | 41.5 1 |
47.7 2 |
49.1 2 |
50.0 2 |
|
We performed additional analyses in model 3 to determine the most appropriate age for the cutoff point. For cutoff at ages 15, 16, 17 and 18 the overall Wald X2 values were 47.9, 48.7, 49.1 and 48.8. We further explored model 3 incorporating other variables describing the incidence of schizophrenia, i.e. paternal age, low social class, year of birth and family history (data not shown); the relative risks and 95% confidence intervals were virtually identical to those shown in table 2. Finally, we explored the effect of taking into account correlation between siblings as described in the methods section, comparing model 3 with or without a robust sandwich estimate of the covariance matrix and analyzing the cohort of 88,237 offspring clustered in 41,056 families with traced and verified mothers. For the RR of 0.88 associated with male sex at ages up to 17, use of the sandwich estimate gave a 95% CI (0.562-1.372) and for the RR of 1.66 associated with male sex from age 17 the 95% CI was (1.425-1.924). Thus, even after taking into account family clustering, the differing effects of sex before and after age 17 are unlikely to be due to chance.
Discussion
This study bears out the well-known observation that generally males have a higher incidence of schizophrenia than females (Leung et al., 2000; McGrath et al., 2004); (Kirkbride et al., 2006); (Jablensky et al., 1992). In our population, however, this excess incidence in males, approximated by first admission for treatment, appears only from age 17, whereas at younger ages there is no significant difference between the sexes.
Furthermore, this lack of variation between males and females before age 17 is unlikely to be due to chance, since the confidence limits for the estimates before and after age 17 do not overlap. We are unaware of any previous population-based study that has presented age- and sex-specific estimates of incidence from birth through middle age. The Aetiology and Ethnicity in Schizophrenia and Other Psychoses (AESOP) study reported age-specific incidence rates of schizophrenia and other selected psychoses by sex, but measured incidence starting at age 16 (Kirkbride et al., 2006). Bresnahan et al. reported on the risk for schizophrenia in males and females using data from the Prenatal Determinants of Schizophrenia study. This cohort had 43 identified cases of schizophrenia; of these 6 males and 1 female were diagnosed at ages 15-19 years old. No cases were diagnosed at a younger age (Bresnahan et al., 2000) and with only one female case diagnosed before age 19, that study cannot be compared with ours.
When we ignored changes over age we estimated that in our population, over all ages combined, the relative risk (RR) in males was 1.6 (95% confidence limits=1.4-1.8), compared with females; this was consistent with the findings in two recent meta-analyses (McGrath et al., 2004); (Aleman, Kahn, & Selten, 2003). Neither of these meta-analyses considered changes in sex-specific incidence over different ages (Aleman et al., 2003; McGrath et al., 2004).
It has been suggested that the sex difference in incidence of schizophrenia follows differences in neurodevelopment in the sexes. Imaging provides evidence that brain development differs in boys and girls. Neuroimaging has shown sex differences in adolescent maturation of the brain (Lenroot et al., 2007). The male brain is larger than the female brain across all the stages of development, and certain structures within the brain differ in size in males and females (Lenroot et al., 2007), (Giedd, Castellanos, Rajapakse, Vaituzis, & Rapoport, 1997). These differences might potentially influence outcomes at any point along the lifespan.
Numerous other sex differences have been observed in schizophrenia. Men are reported to have more negative symptoms than females and worse social and cognitive functioning (Leung et al., 2000). Males are less successful in terms of independent living, self-care, active employment, and social function, and have more and longer re-hospitalizations (Grossman et al., 2006; Leung et al., 2000; Usall et al., 2002). Females tend to experience more affective symptoms, and demonstrate greater response to antipsychotic medications (Leung et al., 2000); (Usall et al., 2002). Men are thought to have an earlier age of onset than women (Leung et al., 2000) though this may only hold true in sporadic cases (Leung et al., 2000). Males and females may also have different risks for childhood onset schizophrenia, though the literature is divided on this (Kyriakopoulos et al., 2007). These differences have led researchers to question whether males and females might suffer from different variants of schizophrenia (Leung et al., 2000). Difference incidence rates in the sexes could support that hypothesis. Similarly, childhood onset schizophrenia may be a variant of the disease, and could potentially have a different sex ratio for incidence. Various cutoffs have been used in analyses of early onset schizophrenia, including onsets before age 13 (Kumra, Shaw, Merka, Nakayama, & Augustin, 2001), 15 (Leung et al., 2000), 17 (Frangou, Hadjulis, & Vourdas, 2008) or 18 (Leung et al., 2000). In our population the ratio of incidence in the sexes changes at age 17, suggesting that there is a change in the disease process at that point. Analyses of other population-based data might also help identify specific ages where there is a change in the ratio of incidences. This information could forward the study of the early onset variant of the disease in a specific population.
It is reported that the majority of new onset schizophrenia occurs before age 30 although there is a second peak after age 40 (Rajji, Ismail, & Mulsant, 2009). Some researchers have observed that in late middle age the incidence of schizophrenia in women increases and catches up to the incidence in men (Leung et al., 2000). The ABC schizophrenia study reported that rate of onset of first psychotic symptoms in women began to exceed that of men at around age 30, while in an analysis of population based first admissions for schizophrenia in Denmark, in 1976, the rate in women surpassed that of men at age 50 (Hafner, Hambrecht, Loffler, Munk-Jergensen & Riecher, 1998). A meta-analysis of studies reporting sex differences in age of onset of schizophrenia showed that overall, in studies with an age cutoff greater than or equal to 64 years, the relative risk in males versus females remained higher. The Jerusalem cohort data on offspring is truncated in the late thirties, so that differential lifetime incidence cannot be evaluated. Our study includes too few cases to address this question with certainty beyond ages 35+; the cohort will have to age before this question can be addressed. Nonetheless, the focus of the current paper is on incidence in younger people and the cohort has passed through that age of risk.
Previous studies of the incidence of schizophrenia have used different operational definitions of schizophrenia; the broader characterization commonly incorporates schizophrenia spectrum disorders while the narrower includes only ICD-10 code F20. Our study found essentially the same ratio of incidence rates in males and females whether the analysis considered the broad or the narrower diagnosis of schizophrenia.
The Jerusalem cohort's exceptional strengths allowed us to use survival analysis to measure the incidence and risk from the youngest ages in both males and females, enabling us to detect changes in the ratio of male to female risk over much of the lifespan. Ninety-eight percent of offspring in the Jerusalem cohort were traced through Israel's national Population Registry and have verified identities. Strengths of the data derive not only from the cohort's size and its being population-based but also from Israel's egalitarian health system. All citizens are insured and admission to psychiatric hospitals is free of charge, as is therapy for schizophrenia. This removes an important barrier to admission to hospital for schizophrenia, and probably contributes to the fact that in Israel almost all patients with psychosis are admitted for care and their data are therefore recorded in the national Psychiatric Case Registry (Levav et al., 1993). Furthermore, Israel has a very high rate of hospital admission for mental health conditions. A prevalence study comparing mental health services in New York State and Israel found a much lower rate of non-residential care in Israel (Siegel C et al, 1993), further insuring that most individuals with psychosis would be admitted to hospital and recorded into the registry.
GeddesJR and Kendell RE used a registry to identify all subjects diagnosed with schizophrenia in Edinburgh, Scotland from 1978-1989. They found that those who were never admitted to hospital had the same male to female ratio as those who were (odds ratio 0.73, 95% confidence interval (0.34-1.55) p=.38). A mean of 5.3%, or 6.7% after adjustment for possible misclassification, of patients diagnosed as suffering from schizophrenia each year were not admitted, and there was no secular trend for this proportion over the years in the study. They concluded that first admission rates for schizophrenia in Scotland are a reasonable approximation of incidence rates (Geddes & Kendell, 1995). Geddes et al.'s findings may be reflected in the Jerusalem cohort, whose members enjoyed a national health system similar to Scotland's. Offspring in the Jerusalem cohort were born in West Jerusalem, and as a result 96.7 of the mothers whose offspring were included in the cohort were Jewish. Of these 15.8% had maternal grandfathers born in Israel, and 29.2% had maternal grandfathers born in Western Asia including the countries of Turkey, Syria, Lebanon, Iraq, Yemen, Iran, as well in North African countries. 32.5% of the cohort was descended from the maternal grandfathers born in Europe and the Americas. Delay or avoidance of treatment, or misdiagnosis, could be biased by ethnic background. However, adjustment for birthplace of maternal grandfather and for socioeconomic status did not change the findings in this study.
Our study faced several limitations. Our data are for admissions for treatment; we could not ascertain whether men and women were equally likely to be admitted when presenting with similar symptoms, or if males presented with types of symptoms that made them more likely to be admitted although they had the same incidence as women, or if these factors change over different ages in each sex. The excess of males hospitalized in mid-adolescence could plausibly reflect a tendency to be referred for more intensive treatment earlier because of behavioral disturbances, whereas girls' psychosis might escape attention for longer, and this could also be the case for sex differences in first admission during early adulthood. With our data we could not determine whether ascertainment and diagnosis of schizophrenia in childhood and adulthood varied, or varied across sexes. Furthermore, there might be a sex difference in length of time to hospitalization after onset of diagnosis. There is little published data on possible sex differences in diagnosis or admission to hospital for children with schizophrenia. Weiser et al.'s study of the psychotic diagnoses in Israel's Psychiatric Case Registry did not specifically address cases in childhood (Weiser et al., 2005), and there is no way to verify whether there is any sex difference in the validity of cases diagnosed in childhood. On the other hand, there is no reason to expect sex would introduce a bias in data on children; one might even expect more males in younger ages because of behavioral disturbances. Another limitation that must be acknowledged is that the only cross-validation data of registry diagnoses show that schizophrenia patients entering biological research studies were found to have concordant diagnoses in the registry, but the broad range of patients with schizophrenia diagnoses in the registry have not been subjected to a validation study. Therefore the findings from Weiser et al.'s validation study are not directly applicable here. It remains possible that increased diagnostic error during adolescence contributes to the findings.
Sex differences in the incidence of schizophrenia over different ages do not have clinical implications at this time. However, these findings suggest that there may be different variants of disease, whether for each sex or for earlier onset cases. Detection of heterogeneity within schizophrenia should advance the study of the etiology, risk factors or response to specific treatments in different segments of the population. Studies in other cohorts are needed to determine if there is a shift from equal incidence in the sexes to male excess occurring sometime in mid-adolescence.
Acknowledgments
We would like to thank the participants in the Jerusalem Study. Supported by grants from the National Institutes of Health: K08 MH085807 (KK); 1R01 MH059114 (DM); 2 K24 MH001699 (DM); 2R01 CA080197 (SH); and from the National Alliance for Research of Schizophrenia and Depression (NARSAD) (DM, SH, MP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–71. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Bresnahan MA, Brown AS, Schaefer CA, Begg MD, Wyatt RJ, Susser ES. Incidence and cumulative risk of treated schizophrenia in the prenatal determinants of schizophrenia study. Schizophr Bull. 2000;26:297–308. doi: 10.1093/oxfordjournals.schbul.a033454. [DOI] [PubMed] [Google Scholar]
- Frangou S, Hadjulis M, Vourdas A. The Maudsley early onset schizophrenia study: cognitive function over a 4-year follow-up period. Schizophr Bull. 2008;34:52–9. doi: 10.1093/schbul/sbm124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JR, Kendell RE. Schizophrenic subjects with no history of admission to hospital. Psychol Med. 1995;25:859–68. doi: 10.1017/s003329170003511x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Grossman LS, Harrow M, Rosen C, Faull R. Sex differences in outcome and recovery for schizophrenia and other psychotic and nonpsychotic disorders. Psychiatr Serv. 2006;57:844–50. doi: 10.1176/ps.2006.57.6.844. [DOI] [PubMed] [Google Scholar]
- Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28(Suppl 2):17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- Harlap S, Davies AM, Deutsch L, Calderon-Margalit R, Manor O, Paltiel O, Tiram E, Yanetz R, Perrin MC, Terry MB, Malaspina D, Friedlander Y. The Jerusalem Perinatal Study cohort, 1964-2005: methods and a review of the main results. Paediatr Perinat Epidemiol. 2007;21:256–73. doi: 10.1111/j.1365-3016.2007.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, Day R, Bertelsen A. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- Kirkbride JB, Fearon P, Morgan C, Dazzan P, Morgan K, Tarrant J, Lloyd T, Holloway J, Hutchinson G, Leff JP, Mallett RM, Harrison GL, Murray RM, Jones PB. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AeSOP study. Arch Gen Psychiatry. 2006;63:250–8. doi: 10.1001/archpsyc.63.3.250. [DOI] [PubMed] [Google Scholar]
- Kumra S, Shaw M, Merka P, Nakayama E, Augustin R. Childhood-onset schizophrenia: research update. Can J Psychiatry. 2001;46:923–30. doi: 10.1177/070674370104601004. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Frangou S. Pathophysiology of early onset schizophrenia. Int Rev Psychiatry. 2007;19:315–24. doi: 10.1080/09540260701486258. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, Thompson PM, Giedd JN. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–73. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Levav I, Kohn R, Dohrenwend BP, Shrout PE, Skodol AE, Schwartz S, Link BG, Naveh G. An epidemiological study of mental disorders in a 10-year cohort of young adults in Israel. Psychol Med. 1993;23:691–707. doi: 10.1017/s0033291700025472. [DOI] [PubMed] [Google Scholar]
- Liang DY, W LJ. The Robust Inference for the Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074–1078. [Google Scholar]
- Lichtenberg P, Kaplan Z, Grinshpoon A, Feldman D, Nahon D. The goals and limitations of Israel's psychiatric case register. Psychiatr Serv. 1999;50:1043–8. doi: 10.1176/ps.50.8.1043. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:286–93. doi: 10.1192/bjp.bp.108.060723. [DOI] [PubMed] [Google Scholar]
- Siegel C, Handelsman M, Haugland G, Popper M, Jouchovitzky T, Katz S. A comparison of the mental health systems of New York State and Israel. Isr J Psychiatry Relat Sci. 1993;30:130–41. [PubMed] [Google Scholar]
- Thorup A, Waltoft BL, Pedersen CB, Mortensen PB, Nordentoft M. Young males have a higher risk of developing schizophrenia: a Danish register study. Psychol Med. 2007;37:479–84. doi: 10.1017/S0033291707009944. [DOI] [PubMed] [Google Scholar]
- Usall J, Haro JM, Ochoa S, Marquez M, Araya S. Influence of gender on social outcome in schizophrenia. Acta Psychiatr Scand. 2002;106:337–42. doi: 10.1034/j.1600-0447.2002.01351.x. [DOI] [PubMed] [Google Scholar]
- Weiser M, Kanyas K, Malaspina D, Harvey PD, Glick I, Goetz D, Karni O, Yakir A, Turetsky N, Fennig S, Nahon D, Lerer B, Davidson M. Sensitivity of ICD-10 diagnosis of psychotic disorders in the Israeli National Hospitalization Registry compared with RDC diagnoses based on SADS-L. Compr Psychiatry. 2005;46:38–42. doi: 10.1016/j.comppsych.2004.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]