Abstract
Background
Declines in hearing acuity, especially in the high frequency range, often appear in middle age. The objective of this study was to characterize genetic and environmental influences that may underlie this pattern.
Methods
One hundred seventy-nine monozygotic and 150 dizygotic twin pairs, ranging in age from 52 to 60 years, were selected from the Vietnam Era Twin Registry and individually tested for hearing acuity in the frequency range from 500 to 8000 Hz. Biometrical modeling was used to quantify genetic and environmental influences.
Results
For individuals’ better ears, approximately two-thirds (65%–70%, 95% confidence interval [CI], 46%–75%) of the variance in hearing acuity in the middle and high frequency ranges could be accounted for by genetic factors. For the individuals’ poorer ear, which would be expected to show lower heritability, approximately one-half (41%–54%, 95% CI, 11%–67%) of the variance in the middle and high frequency ranges could be accounted for by genetic influences. Within a given frequency range, the same genetic factors influenced both the better and poorer ears. In contrast, although there was some overlap of genetic influences on the middle and high frequencies within a given ear, there were also some genetic influences that were specific to each frequency.
Conclusions
Results suggest that genetic effects play an important role in the level of hearing loss that often appears in late middle age. These data have important implications for identifying persons who may be especially vulnerable to environmental risk factors such as noise exposure and medications with ototoxic properties.
Among the changes associated with adult aging is a decline in hearing acuity (presbycusis), especially for the higher sound frequencies (1,2). Within this general age trend, however, the presence and degree of hearing loss can be quite variable from one individual to another (1). In addition to environmental factors such as disease, exposure to noise and ototoxic medication are presumed genetic factors that may affect biological decline in the auditory system and/or susceptibility to effects of environmental insult (3,4). A direct approach to the heritability question is to examine similarity in hearing acuity for adult mono-zygotic (MZ) versus dizygotic (DZ) twin pairs. The difference in similarity between MZ and DZ twin pairs’ acuity enables the estimation of genetic as well as environmental influence. Two past studies with adult twin pairs, one conducted on twin pairs aged 70 to those in their 90s using subjective reports of hearing acuity (5), and one conducted on twin pairs aged 36–80 years using audiometry (6), have demonstrated a statistically significant heritable component to hearing acuity in middle age and older adulthood. The use of objective audiometry controls for a tendency among individuals to underreport hearing loss (7).
In the present study, which was part of the Vietnam Era Twin Study of Aging (VETSA), we used bivariate twin models to examine hearing acuity across the speech frequency range in late middle-aged male twin pairs. One of our interests was to examine genetic influence on acuity for the middle versus the high frequency ranges. The higher frequencies are especially important for the perception of speech, as well as being more sensitive to age-associated hearing decline (1). A second interest was to examine the acuity in the better ear versus the poorer ear. This interest was motivated by the argument that genetic influences should affect both ears equally, with any acuity difference for the poorer ear being likely to reflect environmental contributions added to the genetic effect. Therefore, twin similarity for acuity in the better ear should yield higher heritability estimates than in the poorer ear (4).
Method
The study sample was composed of 179 MZ twin pairs and 150 DZ twin pairs from the Vietnam Era Twin Registry, a sample of male-male twin pairs born between 1939 and 1957 who had both served in the United States military during the Vietnam era (1965–1975) (8,9). Zygosity was determined by a combination of questionnaire and blood group methods (10) (an approach that has been demonstrated to be 95% accurate as measured against DNA analysis) (11,12).
All participants were in their 50s at the time of recruitment. When tested, the MZ twin pairs ranged in age from 52 to 60 (mean = 55.1 years, standard deviation [SD] = 2.3) and the DZ twin-pairs ranged in age from 52 to 59 (mean = 55.3 years, SD = 2.2). Participants were given the option of traveling to Boston University or to the University of California, San Diego, for a day-long series of physical and cognitive assessments as part of the VETSA program.
Beyond basic weapons training, 28.1% of the 658 participants reported some form of combat exposure, with approximately equal proportions in the MZ and DZ groups. The MZ and DZ twin groups also did not differ significantly (using a p < .05 criterion) on age at enlistment, years of formal education, age at time of testing, or on self-reported health variables that included smoking history, hypertension, incidence of diabetes, or history of stroke or other major illness.
Audiometric Assessment
All participants were tested for pure-tone hearing acuity using a Maico M41 Audiometer (Maico Diagnostics, Eden Prairie, MN) at frequencies of 500, 1000, 2000, 4000, and 8000 Hz using standard audiometric procedures (13,14). The lowest sound level detectable at a particular frequency by a person with normal hearing is referenced at 0 dB hearing level (HL), with acuity data plotted as an audiogram showing the loudness level in dB HL necessary for a pure tone to be heard at each of the tested frequencies. Normal hearing is conventionally defined as the ability to hear the tone at, or less than, a loudness of 15 dB HL (14).
Statistical Analysis
Descriptive statistics were calculated using the statistical package SPSS (SPSS, Inc., Chicago, IL), as were tests of potential group differences in mean level. Biometrical twin modeling was conducted utilizing the maximum-likelihood-based structural equation modeling software Mx (15) for the pure tone averages (PTAs) of the middle frequencies (500, 1000, 2000 Hz) and the higher frequencies (4000, 8000 Hz). In order to estimate the genetic and environmental influences on the measures of audiological function, bivariate Cholesky decomposition models were fit to the audiometric data for the two twin groups (16). Within the twin design, the total variance of any trait is accounted for by latent factors representing additive genetic effects (A), shared environmental effects (C), and nonshared environmental effects (E) (16). The Cholesky model estimates the additive genetic, shared environmental, and nonshared environmental covariances between different traits. These covariance parameters can then be used to calculate the correlations between genetic factors (genetic correlations), between shared environmental factors (shared environmental correlations), and between nonshared environmental factors (nonshared environmental correlations).
Four bivariate analyses were conducted examining middle frequency PTAs for the better and poorer ears, high frequency PTAs for the better and poorer ears, the relationship of middle and high frequency performance for the better ear, and the relationship of middle and high frequency performance for the poorer ear. The goodness-of-fit of these nested models was evaluated using a difference chi-square test. The difference in the −2 log-likelihoods (−2LLs) of two models is distributed as a chi-square, with degrees of freedom equal to the difference in degrees of freedom of the two models. Nonsignificant results (p > .05) indicate a nonsignificant reduction in fit between the two models. Akaike’s Information Criterion (AIC) (17) was utilized as an additional fit statistic. The AIC is calculated by the difference in −2LL minus two times the difference in degrees of freedom between two models (AIC = Δ(−2LL) −2(Δdf )). More negative AIC values represent a superior balance between goodness-of-fit and parsimony.
Results
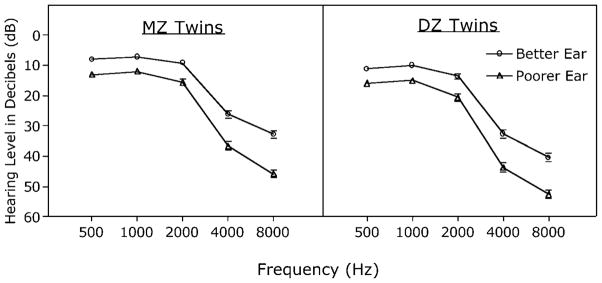
Figure 1 shows the mean audiometric profiles for the better and poorer ears for the MZ and DZ groups plotted in the form of a standard audiogram in which the y axis shows the amplitude (dB HL) necessary for detection of test tones at each frequency. The right ear was the better ear for 64.3% of the MZ twins and 67.0% for the DZ twins.
Figure 1.
Audiometric profiles for the better and poorer ears for the monozygotic (MZ) twins (left) and dizygotic (DZ) twins (right). Ordinate shows the mean amplitude (dB HL) necessary for detection of test tones at each frequency. Error bars represent one standard error; where not shown, they were too small to plot.
Consistent with large-scale studies of hearing acuity for men in this age range (1), Figure 1 shows a descending trend in hearing acuity for both twin groups as one goes from the middle frequency range (500, 1000, 2000 Hz) to the higher frequency range (4000, 8000 Hz). One also sees a small but significant asymmetry in acuity between the two ears as is not uncommon in adult aging. The MZ group’s acuity data were submitted to a 2(Ears: better, poorer) × 5(Frequency: 500, 1000, 2000, 4000, 8000 Hz) analysis of variance (ANOVA) with a Greenhouse–Geisser correction applied to control for sphericity (ε = .637). The MZ group showed significant main effects of ears, F(1,714) = 66.56, p <.001, and of frequency, F(2.55,1818.61) = 647.90, p <.001, verifying the acuity difference between the two ears and the differential decline in hearing acuity at the higher frequencies. The greater ear difference at the higher than at the middle frequencies was confirmed by a significant Ears × Frequency interaction, F(2.55,1818.61) = 11.41, p < .001.
An ANOVA conducted on the DZ twin data in the right panel of Figure 1 (Greenhouse–Geisser correction, ε = .656) showed the same pattern, with significant main effects of ears, F(1,598) = 46.59, p < .001, frequency, F(2.62,1568.13) = 719.15, p < .001, and a significant Ears × Frequency interaction, F(2.62,1568.13) = 8.65, p < .001. As would be expected from the similarity in patterns for the two twin groups evident in Figure 1, there was no Ears × Frequency × Zygosity interaction in a three-way ANOVA that included both twin groups (F < 1)
Levels of hearing loss are descriptively classified for clinical purposes along a continuum referenced as slight, mild, moderate, severe, or profound loss. The PTAs in the middle frequency range (500, 1000, 2000 Hz) for both twin groups’ better ears would be characterized as falling within the range of normal acuity, with the poorer ears showing a hearing loss that would be classified as within the slight hearing loss range (14). For the higher frequency PTAs (4000, 8000 Hz), the range known to be more susceptible to aging effects, the two twin groups’ better ears showed a hearing loss that would be classified as a mild loss, whereas the acuity in their poorer ears fell within the mild to moderate range (14).
Table 1 presents the means and SD values for the middle and high frequency range PTAs for the better and poorer ears in the MZ and DZ twin groups separately. Variance in hearing acuity was not significantly different across zygosity (p > .05); however, the DZ twins tended to have poorer hearing than the MZ twins for both frequency ranges and both ears. Although these differences were small (effect size ≈ .30), they were statistically different from zero. Therefore, means were estimated separately across twin and across zygosity in the biometrical model-fitting analyses. Constraining means to be equal across twin and across zygosity did not change parameter estimates by greater than .02; thus, our analyses of genetic and environmental influences were not biased by the group differences in mean level.
Table 1.
Means, Standard Deviations, and Twin Intraclass Correlations, Stratified by Zygosity
| Pure Tone Average | Better Ear |
Poorer Ear |
||
|---|---|---|---|---|
| MZ Twins | DZ Twins | MZ Twins | DZ Twins | |
| Middle frequencies | ||||
| Mean (SD) | 8.17 (9.96) | 11.76 (11.04) | 13.58 (11.74) | 17.33 (12.88) |
| Twin intraclass correlation | .677* | .330* | .557* | .412* |
| High frequencies | ||||
| Mean (SD) | 29.46 (21.05) | 36.71 (22.11) | 41.20 (22.35) | 48.33 (23.00) |
| Twin intraclass correlation | .674* | .298* | .566* | .319* |
Notes: Means refers to the average hearing level, in decibels, aggregated across frequencies. Middle frequencies = 500, 1000, and 2000 Hz; high frequencies = 4000 and 8000 Hz.
All twin correlations significantly different from zero at p < .001. Ns = 358 MZ twins (179 pairs) and 300 DZ twins (150 pairs).
MZ = monozygotic; DZ = dizygotic; SD = standard deviation.
Table 1 also shows the twin intraclass correlations for hearing acuity for the MZ and DZ twin pairs. It can be seen that the correlations between the hearing thresholds for the better ear for both frequency ranges were roughly twice as large for the MZ twins as for the DZ twins. This same trend holds for the poorer ear, although the differential between better and poorer ears is smaller. This greater similarity observed in MZ versus DZ twins suggests that genetic factors influence hearing acuity for this age group.
Table 2 presents the model-fitting results for the bivariate analyses. In the models estimating the cross-ear correlations for middle frequency and high frequency performance, the genetic correlations could be fixed to unity without significant reductions in model fit. The shared environmental correlation in both models could also be fit at unity. The nonshared environmental correlations for both models could not be eliminated without significant reductions in fit. Subsequently, the best fitting model for both the middle and high frequency performance was obtained by fixing the genetic correlation and the shared environmental correlation to unity. In the analyses examining the relationship between the middle and high frequency acuity within a given ear, the genetic correlations could no longer be fixed at unity without a significant reduction in model fit. Neither could these parameters be fixed to zero, leading to the conclusion that although a significant genetic overlap exists between performance at the middle and high frequencies, there are some genetic influences that are specific to each of the two frequency ranges. In contrast, the shared environmental correlation could be set to unity, as in the above analyses. Finally, nonshared environmental factors could not be set to zero without a significant reduction in fit.
Table 2.
Bivariate Model Fitting Results
| −2LL | df | Δ−2LL | Δdf | p Value | AIC | |
|---|---|---|---|---|---|---|
| Middle frequency performance (better ear and poorer ear) | ||||||
| Full model | 9036.094 | 1299 | — | — | — | — |
| RG = 1 | 9036.094 | 1300 | 0.00 | 1 | * | −2.000 |
| RG = 0 | 9065.203 | 1300 | 29.109 | 1 | < .001 | 27.109 |
| RC = 1 | 9036.094 | 1300 | 0.00 | 1 | * | −2.000 |
| RC = 0 | 9041.710 | 1300 | 5.617 | 1 | .018 | 3.617 |
| RE = 0 | 9259.534 | 1300 | 223.441 | 1 | < .001 | 221.441 |
| RG = 1 and RC = 1 | 9036.094 | 1301 | 0.00 | 2 | * | −4.000 |
| High frequency performance (better ear and poorer ear) | ||||||
| Full model | 10784.213 | 1299 | — | — | — | — |
| RG = 1 | 10784.430 | 1300 | 0.217 | 1 | .641 | −1.783 |
| RG = 0 | 10810.041 | 1300 | 25.828 | 1 | < .001 | 23.828 |
| RC = 1 | 10784.213 | 1300 | 0.00 | 1 | * | −2.00 |
| RC = 0 | 10784.415 | 1300 | 0.201 | 1 | .654 | −1.799 |
| RE = 0 | 11033.796 | 1300 | 249.582 | 1 | < .001 | 247.582 |
| RG = 1 and RC = 1 | 10784.430 | 1301 | 0.217 | 2 | .897 | −3.783 |
| RG = 1 and RC = 0 | 10786.543 | 1301 | 2.330 | 2 | .312 | −1.670 |
| Better ear performance (middle and high frequencies) | ||||||
| Full model | 10456.222 | 1299 | — | — | — | — |
| RG = 1 | 10484.117 | 1300 | 27.895 | 1 | < .001 | 25.895 |
| RG = 0 | 10472.028 | 1300 | 15.806 | 1 | < .001 | 13.806 |
| RC = 1 | 10456.222 | 1300 | 0.00 | 1 | * | −2.000 |
| RC = 0 | 10456.222 | 1300 | 0.00 | 1 | * | −2.000 |
| RE = 0 | 10490.696 | 1300 | 34.474 | 1 | < .001 | 32.474 |
| Poorer ear performance (middle and high frequencies) | ||||||
| Full model | 10739.356 | 1299 | — | — | — | — |
| RG = 1 | 19745.124 | 1300 | 5.769 | 1 | .016 | 3.769 |
| RG = 0 | 10739.359 | 1300 | 4.728 | 1 | .030 | 2.728 |
| RC = 1 | 10739.359 | 1300 | 0.00 | 1 | * | −2.000 |
| RC = 0 | 10739.950 | 1300 | 0.594 | 1 | .441 | −1.406 |
| RE = 0 | 10790.853 | 1300 | 51.497 | 1 | < .001 | 49.497 |
Notes: Full model indicates that all covariance parameters are estimated between the variables. All subsequent models are tested against the fit of the full model.
Indicates that the p value was incalculable due to no change in the −2LL of the model.
RG = genetic correlation; RC = shared environment correlation; RE = environment correlation; −2LL = −2 log likelihood; AIC = Akaike’s Information Criterion.
Figure 2 shows these results in the form of correlated factors models. The output from our bivariate Cholesky models is readily translated into the correlated factors format via simple algebraic transformations (18). To estimate the relative contributions of genetic and environmental factors on variation in each trait, the parameter estimates shown in Figure 2 have to be squared. For descriptive purposes, Figure 2 also presents the phenotypic correlations across variables, calculated from the parameters estimated in our biometrical models. The four bivariate models are shown in panels A–D. At the middle frequencies (A), additive genetic effects accounted for 45% (i.e., .67 * .67; 95% CI, 25%–63%) and 65% (i.e., .81 * .81; 95% CI, 46%–73%) of the total phenotypic variance for the poorer and better ears, respectively. Similar genetic contributions to the phenotypic variance are observed for the high frequencies (B), where the effects accounted for 53% (95% CI, 26%–67%) and 66% (95% CI, 45%–74%) of the variance, respectively. In both models, the genetic correlation between variables is equal to 1.0. In panels C and D, additive genetic effects accounted for 70% (95% CI, 50%–76%) and 68% (95% CI, 49%–75%) of the phenotypic variance for the better ear at middle and high frequencies, respectively. In contrast, for the poorer ear the genetic effect accounted for 41% (95% CI, 11%–66%) and 54% (95% CI, 23%–65%) of the variance at the middle and high frequencies, respectively. The genetic correlations in these models were .51 for the better ear and .57 for the poorer ear. All genetic influences were statistically significant, as the lower bounds of the 95% CI values did not include zero. In contrast, estimates of shared environmental influences were weak, accounting for between 0% and 18% of the variance in any given trait, and all of the 95% CI values included zero, indicating that these influences were not statistically significant.
Figure 2.
Bivariate correlated factors models for middle frequency performance across better ears (A), high frequency performance across better and poorer ears (B), better ear performance across middle and high frequencies (C), and poorer ear performance across middle and high frequencies (D). RG = genetic correlation; RC = shared environment correlation; RE = Nonshared environment correlation. Values adjacent to lines are path coefficients; values in parentheses are 95% confidence intervals for the path coefficients; values in brackets are standardized variance components. h2 = proportion of variance due to genetic influences; c2 = proportion of variance due to shared environment; e2 = proportion of variance due to nonshared environment.
Discussion
Consistent with previous studies (5,6), our results showed that a significant amount of the interindividual variability in hearing acuity that appears in middle age can be attributed to genetic factors. In addition, our results show that although about one-half of the variance in both the middle and high frequencies could be accounted for by genetic influences for an individual’s poorer ear, for the better ear about two-thirds of the variance could be accounted for by genetic influences. This finding is consistent with the previously noted suggestion that, in cases of asymmetric acuity, the better ear represents a better approximation of what the heritability would be prior to the impact of environmental insults. This would be so to the extent that the acuity in the poorer ear will be a compound of environmental effects overlaid upon a heritable baseline that would be expected to be equal for both ears (4).
The pattern of correlations between the latent factors influencing the better and poorer ears indicates that the same genetic factors and shared environmental factors are influencing both ears. The nonshared environmental correlations between the better and poorer ears in both frequency ranges indicate that although there are some aspects of the environment that twins do not share that have an effect on both ears, there are also aspects of the nonshared environment that only affect one ear, accounting for these ear asymmetries. It should be noted in regard to our all-male sample that the incidence of hearing loss, especially in the high frequency range, is higher among men than women (19) and that there may be a somewhat higher relative genetic contribution (i.e., higher heritability) to hearing acuity for women than for men (4). This could be a function of men being more likely than women to experience environmental events that affect hearing (e.g., loud machinery on the job).
It is interesting that we observed a significant, but non-unity, genetic correlation between performance at the middle and high frequencies. That is, although the genes influencing hearing acuity for the middle frequencies also influence hearing acuity for the high frequencies, there are genetic influences specific to each frequency range. It may therefore be the case that the differential loss in the high frequency range relative to the lower frequency range that is the hallmark of presbycusis is due to genetic factors specific to the middle or high frequencies. This finding may also reflect a single genetic factor that affects the two frequency ranges differently as a consequence of the greater general vulnerability to hair cell loss in the high frequency sensitive region of the basilar membrane.
It is important to emphasize that our measurement of hearing acuity was a snapshot in time in a group of men in their late middle years. As such, our results pertain only to genetic influences on the individual differences in the present level of hearing acuity for our participants. Future longitudinal testing of these same individuals as part of the VETSA project will allow us to capture potential differences in rates of loss and genetic predictors of this loss rate as this sample ages. An important question is whether rates of threshold change longitudinally may be related to threshold levels observed at an earlier time (20).
Hearing loss is the third most prevalent chronic disability among older adults after arthritis and hypertension (2), and even mild loss can affect cognitive function due to the attendant perceptual effort drawing resources ordinarily available for higher level comprehension and memory operations (21–23). That is, genes may affect cognition indirectly through their effects on sensory function. That genetic factors can be shown to play an important role in determining vulnerability to age-associated hearing loss may have clinical application to counseling individuals with hearing loss among family members to take special care in terms of environmental risks to hearing and to encourage the search for the genes or gene complexes the expression of which underlies this trait.
Acknowledgments
The VETSA project, of which this is a part, is supported by National Institutes of Health/National Institute on Aging (NIH/NIA) Grants R01 AG018286, R01 AG022381, and R01 AG022982. We also acknowledge support from the NIA (R01 AG19714) to Dr. Wingfield. The U.S. Department of Veterans Affairs has provided support for the development and maintenance of the Vietnam Era Twin Registry.
We gratefully acknowledge the cooperation and participation of the members of the Vietnam Era Twin Registry and their families. Without their contribution this research would not have been possible.
References
- 1.Morrell CH, Gordon-Salant S, Pearson JD, Brant LJ, Fozard JL. Age- and gender-specific reference ranges for hearing level and longitudinal changes in hearing level. J Acoust Soc Am. 1996;100:1949–1967. doi: 10.1121/1.417906. [DOI] [PubMed] [Google Scholar]
- 2.Schneider BA, Pichora-Fuller MK. Implications of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. 2. Mahwah, NJ: Erlbaum; 2000. pp. 155–220. [Google Scholar]
- 3.Fransen E, Lemkens N, Van Laer L, Van Camp G. Age-related hearing impairment (ARHI): environmental risk factors and genetic prospects. Exp Gerontol. 2003;38:353–359. doi: 10.1016/s0531-5565(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 4.Gates GA, Couropmitree NN, Myers RH. Genetic associations in age-related hearing thresholds. Arch Otolaryngol Head Neck Surg. 1999;125:654–659. doi: 10.1001/archotol.125.6.654. [DOI] [PubMed] [Google Scholar]
- 5.Christensen K, Frederiksen H, Hoffman HJ. Genetic and environmental influences on self-reported reduced hearing in the old and oldest old. J Am Geriatr Soc. 2001;49:1512–1517. doi: 10.1046/j.1532-5415.2001.4911245.x. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson KK, Harris JR, Svartengren M. Description and primary results from an audiometric study of male twins. Ear Hear. 1997;18:114–120. doi: 10.1097/00003446-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Pichora-Fuller MK, Carson AJ. Hearing health and the listening experiences of older communicators. In: Hummert ML, Nussbaum JF, editors. Aging, Communication, and Health. Mahwah, NJ: Erlbaum; 2001. pp. 43–74. [Google Scholar]
- 8.Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet Med Gemellol. 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- 9.Henderson WG, Eisen SE, Goldberg J, True WR, Barnes JE, Vitek M. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- 10.Eisen SA, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 11.Nichols RC, Bilbro WCJ. The diagnosis of twin zygosity. Acta Genet Stat Med. 1966;16:265–275. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- 12.Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R. Validation of a telephone zygosity questionnaire in twins of known zygosity. Behav Genet. 1998;28:159–163. doi: 10.1023/a:1021416112215. [DOI] [PubMed] [Google Scholar]
- 13.American Speech-Language-Hearing Association Guidelines for manual pure-tone audiometry. ASHA. 1978;20:297–301. [PubMed] [Google Scholar]
- 14.Harrell RW. Puretone evaluation. In: Katz J, editor. Handbook of Clinical Audiology. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 71–87. [Google Scholar]
- 15.Neale MC, Boker S, Xie G, Maes H. Mx: Statistical Modeling. 5. Richmond, VA: Medical College of Virginia, Department of Psychiatry; 1999. [Google Scholar]
- 16.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 17.Akaike H. Factor analysis and AIC. Psychometrica. 1987;52:317–332. [Google Scholar]
- 18.Loehlin JC. The Cholesky approach: a cautionary tale. Behav Genet. 1996;26:65–69. [Google Scholar]
- 19.Helfer KS. Gender, age, and hearing. Semin Hear. 2001;22:271–286. [Google Scholar]
- 20.Lee FS, Mathews LJ, Dubno JR, Mills JH. Longitudinal study of pure-tone thresholds in older persons. Ear Hear. 2005;26:1–11. doi: 10.1097/00003446-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Murphy DR, Craik FI, Li KZ, Schneider BA. Comparing the effects of aging and background noise on short-term memory performance. Psychol Aging. 2000;15:323–334. doi: 10.1037/0882-7974.15.2.323. [DOI] [PubMed] [Google Scholar]
- 22.Rabbitt PM. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Otolaryngol Suppl. 1991;476:167–176. doi: 10.3109/00016489109127274. [DOI] [PubMed] [Google Scholar]
- 23.Wingfield A, Tun PA, McCoy SL. Hearing loss in older adulthood: what it is and how it interacts with cognitive performance. Curr Dir Psychol Sci. 2005;14:144–148. [Google Scholar]