Abstract
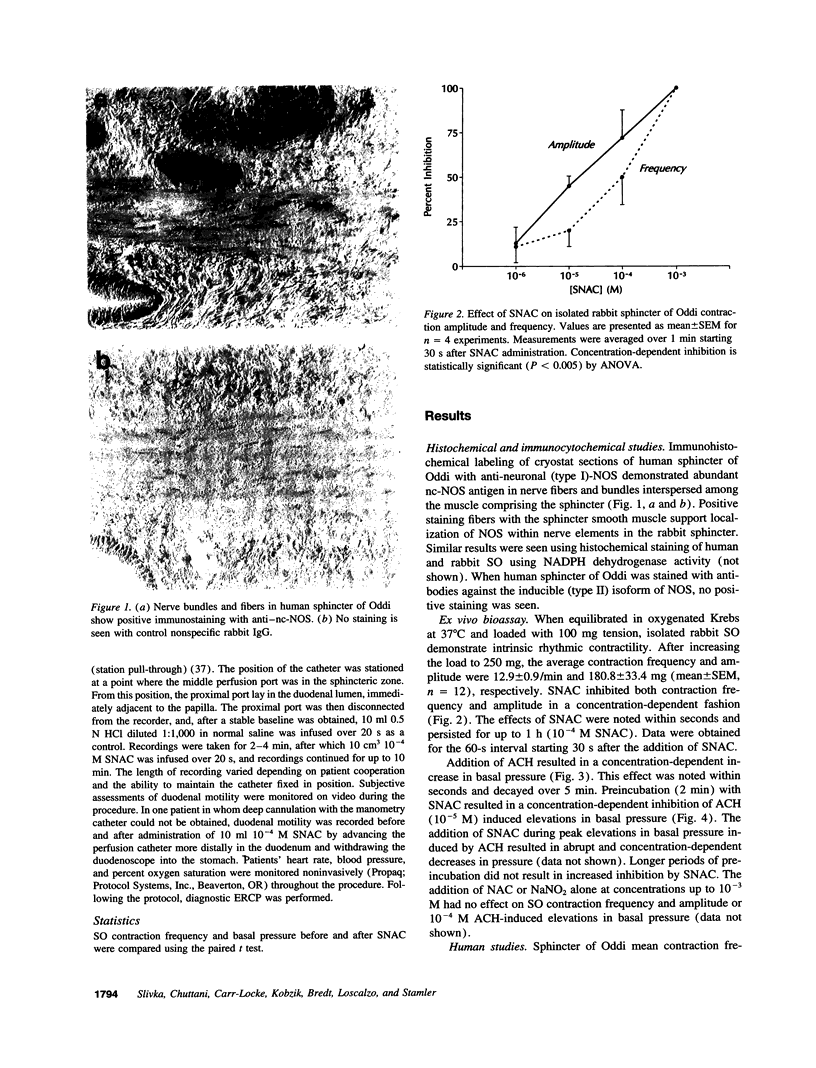
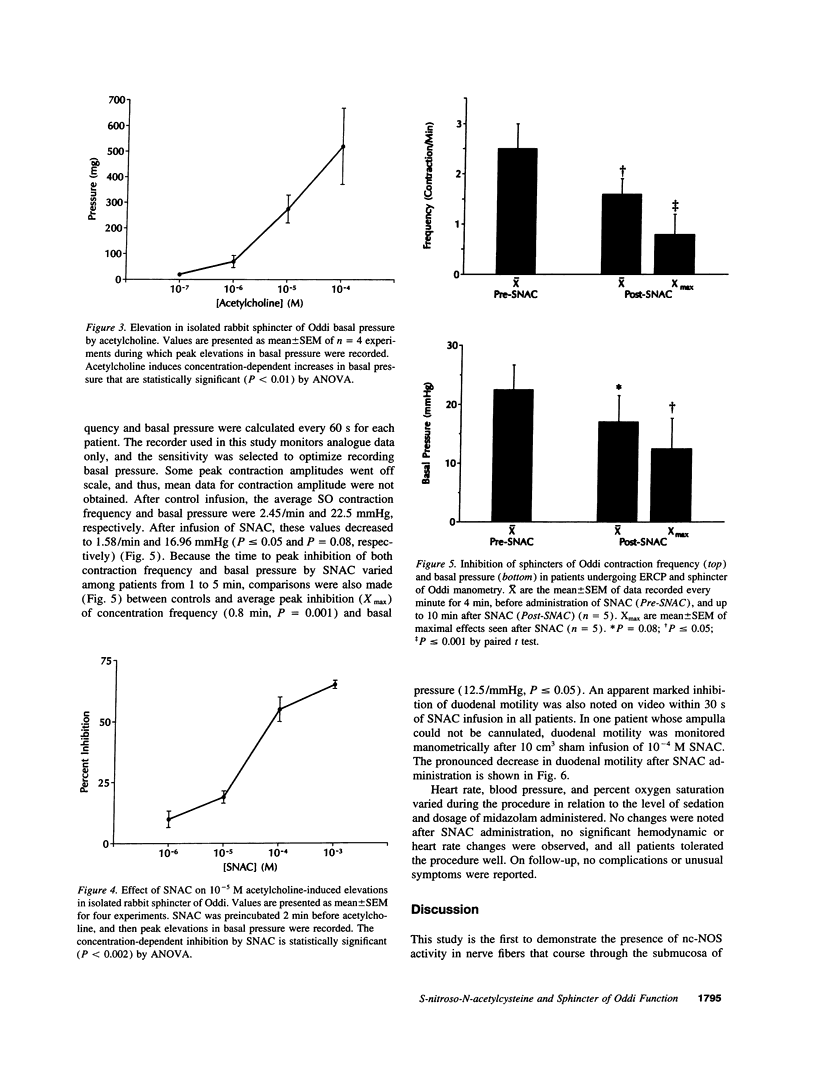
Nitric oxide (NO) is an inhibitor of gastrointestinal smooth muscle. Model systems of the gut predict the NO will complex with biological thiol (SH) groups, yielding S-nitrosothiols (RS-NO), which may limit the propensity to form mutagenic nitrosamines. The inhibitory effects of NO and its biologically relevant adducts on sphincter of Oddi (SO) motility have been inferred from animal studies; however, their importance in regulating human SO is not known. The objectives of this study were to (a) provide histologic confirmation of nitric oxide synthase (NOS) in human SO; (b) characterize the pharmacology of S-nitroso-N-acetylcysteine (SNAC), an exemplary S-nitrosothiol, on SO motility in a rabbit model; and (c) study the effects of topical SNAC on SO motility in humans. Immunocytochemical and histochemical identification of NOS was performed in human SO. The pharmacologic response of SNAC was defined in isolated rabbit SO using a standard bioassay. Topical SNAC was then applied to the duodenal papilla in patients undergoing endoscopic retrograde cholangiopancreatography (ERCP) and biliary manometry. NOS was localized to nerve fibers and bundles of the SO in rabbits and humans. SNAC inhibited spontaneous motility (frequency and amplitude) as well as acetylcholine-induced elevations in SO basal pressure in the rabbit model. In patients undergoing ERCP and biliary manometry, topical SNAC inhibited SO contraction freqency, basal pressure, and duodenal motility. NOS is localized to neural elements in human SO, implicating a role for NO in regulating SO function. Supporting this concept, SNAC is an inhibitor of SO and duodenal motility when applied topically to humans during ERCP. Our data suggest a novel clinical approach using local NO donors to control gastrointestinal motility and regulate sphincteric function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allescher H. D., Tougas G., Vergara P., Lu S., Daniel E. E. Nitric oxide as a putative nonadrenergic noncholinergic inhibitory transmitter in the canine pylorus in vivo. Am J Physiol. 1992 Apr;262(4 Pt 1):G695–G702. doi: 10.1152/ajpgi.1992.262.4.G695. [DOI] [PubMed] [Google Scholar]
- Baker R. A., Saccone G. T., Brookes S. J., Toouli J. Nitric oxide mediates nonadrenergic, noncholinergic neural relaxation in the Australian possum. Gastroenterology. 1993 Dec;105(6):1746–1753. doi: 10.1016/0016-5085(93)91072-p. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Buga G. M., Gold M. E., Wood K. S., Chaudhuri G., Ignarro L. J. Endothelium-derived nitric oxide relaxes nonvascular smooth muscle. Eur J Pharmacol. 1989 Feb 14;161(1):61–72. doi: 10.1016/0014-2999(89)90180-5. [DOI] [PubMed] [Google Scholar]
- Burleigh D. E. Ng-nitro-L-arginine reduces nonadrenergic, noncholinergic relaxations of human gut. Gastroenterology. 1992 Feb;102(2):679–683. doi: 10.1016/0016-5085(92)90120-n. [DOI] [PubMed] [Google Scholar]
- Calabuig R., Ulrich-Baker M. G., Moody F. G., Weems W. A. The propulsive behavior of the opossum sphincter of Oddi. Am J Physiol. 1990 Jan;258(1 Pt 1):G138–G142. doi: 10.1152/ajpgi.1990.258.1.G138. [DOI] [PubMed] [Google Scholar]
- Carr-Locke D. L., Gregg J. A. Endoscopic manometry of pancreatic and biliary sphincter zones in man. Basal results in healthy volunteers. Dig Dis Sci. 1981 Jan;26(1):7–15. doi: 10.1007/BF01307970. [DOI] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C., Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991 Jun 6;351(6326):477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Funch-Jensen P. Sphincter of Oddi motility. Acta Chir Scand Suppl. 1990;553:1–35. [PubMed] [Google Scholar]
- Gaston B., Drazen J. M., Jansen A., Sugarbaker D. A., Loscalzo J., Richards W., Stamler J. S. Relaxation of human bronchial smooth muscle by S-nitrosothiols in vitro. J Pharmacol Exp Ther. 1994 Feb;268(2):978–984. [PubMed] [Google Scholar]
- Gaston B., Reilly J., Drazen J. M., Fackler J., Ramdev P., Arnelle D., Mullins M. E., Sugarbaker D. J., Chee C., Singel D. J. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider J. R., Murthy K. S., Jin J. G., Makhlouf G. M. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am J Physiol. 1992 Apr;262(4 Pt 1):G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Ware K., Gilleland H. E., Jr, Gilleland L. B., Abell C. L., Yamada T. Neutrophil-mediated nitrosamine formation: role of nitric oxide in rats. Gastroenterology. 1992 Oct;103(4):1260–1266. doi: 10.1016/0016-5085(92)91513-4. [DOI] [PubMed] [Google Scholar]
- Hata F., Ishii T., Kanada A., Yamano N., Kataoka T., Takeuchi T., Yagasaki O. Essential role of nitric oxide in descending inhibition in the rat proximal colon. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1400–1406. doi: 10.1016/0006-291x(90)91605-r. [DOI] [PubMed] [Google Scholar]
- Henry P. J., Drummer O. H., Horowitz J. D. S-nitrosothiols as vasodilators: implications regarding tolerance to nitric oxide-containing vasodilators. Br J Pharmacol. 1989 Nov;98(3):757–766. doi: 10.1111/j.1476-5381.1989.tb14603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda R., Toouli J., Dodds W. J., Sarna S., Hogan W. J., Itoh Z. Relationship of sphincter of Oddi spike bursts to gastrointestinal myoelectric activity in conscious opossums. J Clin Invest. 1982 Apr;69(4):770–778. doi: 10.1172/JCI110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Wood K. S. Endothelium-dependent modulation of cGMP levels and intrinsic smooth muscle tone in isolated bovine intrapulmonary artery and vein. Circ Res. 1987 Jan;60(1):82–92. doi: 10.1161/01.res.60.1.82. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Kaufman H. S., Shermak M. A., May C. A., Pitt H. A., Lillemoe K. D. Nitric oxide inhibits resting sphincter of Oddi activity. Am J Surg. 1993 Jan;165(1):74–80. doi: 10.1016/s0002-9610(05)80407-6. [DOI] [PubMed] [Google Scholar]
- Knudsen M. A., Svane D., Tøttrup A. Action profiles of nitric oxide, S-nitroso-L-cysteine, SNP, and NANC responses in opossum lower esophageal sphincter. Am J Physiol. 1992 May;262(5 Pt 1):G840–G846. doi: 10.1152/ajpgi.1992.262.5.G840. [DOI] [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- Lamping K. A., Christensen C. W., Pelc L. R., Warltier D. C., Gross G. J. Effects of nicorandil and nifedipine on protection of ischemic myocardium. J Cardiovasc Pharmacol. 1984 May-Jun;6(3):536–542. doi: 10.1097/00005344-198405000-00024. [DOI] [PubMed] [Google Scholar]
- Leeflang-de Pijper A. M., Hulsmann W. C. Pitfalls in histochemical localization studies of NADPH generating enzymes or enzyme systems in rat small intestine. Histochemistry. 1974 Apr 22;39(2):143–153. doi: 10.1007/BF00492043. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur J Pharmacol. 1990 Dec 4;191(3):303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- Mourelle M., Guarner F., Moncada S., Malagelada J. R. The arginine/nitric oxide pathway modulates sphincter of Oddi motor activity in guinea pigs and rabbits. Gastroenterology. 1993 Nov;105(5):1299–1305. doi: 10.1016/0016-5085(93)90132-v. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Rattan S., Sarkar A., Chakder S. Nitric oxide pathway in rectoanal inhibitory reflex of opossum internal anal sphincter. Gastroenterology. 1992 Jul;103(1):43–50. doi: 10.1016/0016-5085(92)91093-j. [DOI] [PubMed] [Google Scholar]
- Scharfstein J. S., Keaney J. F., Jr, Slivka A., Welch G. N., Vita J. A., Stamler J. S., Loscalzo J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest. 1994 Oct;94(4):1432–1439. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikano K., Long C. J., Ohlstein E. H., Berkowitz B. A. Comparative pharmacology of endothelium-derived relaxing factor and nitric oxide. J Pharmacol Exp Ther. 1988 Dec;247(3):873–881. [PubMed] [Google Scholar]
- Shikano K., Ohlstein E. H., Berkowitz B. A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br J Pharmacol. 1987 Nov;92(3):483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Staritz M., Poralla T., Dormeyer H. H., Ewe K., Meyer zum Büschenfelde K. H. Nitroglycerine dilatation of sphincter of Oddi for endoscopic removal of bileduct stones. Lancet. 1984 Apr 28;1(8383):956–956. doi: 10.1016/s0140-6736(84)92405-x. [DOI] [PubMed] [Google Scholar]
- Staritz M., Poralla T., Dormeyer H. H., Meyer zum Büschenfelde K. H. Endoscopic removal of common bile duct stones through the intact papilla after medical sphincter dilation. Gastroenterology. 1985 Jun;88(6):1807–1811. doi: 10.1016/0016-5085(85)90004-6. [DOI] [PubMed] [Google Scholar]
- Staritz M., Poralla T., Ewe K., Meyer zum Büschenfelde K. H. Effect of glyceryl trinitrate on the sphincter of Oddi motility and baseline pressure. Gut. 1985 Feb;26(2):194–197. doi: 10.1136/gut.26.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Honda R., Dodds W. J., Sarna S., Toouli J., Itoh Z., Chey W. Y., Hogan W. J., Greiff D., Baker K. Effect of motilin on the opossum upper gastrointestinal tract and sphincter of Oddi. Am J Physiol. 1983 Oct;245(4):G476–G481. doi: 10.1152/ajpgi.1983.245.4.G476. [DOI] [PubMed] [Google Scholar]
- Tøttrup A., Glavind E. B., Svane D. Involvement of the L-arginine-nitric oxide pathway in internal anal sphincter relaxation. Gastroenterology. 1992 Feb;102(2):409–415. doi: 10.1016/0016-5085(92)90084-c. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Xue C., Shuttleworth C. W., Bredt D. S., Snyder S. H., Sanders K. M. NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am J Physiol. 1992 Aug;263(2 Pt 1):G277–G284. doi: 10.1152/ajpgi.1992.263.2.G277. [DOI] [PubMed] [Google Scholar]
- Yamato S., Spechler S. J., Goyal R. K. Role of nitric oxide in esophageal peristalsis in the opossum. Gastroenterology. 1992 Jul;103(1):197–204. doi: 10.1016/0016-5085(92)91113-i. [DOI] [PubMed] [Google Scholar]
- Young H. M., Furness J. B., Shuttleworth C. W., Bredt D. S., Snyder S. H. Co-localization of nitric oxide synthase immunoreactivity and NADPH diaphorase staining in neurons of the guinea-pig intestine. Histochemistry. 1992 May;97(4):375–378. doi: 10.1007/BF00270041. [DOI] [PubMed] [Google Scholar]




