Abstract
Background
Published guidelines for the treatment of healthcare-associated pneumonia (HCAP) recommend initial broad-spectrum antibiotics with appropriate de-escalation based on culture results. Guideline recommendations are based on data from intubated patients, in whom cultures are easily obtained. The approach to antibiotic de-escalation for culture-negative patients has not been addressed. Consequently, there are no published reports that describe the current standard of practice.
Patients and methods
All patients admitted to a university hospital with a diagnosis of HCAP, as defined by use of a pneumonia orderset, were identified retrospectively over a 2-year period. Antibiotics prescribed on admission, during hospital stay, and on discharge were recorded. De-escalation was defined as a change in the initial antibiotic therapy from broad- to narrow-spectrum coverage within 14 days of the initial prescription. The Pneumonia Severity Index was used for risk-adjustment.
Results
A total of 102 patients were included in the analysis; of these, 72% (n = 73) were culture-negative. There were more males in the culture-negative than culture-positive group; otherwise, baseline characteristics were similar. Antibiotic therapy was de-escalated in 75% of the culture-negative group and 77% of the culture-positive group (p = 1.00). Culture-negative patients were de-escalated approximately 1 day earlier than culture-positive patients (3.93 vs. 5.04 days, p = 0.03). Culture-negative patients who were de-escalated had a shorter length of hospitalization, lower hospital costs, and lower mortality rates. In 70% of the culture-negative patients, a respiratory fluoroquinolone was chosen for de-escalation.
Conclusion
In this single-center study, most of the patients with culture-negative HCAP were safely de-escalated to a respiratory fluoroquinolone.
Keywords: HCAP, Culture negative, Healthcare-associated pneumonia, Antibiotic de-escalation, Respiratory fluoroquinolone
Introduction
Healthcare-associated pneumonia (HCAP), as defined by guidelines published in 2005 by the American Thoracic Society (ATS) and the Infectious Disease Society of America (IDSA), describes a category of pneumonia in which the risk for multidrug-resistant organisms is higher than for those patients with community-acquired pneumonia (CAP) [1]. Mortality rates for HCAP are twofold higher than those with CAP [2–4]. The risk factors for HCAP are detailed in Table 1.
Table 1.
Risk factors for multidrug-drug resistant pathogens/HCAP
| Bronchiectasis or other structural lung disease |
| Immunosuppressant disease or therapy |
| Antibiotic use within 90 days |
| Currently hospitalized >4 days |
| Hospitalized >1 day in past 3 months |
| Lives in skilled nursing facility/extended care |
| Receives home infusion therapy |
| Received chronic hemodialysis in the past 30 days |
| Receives home wound care |
| Family member with multi-drug resistant pathogen |
Source: Adapted from American thoracic society and infectious diseases society of America [1]
HCAP Healthcare-associated pneumonia
A growing body of evidence demonstrates that the choice of appropriate initial antibiotics has a significant impact on mortality rates for both CAP [5–7] and HCAP [3, 4, 8]. A major focus of the guidelines [1] is to clarify the empiric antibiotic regimens for clinicians that are most likely to ensure adequate broad-spectrum coverage until culture data are available to guide therapy. Unfortunately, for most patients with pneumonia outside the intensive-care unit, data based on cultures are inadequate or negative. Based on data in published studies, blood cultures in patients with pneumonia are positive <10% of the time [9–11]. In addition, only about one-third of non-ventilated patients are able to provide a sputum sample satisfactory for analysis [12, 13], and of those, less than half yield a predominant organism [12].
The guidelines clearly state that initial broad-spectrum antibiotic regimens should be narrowed or “de-escalated” to cover the appropriate organisms identified by cultures [1]. However, antibiotic de-escalation in culture-negative patients is a topic the guidelines do not address. The decision about how and when to de-escalate is left to clinical judgment.
Given this limited benefit of blood and sputum cultures and the high incidence of multidrug-resistant organisms in HCAP, some clinicians may be tempted to continue broad-spectrum antibiotic regimens in patients who show clinical improvement. This practice would need to be tempered by concerns about selective pressure from prolonged exposure to broad-spectrum antimicrobials leading to the development of resistant microorganisms. Others, knowing that patients with pneumonia rarely deteriorate once they have achieved clinical stability [14], may elect to narrow coverage early in stable culture-negative patients.
It is not clear which strategy is the most common or the most appropriate. In the study reported here, we analyzed practice patterns on antibiotic de-escalation for HCAP in order to better understand the current clinical practice landscape.
Patients and methods
A retrospective chart review was conducted at a university medical center between July 2007 and February 2009. A computerized physician order entry system was in place during this period that contained an admission orderset for patients with pneumonia. All patients ≥18 years old admitted with HCAP, as identified by use of the orderset, were enrolled in the study. The study was approved by the university’s Institutional Review Board.
All data were collected through a review of electronic and paper medical records. Demographics, length of stay, inpatient mortality rates, results of sputum and blood cultures, admission and discharge antibiotic prescriptions, time to de-escalation of antibiotic therapy, and total cost of stay were collected for each patient. For each case, the specific risk factors for multidrug-resistant organisms or HCAP, as detailed in Table 1, were recorded.
De-escalation of antibiotic therapy was defined as a change in antibiotic therapy from broad-spectrum HCAP coverage [e.g., coverage of methicillin-resistant Staphylococcus aureus (MRSA) and double-coverage of Pseudomonas] to a narrower spectrum regimen within 14 days of the initial antibiotic prescription. Antibiotic prescription data were reviewed by a licensed pharmacist, who judged whether or not de-escalation had occurred.
Patients were divided into groups based on the results of their blood and sputum cultures. Patients with growth of a respiratory pathogen on any culture were considered to be culture-positive; all others were considered as culture-negative.
In addition, a pneumonia severity index (PSI) score was calculated for each patient to allow for risk-adjustment of the data. The PSI is a validated tool used to predict 30-day mortality for pneumonia patients [15]. PSI components are assigned a numeric value and consist of age, sex, vital signs (temperature, blood pressure, heart rate, respiratory rate, altered mental status), past medical history (active cancer, congestive heart failure, cerebrovascular disease, chronic kidney/liver disease, residence at a skilled nursing facility), laboratory parameters [glucose, blood urea nitrogen, sodium, hematocrit, pH, pO2 or oxygen saturation], and the presence of pleural effusion. All components are tallied and assigned a PSI class ranging from I to V. PSI classes I–III confer a low mortality risk, whereas, classes IV and V are associated with 30-day mortality rates of 9 and 27%, respectively.
Baseline and outcome data were presented using means ± the standard deviation (SD) and analyzed with the t test with 95% confidence interval (CI) of the difference for continuous normally distributed data. Medians and ranges and the Wilcoxon rank-sum test were used for non-normally distributed continuous data. Proportions were analyzed with the Fischer’s exact test. A p value of <0.05 was considered to be statistically significant for all tests. The Excel (Microsoft, Redmond, WA) spreadsheet and the SPlus 6.2 (Insightful, Seattle, WA) statistical software package were used for all data management and analysis.
Results
A total of 122 patients with an initial diagnosis of HCAP were identified between July 2007 and February 2009. Of these 122 patients, 102 were included in the analysis; the remaining patients were excluded because HCAP was ultimately ruled out as a diagnosis (Fig. 1).
Fig. 1.
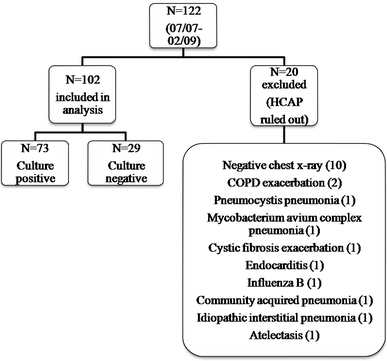
Patients included in the analysis. HCAP Healthcare-acquired pneumonia, COPD chronic obstructive pulmonary disease
Of the 102 patients, 72% (n = 73) fell in the culture-negative group versus 28% (n = 29) in the culture-positive group. There were significantly more males in the culture-negative group (p = 0.03); otherwise, baseline characteristics and severity of illness were similar between the groups (Table 2).
Table 2.
Characteristics of culture-negative and culture-positive patients with HCAP
| Patient characteristics | Culture negative (n = 73) | Culture positive (n = 29) | p value | Difference (95% CI) |
|---|---|---|---|---|
| Male sex, n (%) | 41 (56) | 9 (31) | 0.03 | 25 (−7 to 52) |
| Age, years (mean ± SD) | 58.4 ± 16.4 | 59.4 ± 16.9 | 0.80 | −1.0 (−8.32 to 6.44) |
| HCAP RF (median) | 2.0 | 2.0 | 0.54 | 0.0 (−0.01 to 0.01) |
| PSI score (mean ± SD) | 87.6 ± 36.2 | 95.2 ± 39.4 | 0.37 | −7.6 (−24.56 to 9.41) |
| LOS, days (mean ± SD) | 7.9 ± 7.8 | 10.2 ± 13.2 | 0.39 | −2.3 (−7.48 to 2.99) |
| Readmission within 30 days, n (%) | 9 (12) | 3 (10) | 1.00 | 2 (−21 to 20) |
| Total cost, $ (median) | 35,704 | 41,084 | 0.095 | −5,380 (−22,654 to 2,422) |
| Inpatient mortality, n (%) | 9 (12) | 0 (0) | 0.06 | 12 (−7 to 23) |
CI Confidence interval, SD standard deviation, RF risk factors; PSI pneumonia severity index; LOS length of stay
Culture-negative and culture-positive patients had similar lengths of stay, 30-day readmission rates, and inpatient mortality. The number of patients whose pneumonia was fully treated prior to discharge were similar between groups.
De-escalated patients
De-escalation to a narrow-spectrum antibiotic regimen occurred in 75% (n = 55) of culture-negative patients and 77% (n = 22) of culture-positive patients (p = 1.00). However, culture-negative patients were de-escalated 1 day sooner. Median time to de-escalation was 4 days in the culture-negative group versus 5 days in the culture-positive group, but this did not reach statistical significance (p = 0.17). The median number of HCAP risk factors was higher in patients who were not de-escalated (2 vs. 1, p = 0.003).
Moxifloxacin was the most common antibiotic used when antibiotic therapy was de-escalated. Overall, in the combined groups, 62% of patients were de-escalated to moxifloxacin. In the culture-negative group, 70% of patients transitioned to moxifloxacin versus 41% in the culture-positive group. There was no consistent pattern in the use of other antibiotics for de-escalation (Fig. 2). A combination of two or more antibiotics was used to de-escalate 27% of patients in the culture-positive group and 9% in the culture-negative group (Fig. 3).
Fig. 2.
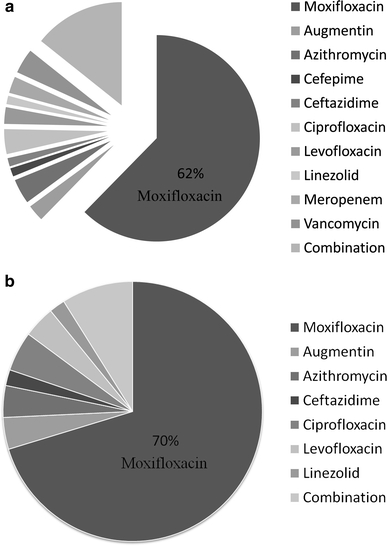
Prescribing patterns for de-escalation therapy. a Combined groups (culture-positive + culture-negative patients; n = 77). b Culture-negative patients (n = 55)
Fig. 3.
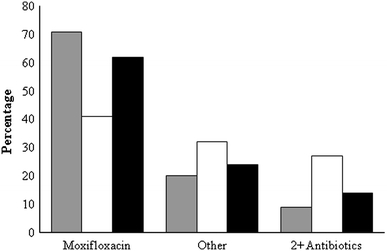
Antibiotic utilization for de-escalation therapy in patients with HCAP. Gray-shaded column Culture-negative patients (n = 55), open column culture-positive patients (n = 22), black- shaded column combined groups (culture-positive + culture-negative patients; n = 77). Other Augmentin, azithromycin, cefepime, ceftazidime, ciprofloxacin, levofloxacin, linezolid, meropenem, vancomycin
Overall, patients whose antibiotic therapy was de-escalated showed improved HCAP outcomes. For patients in the de-escalated group, the hospital stay was shorter (7.1 vs. 13.0 days, p = 0.02) and total cost of care was lower ($31,644 vs. $62,524, p < 0.001) than those in the non-de-escalated group. At the same time, there was no difference in readmission rates within 30 days of hospital discharge (p = 0.73) between the groups. Inpatient mortality was significantly lower in de-escalated patients (Table 3).
Table 3.
Characteristics of de-escalated patients with HCAP
| Patient characteristics | De-escalation (n = 77) | No de-escalation (n = 25) | p value | Difference (95% CI) |
|---|---|---|---|---|
| Male sex, n (%) | 39 (51) | 11 (44) | 0.65 | 7 (−26 to 38) |
| Age, years (mean ± SD) | 59.0 ± 16.1 | 57.7 ± 17.7 | 0.75 | 1.3 (−9.39 to 6.78) |
| HCAP RF (median) | 1.0 | 2.0 | 0.003 | −1.0 (−1.01 to −0.01) |
| PSI score (mean ± SD) | 87.3 ± 36.8 | 97.3 ± 37.7 | 0.25 | −10 (−7.44 to 27.41) |
| LOS, days (mean ± SD) | 7.1 ± 8.6 | 13.0 ± 11.0 | 0.02 | −5.9 (−10.77 to −0.96) |
| Readmission within 30 days, n (%) | 10 (13) | 2 (8) | 0.73 | 5 (−20 to 22) |
| Total cost, $ (median) | 31,644 | 62,524 | <0.001 | −30,880 (−54,967 to −14,621) |
| Inpatient mortality, n (%) | 2 (3) | 7 (28) | <0.001 | −25 (−49 to −3) |
CI confidence interval; SD standard deviation; HCAP healthcare-associated pneumonia; RF risk factors; PSI pneumonia severity index; LOS length of stay
Culture-negative patients
Baseline characteristics and severity of illness in culture-negative patients who were de-escalated were similar to those who were not (Table 4). In the culture-negative group, patients who were de-escalated had a shorter hospital stay (5.8 vs. 14.2 days, p = 0.005) and lower total cost of care ($28,286 vs. $80,791, p < 0.001) than patients who were not. At the same time, there was no difference in 30-day readmission rates (p = 1.00). Inpatient mortality was significantly lower in culture-negative de-escalated patients (Table 4).
Table 4.
Characteristics of culture-negative patients with HCAP by antibiotic de-escalation status
| Patient characteristics | De-escalation (n = 55) | No de-escalation (n = 18) | p value | Difference (95% CI) |
|---|---|---|---|---|
| Male sex, n (%) | 33 (60) | 8 (44) | 0.28 | 16 (−23 to 51) |
| Age, years (mean ± SD) | 59 ± 16 | 57 ± 18 | 0.65 | 2 (−11.75 to 7.49) |
| HCAP RF (median) | 1.0 | 2.0 | 0.086 | −1.0 (−0.01 to 0.99) |
| PSI score (mean ± SD) | 84.4 ± 33.5 | 97.4 ± 42.8 | 0.25 | −13.0 (−9.4 to 35.74) |
| LOS, days (mean ± SD) | 5.8 ± 5.0 | 14.2 ± 10.9 | 0.005 | −8.4 (−13.87 to −2.79) |
| Readmission rates within 30 days, n (%) | 7 (13) | 2 (11) | 1.00 | 2 (−29 to 23) |
| Total cost, $ (median) | 28,286 | 80,791 | <0.001 | −52,505 (−115,303 to −30,084) |
| Inpatient mortality, n (%) | 2 (4) | 7 (39) | <0.001 | −35 (−63 to −4) |
CI confidence interval; SD standard deviation; HCAP healthcare-associated pneumonia; RF risk factors; PSI pneumonia severity index; LOS length of stay
Discussion
In this observational study of antibiotic de-escalation for patients with culture-negative HCAP, the majority of patients had therapy narrowed to the hospital’s formulary respiratory fluoroquinolone, moxifloxacin. Less than 10% of culture-negative patients were de-escalated to a multidrug regimen. Despite de-escalated patients having a shorter stay in the hospital, there was no excess in 30-day readmission rates, and inpatient mortality was lower by almost a factor of 10 compared to the non-de-escalated group (3 vs. 28%).
Approximately 20% of patients in our study continued broad-spectrum therapy throughout their treatment course. The design of our study did not allow us to determine the reason why such patients were not de-escalated. The severity of illness as measured by the PSI was not different between groups; however, the number of HCAP risk factors was higher in the non-de-escalated group, suggesting that factors other than the pneumonia likely impacted the decision about therapy. It is reasonable to assume that at least some patients had co-morbidities that required prolonged hospitalization or other medical diagnoses that warranted continued use of broad-spectrum therapy despite negative cultures.
However, it is notable that culture-positive patients were not de-escalated any more frequently than culture-negative patients, despite the clear direction in the guidelines to narrow broad-spectrum therapy based on culture data [1]. Furthermore, despite positive cultures, clinicians took 1 day longer on average to narrow therapy in these patients. Severity of illness was the same between groups. Again, the retrospective nature of our study does not allow us to clearly understand the reason for this delay. However, the significant cost differential (over $50,000 per case) raises concerns about unnecessary delays in narrowing therapy, especially in light of the fact that mortality rates were lower in de-escalated patients.
We have not identified any prospective studies comparing treatment approaches for culture-negative HCAP in the current literature. Expert consensus panels have convened to review the evidence and found that there is currently insufficient data on which to determine best practice [16]. However, there was strong consensus among panelists that de-escalation of culture-negative HCAP patients is appropriate when clinical improvement is observed. Further outcomes-based studies in this area were recommended.
This study is limited by a small sample size and single-center design and should be seen as a framework within which to guide further research. Furthermore, the retrospective design made identification of HCAP patients challenging. There are currently no searchable ICD codes for HCAP, which limits the utility of administrative data.
The use of the pneumonia orderset to identify patients with HCAP risk factors was likely accurate, but physicians admitting patients with pneumonia do not always use it to enter the orders. Therefore, some patients would have been missed with our study methodology. We did not do independent chart confirmation of the diagnosis of pneumonia; use of the orderset by the admitting physician was the main inclusion criteria. Although this may have led to the inclusion of patients who did not actually have pneumonia, this is unlikely, as the admission chest X-rays were reviewed, and patients without abnormalities were excluded (see Table 1).
Also, we were only able to identify readmission to our hospital; the 30-day readmission rates may be an underestimate if patients were re-hospitalized elsewhere. A prospective study would allow for a more granular understanding of the issue of de-escalation, including data on the reasons for antibiotic choices, for prolonged broad-spectrum therapy, and for prolonged length of stay.
Healthcare-associated pneumonia is relatively common among hospitalized pneumonia patients, representing up to 25% of inpatient pneumonias in some studies [4]. At our institution, the majority of stable culture-negative patients with HCAP were safely de-escalated to a respiratory fluoroquinolone relatively early in the hospital course. Whether this represents best practice is yet to be determined. Larger, prospective, multicenter studies will help clarify this question.
Acknowledgments
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
M. Schlueter, Phone: +1-858-6576424, FAX: +1-858-6576481, Email: mlschlueter@ucsd.edu
C. James, Email: cljames@ucsd.edu
A. Dominguez, Email: ardominguez@ucsd.edu
L. Tsu, Email: tsu.laura@gmail.com
G. Seymann, Email: gseymann@ucsd.edu
References
- 1.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. [DOI] [PubMed]
- 2.Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 3.Micek ST, Kollef KE, Reichley RM, et al. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–3573. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venditti M, Falcone M, Corrao S, et al. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med. 2009;150:19–26. doi: 10.7326/0003-4819-150-1-200901060-00005. [DOI] [PubMed] [Google Scholar]
- 5.Frei C, Restrepo M, Mortensen E, et al. Impact of guideline-concordant empiric antibiotic therapy in community-acquired pneumonia. Am J Med. 2006;119:865–871. doi: 10.1016/j.amjmed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Mortensen E, Restrepo M, Anzueto A, et al. Effects of guideline-concordant antimicrobial therapy on mortality among patients with community-acquired pneumonia. Am J Med. 2004;117:726–731. doi: 10.1016/j.amjmed.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Mortensen EM, Restrepo MI, Anzueto A, et al. Antibiotic therapy and 48-hour mortality for patients with pneumonia. Am J Med. 2006;119:859–864. doi: 10.1016/j.amjmed.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Zilberberg MD, Shorr AF, Micek ST, et al. Antimicrobial therapy escalation and hospital mortality among patients with health-care-associated pneumonia: a single-center experience. Chest. 2008;134:963–968. doi: 10.1378/chest.08-0842. [DOI] [PubMed] [Google Scholar]
- 9.Campbell SG, Marrie TJ, Anstey R, et al. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest. 2003;123:1142–1150. doi: 10.1378/chest.123.4.1142. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932–936. doi: 10.1378/chest.108.4.932. [DOI] [PubMed] [Google Scholar]
- 11.Corbo J, Friedman B, Bijur P, et al. Limited usefulness of initial blood cultures in community acquired pneumonia. Emerg Med J. 2004;21:446–448. [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Vazquez E, Marcos MA, Mensa J, et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med. 2004;164:1807–1811. doi: 10.1001/archinte.164.16.1807. [DOI] [PubMed] [Google Scholar]
- 13.Roson B, Carratala J, Verdaguer R, et al. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis. 2000;31:869–874. doi: 10.1086/318151. [DOI] [PubMed] [Google Scholar]
- 14.Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452–1457. doi: 10.1001/jama.279.18.1452. [DOI] [PubMed] [Google Scholar]
- 15.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 16.Kollef M, Morrow L, Baughman R, et al. Health care-associated pneumonia (HCAP): a critical appraisal to improve identification, management, and outcomes—proceedings of the HCAP summit. Clin Infect Dis. 2008;46:S296–S334. doi: 10.1086/526355. [DOI] [PubMed] [Google Scholar]


