Abstract
Background
Menopause is associated with both a loss of muscle mass and a worsening of insulin sensitivity (IS). Although eccentric resistance exercise (ECC) can effectively improve muscle mass over time, a single bout of ECC can worsen IS. This study assessed the effect of repeated ECC on IS, muscle mass, and function in postmenopausal women with impaired glucose tolerance (IGT).
Methods
Sixteen PM women (aged 56 years ± 6.4) with IGT were randomly assigned to a 12-week, knee extensor ECC program (n = 10) or a nonexercise control group (CON) (n = 6). Participants underwent hyperinsulinemic-euglycemic clamps, dual-energy x-ray (DEXA) absorptiometry, quadriceps strength assessment, 6-minute walk (6MW) tests, and an assessment of steps taken per day before and after training.
Results
ECC participants experienced greater increases in leg lean soft tissue mass (ECC, 0.41 kg; CON, −0.53 kg; p = 0.03), quadriceps strength (ECC, 9.3 kg force; CON, −2.9 kg force; p = 0.02), and 6MW distance (ECC, 56.4 meters; CON, 3.3 meters; p = 0.03) than CON participants and demonstrated a trend toward more steps taken per day posttraining (ECC, +1747 steps; CON, +339 steps; p = 0.10). IS was unchanged.
Conclusions
This novel exercise improves muscle mass and function without worsening IS in postmenopausal women with IGT. Because it can be performed at low levels of exertion and improves muscle mass and function without impairing IS, ECC should be used to ameliorate muscle loss in physically inactive postmenopausal women. The impact of longer-term ECC on IS should be investigated. Demonstrating that ECC does not worsen IS in this population is significant because it has promise to combat the muscle-mediated impairments common in aging women.
Introduction
Aging is associated with a decline in skeletal muscle mass and function, collectively termed sarcopenia,1,2 and adversely impacts up to 50% of older individuals.3,4 Skeletal muscle is the largest reservoir for glucose in the body,5 accounting for up to 80% of the glucose disposal rate (GDR). Sarcopenia is accelerated in postmenopausal women,6 exacerbates the problems of skeletal muscle glucose uptake, and often is associated with subsequent insulin resistance and type 2 diabetes.7 Moreover, sarcopenia also manifests in reduced strength and an increased risk of impaired mobility and fall-related injuries in older people,8,9 although the relationship between muscle mass and physical function is variable.10,11 Nevertheless, resistance or strength training in women has been shown to improve muscle mass, strength, function, and mobility as well as insulin sensitivity (IS),12–14 although high muscle force production, a goal of resistance exercise programs, has been shown to worsen IS.15,16
The magnitude of the muscle mass and strength improvements following a resistance training program is linked to the magnitude of muscle forces produced.17,18 The greatest force production, hence stimulus to increase muscle mass and strength, is possible when an external force exceeds a muscle's maximum isometric force production, resulting in muscle lengthening or eccentric muscle contraction. Functional activities, such as negotiating obstacles and descending stairs, rely almost exclusively on eccentric muscle contractions.19 Eccentric muscle contractions can result in two to three times greater force production than the more traditional isometric or concentric muscle contractions.18,20 It is important to note that during eccentric resistance exercise (ECC), the external force (e.g., weight) must progressively increase to a magnitude whereby one cannot move the weight with concentric muscle shortening but can control the weight with eccentric muscle lengthening. Because this type of ECC can produce high muscle forces at relatively low perceived exertion levels,21,22 it makes for an ideal therapeutic countermeasure for aging, overweight, physically inactive women. In other words, ECC produces high force at low metabolic costs.
ECC is a promising solution for those suffering the mobility-related consequences of sarcopenia; however, a worsening of IS following a single exposure to eccentric muscle contractions has been reported.15,23 Exercise-induced muscle injury has been implicated as a potential cause of the detrimental effects of ECC on IS.15 The influence of nondamaging ECC, possible with repeated and progressive exposures over 10–12 weeks, on IS has not been studied in older individuals, specifically women with impaired glucose tolerance (IGT). Because ECC can be performed easily by overweight, physically inactive, aging individuals and it can effectively combat the muscle-related mobility decline that accompanies aging, investigating its impact on IS in older individuals, many who have metabolic abnormalities, is necessary.
In this report, we summarize the effects of a repeated (three times per week for 12 weeks), progressively increased ECC program on IS, body composition, strength, and physical function in overweight postmenopausal women with IGT. We hypothesized that repeated exposure to a progressively increased ECC would improve body composition (increase lean tissue, decrease fat tissue), muscle strength, and physical function (mobility) while not adversely impacting IS.
Materials and Methods
Subjects
Sixteen overweight or obese postmenopausal women (aged 56.1 ± 6.4 years, body mass index [BMI] 29.9 ± 4.1), with serum follicle-stimulating hormone (FSH) levels >30 IU/L and IGT defined by plasma glucose levels between 140 and 200 mg/dL 2 hours after ingesting a 75-g glucose load, volunteered to participate in this study. Eligibility required all participants not to be taking hormone replacement therapy (HRT), using nicotine, or participating in a regular resistance training program for the past 12 months. In addition, all were weight stable over the previous 12 months, and none had unstable medical conditions (i.e., no medication changes, newly diagnosed medical conditions in the previous 12 months; no cardiovascular, orthopedic, or neuromuscular conditions that would prevent them from participating in an exercise program.)
Measurements
Prior to participation, all women signed an Institutional Review Board-approved informed consent document. The initial screening examination included a medical history, physical examination, blood sample draw (to determine FSH levels), and oral glucose tolerance test (OGTT). Those meeting the study eligibility requirements then participated in an overnight pretraining testing session in the General Clinical Research Center (GCRC). This testing consisted of measurements of body composition, strength, and physical function as well as a hyperinsulinemic-euglycemic (HE) clamp study. An identical, posttraining testing session was completed at the end of the 12-week training program. Participants kept a diet record for the 3 days prior to their pretraining testing and repeated this diet for 3 days prior to their posttraining testing.
Procedures
After completion of the pretraining testing, the participants were randomly assigned to either the ECC group (n = 10) or a no exercise control group (CON, n = 6). Both the ECC and CON groups were individually instructed and were provided written handouts on diet and exercise according to the clinical guidelines of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The CON group did not participate in a supervised exercise program. The ECC group participated in an exercise program that involved high-force lower extremity extensor muscle contractions for 3 nonconsecutive days per week for 12 weeks.24 Eccentric exercising of muscle occurs anytime the magnitude of a force applied to a muscle exceeds that produced by the muscle and the muscle lengthens, that is, undergoes an eccentric contraction. Conversely, when a muscle's force production is greater than that applied to the muscle, the muscle shortens, that is, undergoes a concentric contraction. The former eccentric contraction can thus produce greater muscle forces than the latter concentric contraction and, hence, causes greater gains in muscle size and strength.
In this paper, we report on eccentric exercise to the knee extensors, where the muscle is lengthened as the subject attempts to slow down the external load being applied to the muscle by the motorized ergometer movement. This is quite different from a typical strengthening exercise whereby the muscle must work in a concentric fashion (e.g., lifting a weight) prior to being exercised eccentrically (e.g., lowering a weight). This requisite preceding concentric action is eliminated in the eccentric exercise described here. By eliminating the preceding concentric action, the muscle can exercise eccentrically against an external load that could never be moved concentrically, and the muscle can produce high forces that ultimately lead to beneficial muscle changes. For ECC to achieve the beneficial gains noted, it is important to be aware that the external load required must be greater than what one could move concentrically.
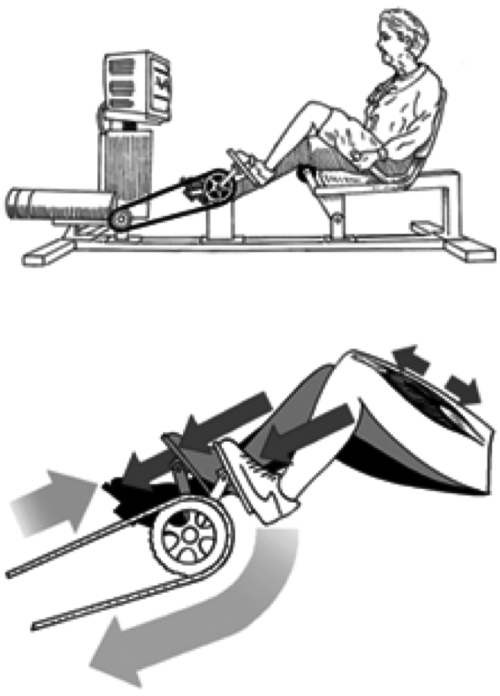
A physical therapist and exercise physiologist supervised the exercise sessions. Ratings of perceived exertion (RPE), using the Borg scale,25 were obtained from the subject at the end of each training session. This ECC program was titrated to progressively increase in intensity while avoiding muscle damage. The progression proceeded from a perceived exertion level of very very light to somewhat hard and from a duration of 5 minutes to 30 minutes per day and was performed on a high-force eccentric ergometer, described previously26 (Fig. 1). As the ergometer pedals moved, subjects attempted to slow the pedals by applying force, resulting in high-force eccentric muscle contractions of the lower extremity extensor muscles. The progression of eccentric training is summarized in the Appendix. Total work on the ergometer was calculated by integrating the work in joules determined directly from a 0–10-V output from the ergometer motor and the distance the pedal arm moved, both calibrated to a known work value. The amount of work in kilojoules (kJ) was monitored and recorded throughout the study.
FIG. 1.
Eccentric ergometer. (Top) High muscle forces are generated on an eccentric ergometer powered by a 3-hp motor that drives the pedals in a backward rotation. (Bottom) As the pedals move toward the participant (at right), she resists by applying force to the pedals. Because the magnitude of force produced by the motor exceeds that produced by the participant, the leg extensors lengthen, creating eccentric muscle contractions. (Adapted from LaStayo et al., 2003.)35
Participants were assessed for body composition (lean and fat tissue) by dual energy x-ray absorptiometry (DEXA) (Hologic QDR-4500A; Waltham, MA). In addition, a specific region of interest from the upper edge of the second lumbar vertebra to the lower edge of the fourth lumbar vertebra was used to assess abdominal fat mass.27
The computer-based system, Quantitative Muscle Assessment (Computer Source, Gainesville, GA), was used to quantify muscle strength. Maximal isometric force measures of the knee extensors, when the knee was fixed at 90° flexion, were taken as the participant sat on a dynamometer. Three measures were obtained for each leg to determine a mean value. The 6-minute walk test (6MW), a standard measure of the distance a subject walks in 6 minutes, was used to assess, overall, both locomotor ability and fatigue.28 Pedometer measurements29 (Yamax Digiwalker SW-701, San Antonio, TX) of average steps taken per day over a 7-day period before and after the 12-week training program were also collected as an additional measure of physical function.30
Peripheral insulin resistance was quantified by a 2-step HE clamp test that was performed the morning after an overnight stay and 12-hour fast in the GCRC.31 To minimize the immediate effects of the most recent bout of ECC on the posttraining assessment of IS, the posttraining HE clamp was performed 72 hours after the last exercise bout. Briefly, a catheter was placed into the antecubital vein for administration of glucose, insulin, potassium, and saline. Arterialized venous blood samples were obtained from an indwelling catheter placed in a vein of the opposite hand that was wrapped in a warming device. Two blood samples were taken from −20 minutes to time 0 to measure basal insulin and glucose. At time 0, the HE clamp was started and continued for approximately 120 minutes. Blood samples were taken at 5-minute intervals for plasma glucose determination using a YSI-2300 STAT Plus glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Insulin was infused at a predetermined rate based on the subject's body surface area. Plasma glucose determinations were used to titrate the infusion rate of 20% glucose as needed to maintain euglycemia. To obtain two different target insulin levels, insulin was increased after both blood glucose levels and glucose infusion rates had reached a steady state (< ± 5% of 95 mg/dL for six consecutive readings, 5 minutes apart), approximately 75 minutes after initiating the clamp. The initial infusion level was 40 mU/m2/min to achieve insulin levels of approximately 90 uU/mL (one-half maximum glucose disposal). The final infusion level was 200 mU/m2/min to achieve insulin levels of approximately 300 uU/mL (maximum glucose disposal). The clamp was stopped by the same criterion used to increase the insulin level, steady-state glucose near 95 mg/dl for six consecutive readings. Insulin levels were measured by radioimmunoassay using a double-antibody technique (Coat-A-Count; Diagnostic Products Corp., Los Angeles, CA). Results of the HE clamp studies are expressed as the glucose infusion rate (GIR) during each of the two clamp stages.
Data were analyzed with the Statistical Package for the Social Sciences version 13.0 (SPSS Inc, Chicago, IL). Descriptive statistics were calculated for baseline demographic characteristics. In the analyses, we evaluated the training effect on IS, body composition, strength, and physical function. To assess between-group differences at baseline, the groups were compared on all outcome variables using nonparametric tests for two independent groups. We hypothesized that ECC would produce greater changes in strength, physical function, and muscle mass. Because previous research has suggested that muscle damage has worsened IS, we hypothesized that our nondamaging repetitive eccentric intervention would result in our acceptance of the null hypothesis; that is, there would be no between-group differences in IS. To examine these outcomes, the change scores (posttraining values – pretraining values) were calculated for each dependent variable. Between-group comparisons of these change scores were analyzed using separate nonparametric tests for two independent groups. The level of significance was set at p < 0.05. To gain a clearer picture of the differential response of the groups, the within-group and between-group magnitudes of effect were estimated using calculations of effect size and percent change for all dependent variables.
Results
All 16 participants completed the trial (10 ECC, 6 CON); there was no study attrition. Both groups were similar in age, pretraining BMI, waist circumference, IGT status, fasting plasma glucose, and fasting plasma insulin. In only one outcome measure, average steps per day, were there significant between-group differences at baseline (Table 1).
Table 1.
Pretraining Characteristics
| Eccentric (n = 10) | Control (n = 6) | |
|---|---|---|
| Age (years) | 56.3 ± 6.4 | 53.2 ± 6.5 |
| (51.7–60.9)a | (46.4–60.0) | |
| BMI (kg/m2) | 28.5 ± 3.7 | 32.2 ± 4.0 |
| (25.8–31.2) | (28.0–36.4) | |
| Waist circumference (cm) | 93.0 ± 8.0 | 97.2 ± 6.8 |
| (87.3–98.8) | (90.0–104.4) | |
| 2-hour postchallenge glucose (mg/dL) | 171.0 ± 22.8 | 153.0 ± 10.6 |
| (153.6–188.6) | (140.4–170.3) | |
| Fasting plasma glucose (mg/dL) | 98.6 ± 5.0 | 99.2 ± 6.4 |
| (94.8–102.5) | (92.5–105.9) | |
| Fasting plasma insulin (μU/mL) | 10.9 ± 9.7 | 8.5 ± 4.2 |
| (3.4–18.3) | (4.1–12.9) | |
| Steps per day | 5949 ± 2170 | 7873 ± 778* |
| (4396–7501) | (6906–8839) |
Means ± SD (95% CI).
p < 0.05.
All ECC participants completed 36 training sessions over 12 weeks; that is, there was 100% adherence. Work increased from 20.3 kJ to 229.7 kJ, and perceived exertion was incrementally increased from very light (8.5) to somewhat hard (13.0) over the first 3 weeks of training. Subjects maintained their perceived exertion levels at somewhat hard throughout the rest of the 12-week program.
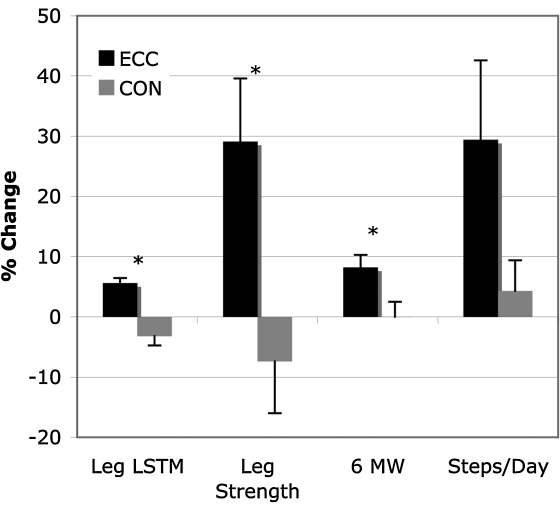
The mean change in leg soft tissue lean mass (STLM) was significantly different between groups [p = 0.03, between-group effect size (ES) = 1.32]. The leg STLM improvements by the participants in the ECC group exceeded those in the CON group (ECC +5.6%, ES= 0.85; CON −3.2%, ES = 0.28)(Fig. 2). Although there was no significant between-group difference in change in abdominal fat, the participants in the ECC group lost 3.7% (ES = 0.11), whereas the CON group mean increased by 2.5% (ES = 0.13) (p = 0.15) (Table 2).
FIG. 2.
Percent change in leg soft tissue lean mass (STLM), leg strength, 6-minute walk distance (6MW), and steps taken per day between ECC and CON groups. *p < 0.05; error bars represent SD.
Table 2.
Training Outcomesa
| |
Eccentric (n = 10) |
|
Control (n = 6) |
|
||
|---|---|---|---|---|---|---|
| Group | Pre | Post | Mean difference (95% CI) | Pre | Post | Mean difference (95% CI) |
| Leg STLMb (kg)* | 7.33 (0.5) | 7.75 (0.5) | 0.41 (0.01–0.80) | 8.52 (1.1) | 8.25 (0.98) | −0.53 (−1.60–0.55) |
| Quadriceps strength (kg)* | 31.8 (7.4) | 41.1 (8.1) | 9.30 (3.70–14.80) | 39.0 (17.7) | 36.1 (13.9) | −2.9 (−11.30–5.51) |
| 6MW (m)* (meters) | 687.1 (43.9) | 743.4 (60.2) | 56.40 (20.20–92.50) | 696.2 (75.5) | 699.6 (66.5) | 3.30 (−25.76–32.43) |
| Steps/day | 5949 (2170) | 7976 (2467) | 1747 (321–3174) | 7873 (778) | 8212 (1612) | 339 (−760–1438) |
| Abdominal fat (kg) | 1.62 (0.5) | 1.56 (0.6) | −0.06 (−0.19–0.05) | 2.00 (.41) | 2.05 (0.43) | 0.05 (−0.24–−0.35) |
| Fasting plasma glucose (mg/dL) | 98.6 (5.0) | 98.4 (7.5) | −0.002 (−0.05–0.04) | 99.2 (6.4) | 96.4 (8.1) | 0.03 (−0.10–0.05) |
| Fasting plasma insulin (μU/mL) | 10.9 (9.7) | 10.6 (10.7) | 1.73 (−2.02–5.48) | 8.5 (4.2) | 8.3 (6.6) | 0.11 (−0.96–1.12) |
| Maximum GIR (mg · kg FFM−1 · min−1) | 10.99 (2.7) | 10.80 (4.5) | −0.04 (−0.22–0.14) | 15.05 (4.4) | 15.01 (3.9) | 0.01 (−0.14–0.15) |
| GIR at 40 mU/m2/minc (mg · kg FFM−1 · min−1) | 5.34 (2.5) | 6.36 (3.8) | 0.18 (−0.27–0.62) | 4.87 (0.15) | 6.26 (1.38) | 0.29 (−0.43–1.01) |
Values are means ± (SD).
STLM, soft tissue lean mass; 6MW, 6-minute walk; GIR, glucose infusion rate.
n = 8; 3 control, 5 eccentric.
Significant differences between the eccentric group mean change scores and the control group mean change scores (p < 0.05).
Relative to physical function, there were significant differences in the change scores for both quadriceps muscle strength and 6MW distance (quadriceps muscle strength, p = 0.01, between-group ES = 1.66; 6MW distance, p = 0.02, between-group ES = 1.29). There was a trend toward significance in steps per day (p = 0.08, between-group ES = 0.97) (Table 2). The ECC group showed greater improvements in quadriceps muscle strength, 6MW distance, and steps per day than did the CON group (ECC quadriceps muscle strength +29.1%, ES = 1.26; 6MW distance +8.2%, ES = 1.13; steps/day +29.4%, ES = 0.79; CON quadriceps muscle strength −7.4%, ES = 0.2; 6MW distance +0.01%, ES = 0.05; steps/day +4.3%, ES = 0.29) (Fig. 2).
No significant differences in the change scores between groups were noted for either fasting plasma glucose (p = 0.47) or fasting plasma insulin levels (p = 0.44).
Progressively increased ECC had no impact on IS. No significant differences in change scores between groups were found for maximum GIR (p = 0.91) or for the GIR at the lower insulin infusion level (p = 0.65). The fact that the 95% confidence intervals of the mean change scores for GIR values for both groups overlap and that they encompass 0 supports that there was no change in IS in either group.32 Calculation of the within-group effect size for the ECC group was 0.06. The unanimous agreement of all these statistical results led us to conclude that no meaningful or clinically significant change in IS occurred posttraining, that is, no worsening of IS following repeated exposure to eccentric muscle activity.
Discussion
ECC is an easily tolerated yet potent intervention that can potentially mitigate worsening physical function and mobility-related consequences of sarcopenia in aging women. However, the association of a single exposure to eccentric exercise with a worsening of IS, thought to occur in parallel with damage to muscle,15,16 has been a barrier to its clinical use in this population. Because progressive and repeated exposure to ECC protects skeletal muscle from damage,26,33,34 we hypothesized that repeated exposure to a progressively increased ECC program over 12 weeks would improve body composition, strength, and function without adversely impacting IS. Our data demonstrate significant improvements in leg STLM, strength, and mobility without adversely impacting IS following 12 weeks of ECC in aging, overweight, physically inactive women with IGT.
Our results are in contrast to the previously reported worsening of IS after a single exposure to eccentric exercise, thought to occur in concert with damage to muscle.15,23 The goal of our study was to explore a 12-week protocol of repeated exposures to high-muscle forces via ECC that has been described previously as safe, feasible, and nondamaging to muscle in older, impaired, physically inactive populations.26,35 Because our aim was to determine if IS worsens (as it does after a single exposure) after an established, nondamaging, repeated exposure to eccentric muscle activity, we did not specifically monitor markers of muscle damage, hence cannot comment on any potential influence of damage. All indications are that the ECC did not adversely impact muscle or IS.
Although IS in our study did not worsen as a result of exposure to ECC, neither did it improve. These results are in contrast to previously published positive effects of traditional resistance training programs on IS in postmenopausal women.13,14,36,37 These improvements have been attributed to both increases in muscle mass and decreases in abdominal adiposity. The specific types of resistance-training programs and the posttraining measurements of IS vary across studies, however, making it difficult to determine the degree and mechanism of effects on IS. Of the studies that report improved IS or glycemic control in postmenopausal women following resistance training, only two report improvements ≥ 24 hours after the final exercise bout, and both were of 16-weeks duration.14,36 Conversely, Goulet et al.38 reported no sustained effect on IS 96 hours after the final exercise bout of a 6-month ECC program.38
Several aspects of the training regimen used in our study may account for the lack of change in IS. First, the stability of IS may have been related to the length of training time. Previous studies14,39 have employed training regimens for up to 6 months.12,38 Second, the amount of muscle recruited and the amount of muscle mass increase from the exercise may have been insufficient to induce systemic IS changes; that is, the women in this study exercised only the lower extremities, whereas studies reporting IS changes exercised several major muscle groups.13,14 Whether significant changes in total body composition as a result of resistance training are needed to positively impact IS is still unclear,14,36 but this remains an alluring and testable hypothesis. This is especially true considering that the resistance-trained participants reported by Cauza et al.,36 who demonstrated a 6.5% improvement in fat free mass (FFM) and a 9.7% decrease in fat mass (FM), also demonstrated significant improvements in long-term glycemic control.
It is important to note that the timing of the posttesting (72 hours after the final exercise bout) in our study was deliberate in order to determine if repeated ECC and not simply a single exposure would worsen IS; any long-lasting IS effects of the last exercise session may have been masked. Finally, the current study was not designed to make a comparison of resistance exercise modes; rather, we intended to determine if repeated exposure to eccentric muscle activity would improve body composition, strength, and function while not adversely impacting IS in postmenopausal women with IGT. Future research should compare an ECC program with both a traditional resistance training program and aerobic training, another exercise mode previously shown to positively impact IS.
Some limitations notable in the present study include the small sample size, the potential impact of differing initial levels of spontaneous physical activity (i.e., steps per day) between groups, and the between-group differences in the pretraining maximum GIR. Because the CON group had higher initial levels of spontaneous physical activity, we cannot rule out that they did not change posttraining because of this initial difference. Because we observed no within-group changes in maximum GIR in either group, however, we are confident that the differences that were observed pretraining in this measure of IS did not impact our results.
Aging women are subject to loss of muscle mass, strength, and physical function. These deficits are often accompanied by insulin resistance. Our report illustrates that ECC is feasible and improves muscle mass and strength in older women. Moreover, these changes are clinically meaningful in that they were accompanied by improvements in physical function that may enable these women to be more active. Demonstrating that ECC does not worsen IS in this population is significant because it has promise to combat the muscle-mediated impairments common in aging women.
Appendix:
Eccentric Group Resistance Training Progression
| Week | Times/week | Training duration | RPEa |
|---|---|---|---|
| 1 | 3 | 5 minutes | 7 (very, very light) |
| 2 | 3 | 5–10 minutes | 9–11 (very light to fairly light) |
| 3 | 3 | 10–15 minutes | 11–13 (fairly light to somewhat hard) |
| 4 | 3 | 15–20 minutes | 13 (somewhat hard) |
| 5–12 | 3 | 20–30 minutes | 13 (somewhat hard) |
RPE, rating of perceived exertion for the lower extremities.
Footnotes
Portions of this study are published in abstract form in the Gerontologist 2005;45(52):297–298.
Acknowledgments
Our appreciation is extended to the women who participated in the program; to the General Clinical Research Center (NCRR-00064), specifically Deanna Palma and Laurene Jones for their expertise and care during the HE clamp studies; to Dan Williams for critical discussions about project design and implementation; and to J. Parry Gerber for his assistance with training and manuscript preparation.
This research was supported by the Utah Building Interdisciplinary Research Careers in Women's Health Program (NIH grant 5K12HD043449-04).
Disclosure Statement
No competing financial interests exist.
References
- 1.Hughes VA. Roubenoff R. Wood M. Frontera WR. Evans WJ. Fiatarone Singh MA. Anthropometric assessment of 10–y changes in body composition in the elderly. Am J Clin Nutr. 2004;80:475–482. doi: 10.1093/ajcn/80.2.475. [DOI] [PubMed] [Google Scholar]
- 2.Raguso CA. Kyle U. Kossovsky MP, et al. A 3–year longitudinal study on body composition changes in the elderly: Role of physical exercise. Clin Nutr. 2006;25:573–580. doi: 10.1016/j.clnu.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner RN. Koehler KM. Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 4.Iannuzzi-Sucich M. Prestwood KM. Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–777. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA. Jacot E. Jequier E. Maeder E. Wahren J. Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 6.Frontera WR. Hughes VA. Lutz KJ. Evans WJ. A cross-sectional study of muscle strength and mass in 45– to 78–yr-old men and women. J Appl Physiol. 1991;71:644–650. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 7.Karakelides H. Sreekumaran Nair K. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005;68:123–148. doi: 10.1016/S0070-2153(05)68005-2. [DOI] [PubMed] [Google Scholar]
- 8.Visser M. Goodpaster BH. Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 9.Janssen I. Heymsfield SB. Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 10.Bouchard DR. Beliaeff S. Dionne IJ. Brochu M. Fat mass but not fat-free mass is related to physical capacity in well-functioning older individuals: Nutrition as a determinant of successful aging (NuAge) The Quebec Longitudinal Study. J Gerontol A Biol Sci Med Sci. 2007;62:1382–1388. doi: 10.1093/gerona/62.12.1382. [DOI] [PubMed] [Google Scholar]
- 11.Lebrun CE. van der Schouw YT. de Jong FH. Grobbee DE. Lamberts SW. Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause. 2006;13:474–481. doi: 10.1097/01.gme.0000222331.23478.ec. [DOI] [PubMed] [Google Scholar]
- 12.Dionne IJ. Melancon MO. Brochu M. Ades PA. Poelhman ET. Age-related differences in metabolic adaptations following resistance training in women. Exp Gerontol. 2004;39:133–138. doi: 10.1016/j.exger.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Fenicchia LM. Kanaley JA. Azevedo JL, Jr, et al. Influence of resistance exercise training on glucose control in women with type 2 diabetes. Metabolism. 2004;53:284–289. doi: 10.1016/j.metabol.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Ryan AS. Pratley RE. Goldberg AP. Elahi D. Resistive training increases insulin action in postmenopausal women. J Gerontol A Biol Sci Med Sci. 1996;51:M199–205. doi: 10.1093/gerona/51a.5.m199. [DOI] [PubMed] [Google Scholar]
- 15.Kirwan JP. Hickner RC. Yarasheski KE. Kohrt WM. Wiethop BV. Holloszy JO. Eccentric exercise induces transient insulin resistance in healthy individuals. J Appl Physiol. 1992;72:2197–2202. doi: 10.1152/jappl.1992.72.6.2197. [DOI] [PubMed] [Google Scholar]
- 16.Asp S. Daugaard JR. Kristiansen S. Kiens B. Richter EA. Eccentric exercise decreases maximal insulin action in humans: Muscle and systemic effects. J Physiol. 1996;494:891–898. doi: 10.1113/jphysiol.1996.sp021541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hortobagyi T. Hill JP. Houmard JA. Fraser DD. Lambert NJ. Israel RG. Adaptive responses to muscle lengthening and shortening in humans. J Appl Physiol. 1996;80:765–772. doi: 10.1152/jappl.1996.80.3.765. [DOI] [PubMed] [Google Scholar]
- 18.Lindstedt SL. LaStayo PC. Reich TE. When active muscles lengthen: Properties and consequences of eccentric contractions. News Physiol Sci. 2001;16:256–261. doi: 10.1152/physiologyonline.2001.16.6.256. [DOI] [PubMed] [Google Scholar]
- 19.McFadyen BJ. Winter DA. An integrated biomechanical analysis of normal stair ascent and descent. J Biomech. 1988;21:733–744. doi: 10.1016/0021-9290(88)90282-5. [DOI] [PubMed] [Google Scholar]
- 20.Komi PV. Buskirk ER. Effect of eccentric and concentric muscle conditioning on tension and electrical activity of human muscle. Ergonomics. 1972;15:417–434. doi: 10.1080/00140137208924444. [DOI] [PubMed] [Google Scholar]
- 21.Bigland-Ritchie B. Woods JJ. Integrated electromyogram and oxygen uptake during positive and negative work. J Physiol. 1976;260:267–277. doi: 10.1113/jphysiol.1976.sp011515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott BC BB. Ritchie JM. The physiological cost of negative work. J Physiol (Lond) 1952;117:380–390. doi: 10.1113/jphysiol.1952.sp004755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Aguila LF. Krishnan RK. Ulbrecht JS, et al. Muscle damage impairs insulin stimulation of IRS-1, PI 3-kinase, and Akt-kinase in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E206–212. doi: 10.1152/ajpendo.2000.279.1.E206. [DOI] [PubMed] [Google Scholar]
- 24.Dibble LE. Hale TF. Marcus RL. Droge J. Gerber JP. Lastayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson's disease. Mov Disord. 2006;21:1444–1452. doi: 10.1002/mds.20997. [DOI] [PubMed] [Google Scholar]
- 25.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 26.Dibble LE. Hale T. Marcus RL. Gerber JP. Lastayo PC. The safety and feasibility of high-force eccentric resistance exercise in persons with Parkinson's disease. Arch Phys Med Rehabil. 2006;87:1280–1282. doi: 10.1016/j.apmr.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Park YW. Heymsfield SB. Gallagher D. Are dual-energy x-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Rel Metab Disord. 2002;26:978–983. doi: 10.1038/sj.ijo.0801982. [DOI] [PubMed] [Google Scholar]
- 28.Bean JF. Kiely DK. Leveille SG, et al. The 6–minute walk test in mobility-limited elders: What is being measured? J Gerontol A Biol Sci Med Sci. 2002;57:M751–756. doi: 10.1093/gerona/57.11.m751. [DOI] [PubMed] [Google Scholar]
- 29.Crouter SE. Schneider PL. Karabulut M. Bassett DR., Jr. Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35:1455–1460. doi: 10.1249/01.MSS.0000078932.61440.A2. [DOI] [PubMed] [Google Scholar]
- 30.Tudor-Locke CE. Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Med. 2001;31:91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA. Tobin JD. Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 32.Blackwelder WC. “Proving the null hypothesis” in clinical trials. Control Clin Trials. 1982;3:345–353. doi: 10.1016/0197-2456(82)90024-1. [DOI] [PubMed] [Google Scholar]
- 33.Newham DJ. Jones DA. Clarkson PM. Repeated high-force eccentric exercise: Effects on muscle pain and damage. J Appl Physiol. 1987;63:1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- 34.Lastayo P. McDonagh P. Lipovic D, et al. Elderly patients and high force resistance exercise—A descriptive report: Can an anabolic, muscle growth response occur without muscle damage or inflammation? J Geriatr Phys Ther. 2007;30:128–134. doi: 10.1519/00139143-200712000-00008. [DOI] [PubMed] [Google Scholar]
- 35.LaStayo PC. Ewy GA. Pierotti DD. Johns RK. Lindstedt S. The positive effects of negative work: Increased muscle strength and decreased fall risk in a frail elderly population. J Gerontol A Biol Sci Med Sci. 2003;58:M419–424. doi: 10.1093/gerona/58.5.m419. [DOI] [PubMed] [Google Scholar]
- 36.Cauza E. Hanusch-Enserer U. Strasser B, et al. The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil. 2005;86:1527–1533. doi: 10.1016/j.apmr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Cuff DJ. Meneilly GS. Martin A. Ignaszewski A. Tildesley HD. Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26:2977–2982. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 38.Goulet ED. Melancon MO. Dionne IJ. Leheudre MA. No sustained effect of aerobic or resistance training on insulin sensitivity in nonobese, healthy older women. J Aging Phys Act. 2005;13:314–326. doi: 10.1123/japa.13.3.314. [DOI] [PubMed] [Google Scholar]
- 39.Ryan AS. Hurlbut DE. Lott ME, et al. Insulin action after resistive training in insulin-resistant older men and women. J Am Geriatr Soc. 2001;49:247–253. doi: 10.1046/j.1532-5415.2001.4930247.x. [DOI] [PubMed] [Google Scholar]