Abstract
The impact of left ventricular ejection fraction (EF) on outcome in patients with heart failure (HF) undergoing non-cardiac surgery has not been extensively evaluated. 174 subjects (mean age 75±12 years, 47% male, mean EF (47±18%) underwent intermediate or high risk non-cardiac surgery. Patients were stratified by EF and adverse perioperative complications were identified and compared. Adverse perioperative events occurred in 53 (30.5%) of subjects, including 14 (8.1%) deaths within 30 days, 26 (14.9%) myocardial infarctions, and 44 (25.3%) HF exacerbations. Among the factors associated with adverse perioperative outcomes in the first 30 days were advanced age (e.g. >80 years), diabetes and a severely decreased EF (e.g. <30%). Long term mortality was high and Cox proportional hazards analysis demonstrated that EF was an independent risk factor for long term mortality.
Keywords: Risk Stratification, Heart Failure, Non-cardiac surgery
Introduction
Patients with clinical heart failure (HF) or a history of HF are at significant risk for perioperative complications (1–3) and several well known and widely used indices of cardiac risk(4–6) include heart failure as an independent prognostic variable. Using Medicare claims data, patients with HF, independent of the presence of coronary artery disease, have been reported to suffer significant perioperative morbidity and mortality despite advances in perioperative care(2;7). After accounting for demographic characteristics, types of surgery, and co-morbid conditions, the risk adjusted thirty-day mortality was nearly double in patients with HF compared to patients with coronary artery disease and to an elderly control without a history of coronary artery disease or HF. Both these studies(2;7) were limited by the absence of clinical parameters and patient level data by which assessment of risk could be further evaluated and quantified. Data from a single center study among individuals with clinically stable heart failure undergoing elective non-cardiac surgery did not demonstrate a high perioperative mortality whether the EF was >40% or <40%.(8) However, all patients in this latter study were all seen in a preoperative assessment, consultation and treatment program, which did not include many of the high risk patients. Additionally, the impact of EF was evaluated only as a dichotomous variable (e.g. <40 or >40%). Accordingly, the purpose of this study is to evaluate the impact of ejection fraction on perioperative outcomes and long term mortality in subjects with HF undergoing intermediate to high risk non cardiac surgical procedures after controlling for other risk factors.
Methods
This study is a retrospective chart review of patients at Columbia University Medical Center diagnosed with HF who underwent intermediate or high-risk non-cardiac surgery from 2001–2004. Institutional IRB approval was obtained for the study.
Study Population and Design
All subjects who underwent either an intermediate or high-risk non-cardiac surgical procedure(1;9) including peripheral vascular surgery, aortic repair–including dissection, carotid endarterectomy, head and neck surgery, intraperitoneal surgery, non-cardiac intrathoracic surgery, orthopedic surgery, or prostate surgery and had a physician visit or hospitalization coded for HF [including the following ICD-9 codes: 425.4, 428.0, 428.3, 428.9] in the two years prior to the surgical procedure were included in the initial cohort. Analysis of a central data repository with the aforementioned ICD-9 codes and relevant CPT codes was used to identify potential subjects.
Among this initial cohort of 410 patients, the diagnosis of HF was further investigated by an explicit chart review. All 174 patients subsequently included in the study met the Framingham criteria for HF.(10) From the initial cohort a total of 236 patients were excluded. Reasons for exclusion were the following: (1) history of HF not clearly documented by Framingham criteria, or the diagnosis of HF was made postoperatively, n=177, (2) history of a prior organ transplant, n=26, (3) hospital admission not specifically for the surgical procedure, n=28 or (4) the surgery was emergent, n=5.
Demographic information, clinical characteristics, preoperative and postoperative laboratory values, and chest x-ray, electrocardiogram and non-invasive testing reports, including echocardiograms used to determine the ejection fraction, were recorded on standard data abstraction forms for each patient. Intraoperative data were also recorded for each patient, as well as preoperative and postoperative medications. Surgery was classified as elective if it was necessary but admission for surgery could be delayed for at least 24 hours, as urgent if a delay of surgery of more than 24 hours was likely to result in the condition deteriorating, and as emergent if death was likely to result without immediate operative intervention (the latter group was excluded from this analysis). The primary outcomes were myocardial infarction and HF exacerbation occurring during the incident hospitalization, and/or death occurring within 30 days. Myocardial infarction was defined as elevation of postoperative troponins with concomitant clinical signs and symptoms and/or changes on electrocardiograms.(11;12) A HF exacerbation was defined as new or increasing rales noted on clinical exam with a chest x-ray showing worsening peripheral vascular congestion or edema. Using the Social Security Death Index, long term (~4.5 years) survival was classified as dead, alive or unknown. The latter category constituted 11 subjects (6.3%) for whom vital statistics could not be definitively ascertained.
Statistical Analysis
Demographic and clinical characteristics of study subjects were compared between groups stratified by EF as normal, EF>50%, mildly reduced, EF 40–50%; moderately reduced, EF 30–40%; and severely reduced, EF < 30%. To determine the impact of ejection fraction on adverse outcomes, we evaluated EF as categorical variable (normal, mildly reduced, moderately reduced, and severely reduced) on the composite perioperative endpoint of myocardial infarction and HF exacerbation, evaluated during the index hospitalization, or death, evaluated within 30 days of the operation. In addition to ventricular function, demographic and clinical characteristics, preoperative, intra-operative, and postoperative variables were evaluated for univariate associations with the composite endpoint as well. Specifically, to evaluate renal function, we calculated estimated glomerular filtration rate using the Cockroft-Gault formula. In order to determine which factors independently contributed to adverse outcomes, a multivariate logistic regression model was employed. All variables found to be associated with adverse outcomes at a threshold (p < 0.05) were included in a multivariable logistic regression model, retaining in the final model only those variables that contained at least 1 statistically significant category (P < 0.05). Missing data was not imputed. Finally, long term mortality was evaluated in subjects stratified by EF as a categorical variable (normal, EF>50%; mildly reduced, EF 40–50%; moderately reduced, EF 30–40%; and severely reduced, EF < 30%) using Kaplan-Meier survival curves, excluding subjects with active malignancy. Cox proportional hazards model was performed to evaluate the impact of ejection fraction on long term mortality as a continuous variable after controlling for other risk factors. SAS 9.1 was used for all analyses.
Results
The 174 subjects were 75±12 years of age with an almost equal number of males and females. Most of the subjects were NYHA class II to III, with an average EF of 47±18%. They had a significant burden of cardiovascular risk factors and the etiology of HF was ischemic in slightly less than half of the subjects. Additionally, subjects were afflicted by several co-morbid conditions characteristic of a hospitalized cohort (Table 1). Subjects with HFNEF differed from those with heart failure with a low (<50%) ejection fraction (HFLEF) in that they were older, more often female, less often afflicted with atherosclerotic disease (e.g. CAD, MI and PVD) and less often had an ischemic etiology than cohorts with a reduced EF.
Table 1.
Baseline Demographics and Clinical Characteristics
| Heart Failure subgroups by Ejection Fraction | |||||
|---|---|---|---|---|---|
| HFNEF | HFLEF | ||||
| Demographics | Overall (n=174) | EF≥50% (n=90) | 40–49% (n=23) | 30–39% (n=33) | <30% (n=28) |
| Age – yr | 75±12 | 78±12† | 71±9 | 73±14 | 70±10 |
| Male sex - %* | 47 | 39 | 61 | 45 | 56 |
| Race - no. (%) | |||||
| Caucasian | 61 | 68 | 70 | 58 | 43 |
| Hispanic | 30 | 23 | 22 | 36 | 46 |
| African American | 9 | 9 | 9 | 6 | 11 |
| Cardiac Risk Factors (%) | |||||
| Hypertension | 82 | 84 | 87 | 82 | 68 |
| Diabetes Mellitus | 39 | 33 | 52 | 39 | 43 |
| History of Tobacco Use | 39 | 31 | 48 | 42 | 50 |
| Hyperlipidemia* | 51 | 42 | 48 | 67 | 64 |
| Other Medical Problems (%) | |||||
| Coronary Artery Disease* | 56 | 40 | 87 | 64 | 75 |
| Prior Myocardial Infarction* | 42 | 28 | 59 | 55 | 57 |
| Peripheral Vascular Disease* | 38 | 26 | 52 | 55 | 46 |
| History of Stroke | 13 | 13 | 22 | 12 | 4 |
| Atrial Fibrillation | 31 | 34 | 39 | 10 | 14 |
| Valvular Heart Disease | 25 | 27 | 4 | 30 | 32 |
| End Stage Renal Disease | 6 | 7 | 13 | 0 | 7 |
| COPD | 12 | 13 | 9 | 9 | 14 |
| Active Malignancy | 7 | 8 | 13 | 0 | 17 |
| Prior Malignancy | 10 | 10 | 13 | 12 | 6 |
| Characteristics of Heart Failure | |||||
| Etiology (Ischemic) (%)* | 42 | 20 | 74 | 48 | 53 |
| NYHA Class I/II/III (%) | 3/61/35 | 4/72/23 | 9/65/26 | -/34/66 | -/54/46 |
| Ejection Fraction | 47±18 | 63±7 | 42±3 | 32±2 | 22±4 |
| Type of Surgery (%) | |||||
| Vascular* | 47 | 34 | 57 | 61 | 61 |
| Orthopedic | 39 | 52 | 26 | 30 | 21 |
| Other† | 14 | 13 | 17 | 9 | 18 |
p < 0.05 for chi-square analysis with Fisher's exact test.
p < 0.05 for comparison with normal EF cohort in comparison with mild and severely decreased EF
All values are % unless otherwise noted
† Includes abdominal, urologic and thoracic procedures
A majority of the operative interventions were vascular. 82 (47%), and orthopedic, 69 (39%), with a minority being for abdominal, 17 (10%), urologic, 4 (2.3%), and thoracic 2 (1%) procedures. Subjects with heart failure and an EF≥50% more often underwent orthopedic procedures (52% vs. 25%) while those with an EF <50% more often underwent vascular surgery (60% vs. 34%). The Average American Society of Anesthesiologists score (ASA score) was 3.0±0.5 and did not differ between the cohorts. Forty-eight (28%) of the surgeries were elective while the remaining 126 (72%) were urgent in nature and did not differ based on EF cohort. General anesthesia was administered to 103 (59%) of the patients. Epidural, regional, or spinal anesthesia was administered to 25 (14%), 12 (7%), and 34 (20%) patients respectively. These did not differ across EF cohorts. Intra-operative hemodynamic monitoring was performed in 8 subjects (4.6%), 5 (5.9%) who had HFLEF and 3 (3.5%) who had HFNEF.
Standard therapies, including beta-blockers (57%) and angiotensin-converting enzyme (ACE) inhibitors (41%) were used in the preoperative period. These agents were prescribed more frequently to those with HFLEF compared to HFNEF. The average preoperative creatinine was 1.7±1.6 and the average hemoglobin was 12.1±2.9 g/dl. A preoperative cardiology consult was obtained in 112 (64%) of patients. A general medicine consultation was obtained in 54 (31%). 20% had neither consultation. The rate of consultation did not differ based on EF.
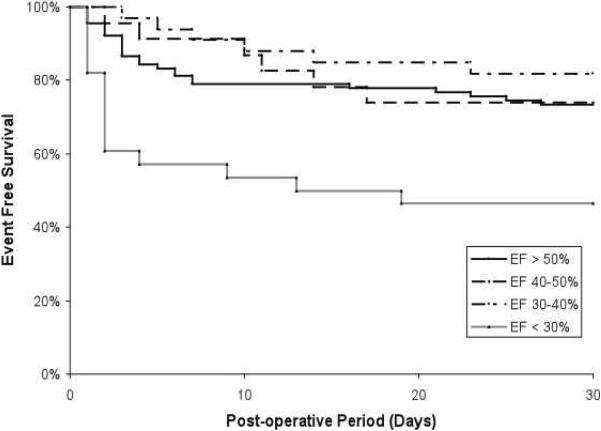
Fifty-three subjects (30.5%) had at least one perioperative event including 14 (8.1%) deaths, 26 (14.9%) myocardial infarctions, and 44 (25.3%) HF exacerbations. Perioperative deaths occurred on average 15±10 days post-operatively (median 17 days) with 8 deaths occurring prior to discharge and 6 deaths post-discharge. Subjects with a severely reduced EF (<30%) had a significantly higher risk of adverse perioperative events than subjects with a normal EF (Table 2 and Figure 1). In multivariate analysis, the presence of a severely reduced EF (< 30%) was independently associated with adverse perioperative events along with age > 80 years and the presence of diabetes.
Table 2.
Rates (95% CI) of Adverse Perioperative Outcomes Stratified by Ejection Fraction
| Subgroup by Ejection Fraction | Death | Myocardial Infarction | Heart Failure Exacerbation | Composite End Point (Death, MI or HF Exacerbation) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | N | % | 95% CI | |
| EF > 50% | 7 | 7.8 | 2.3 – 13.3 | 13 | 14.4 | 7.2 – 21.7 | 24 | 26.7 | 17.5 – 35.8 | 26 | 28.9 | 19.5 – 38.3 |
| 40–50% | 0 | 0 | - | 2 | 8.7 | 0 – 22.2 | 5 | 21.7 | 4.9 – 38.6 | 6 | 26.1 | 8.1 – 44.0 |
| 30–40% | 3 | 9.1 | 0 – 18.9 | 4 | 12.1 | 1.0 – 23.1 | 4 | 12.1 | 1.0 – 23.1 | 6 | 18.2 | 5.0 – 31.3 |
|
| ||||||||||||
| <30% | 4 | 14.3 | 1.3–27.3 | 7 | 25.0 | 9.0 – 41.0 | 11 | 39.3 | 21.2 – 57.4 | 15 | 53.6 | 35.1–72.0 |
Figure 1.
Perioperative outcomes among HF subjects undergoing non-cardiac surgery stratified by Ejection Fraction.
During the follow-up period, excluding those subjects with active cancer at baseline (n= 12, 6.9%), survival among this cohort was 73% (95% CI 65–80%) at one year, 58% (95% CI 49–65%) at 3 years and 40% (95% CI: 32–49%) at five years after surgical intervention. Long term mortality differed when stratified by the presence of a normal, mildly, moderately or severely reduced EF and formal statistical testing demonstrated that there was an increased risk of long term mortality in HF subjects the lower the EF group (Hazard Ratio of 1.25, 95% CI of 1.04–1.49). In a Cox proportional hazards model, the following variables remained significant associated with long term mortality: Age > 80 years (Hazard ratio: 2.04, 95% CI 1.3 – 3.19), Blood urea nitrogen > 50 mg/dl (Hazard ratio: 2.50, 95% CI 1.42 – 4.41) and ejection fraction (Hazard ratio: 1.30, 95% CI 1.08 – 1.57) per declining EF subgroup.
Discussion
The principal finding of the present study is that patients with HF undergoing intermediate and high risk non-cardiac surgery are at significant risk for perioperative complications including death, myocardial infarction and worsening HF. Independent risk factors for adverse perioperative events include advanced age (e.g. >80 years), diabetes and a severely reduced EF (<30%), but not a normal, mildly or moderately decreased ejection fraction. Additionally, these same patients have a high mortality over the ensuing years after operative intervention. Decreasing EF was an independent risk factor for increased long term mortality.
In the original Goldman study published in 1977, an S3 gallop or JVD were among the nine risk factors associated with life threatening cardiac complications and perioperative cardiac death. In addition, this study found that the rate of perioperative HF was 2% among patients with no history of HF, 6% among those with a prior history of HF and 35% among those with HF based on clinical and radiological evidence prior to surgery.(4) However, cardiac mortality was only 1.9% in that original cohort and only 5.8% experienced either cardiac death or major complications. More than thirty years later, with a rapidly aging population, surgical interventions are being undertaken more often in frailer adults with concomitant heart failure and various degrees of ventricular dysfunction resulting in significantly greater perioperative risk. Hernandez et al(2) identified higher mortality in the modern era by using a large Medicare database. He found that elderly patients with HF undergoing non-cardiac surgery had a risk adjusted 30 day mortality of 11.7% in comparison to a 30 day mortality of 6.6% found in patients with coronary artery disease. Our data are concordant with the findings of Hernandez (2;7), with 30% of our patients having at least one significant cardiovascular complication and 8% of them dying during the 30 day post-operative period.
Data from a single center study did not confirm the high mortality observed in our data and those from the Medicare database.(8) In this study, all of the patients were clinically stable and evaluated in a preoperative assessment and management program, resulting in a potential referral bias. Additionally, many of the surgical procedures performed were not high risk, with more than half of all interventions including laparoscopic, arthroscopic, thyroidectomy, laminectomy or total hip of knee replacements. Finally, all of the surgical procedures in that study were elective as compared to our cohort in which most procedures were urgent. These and other factors are likely explanations for the differences observed. Concordant with our findings, perioperative outcomes were not demonstrably different in the cohort with a preserved (e.g. ≥40%) versus reduced (<40%) EF and long term mortality was dependent on EF, similar to our data.
We found that a severely reduced ejection fraction was independently associated with adverse outcomes in the perioperative period and that EF was an independent predictor of mortality in the longer term. This finding was independent of surgical procedure, type of anesthesia and other co-morbid conditions. Among the cohort of older adult patients with a normal EF, which was a majority of the subjects in our study, perioperative outcomes were not significantly different from subjects with mild to moderate reductions in the their ventricular systolic function. Individuals with heart failure and a normal EF are often older women with multiple co-morbid conditions, as was the case in our data. While this population is less often afflicted with coronary artery disease and previous myocardial infarction, other co-morbid conditions that confer an increased perioperative risk including renal dysfunction (50%), diabetes (48%) and anemia (64%) were highly prevalent.
The long term mortality rates that we observed were consistent with other published data.(13) Among elderly patients who have been hospitalized with HF, the one-year mortality rate was about 33%, and five-year mortality rates were reported as high as 79% in men and 70% in women,(14) similar to but worse than our study population.
This study is a small, retrospective chart review from a single institution without a control group of subjects without heart failure. Our overall results are concordant with those from Medicare data showing an increased event rate in this population as compared to controls without heart failure,(2) despite the fact that our data was restricted to patients who not only had claims codes for heart failure but also met clinical criteria(10). We did not compare outcomes among the patients who did and did not meet clinical criteria for heart failure despite having a claim for heart failure and thus can not address the potential differences between these populations. This should be a target for future studies. These data obtained were abstracted from charts and relied on previously transcribed medical data. However, we employed a standardized method for data abstraction and pre-specified definitions to minimize variability. Additionally, the analysis of patient level data in the population and the comparison of outcomes among those stratified by EF after controlling for relevant confounders permitted the assessment of the impact of EF on outcomes. Several other measures (e.g. diastolic function and biomarkers) that might have predictive value were not routinely collected in the subjects studied and thus their role in risk stratification could not be assessed. While limited by small sample size and hence statistical power, we have corroborated the high risk of adverse events in such patients in the perioperative period and demonstrated the impact of EF on outcomes among those undergoing intermediate to high risk non-cardiac surgery.
In summary, patients with HF undergoing intermediate or high risk non-cardiac surgery are at significant risk for adverse perioperative outcomes and have a high mortality in the longer term. Several clinical variables are independent risk factors for adverse outcomes in such patients including a severely but not mildly or moderately decreased ejection fraction. Additionally, EF is independently associated with long term mortality. Further investigations aimed at confirming and extending these findings will be essential in order to facilitate much needed guidelines for managing this important cohort.
Table 3.
Odds Ratios and 95% CI for Adverse Peri-operative Events*
| Risk Factor | Univariate | Multivariate |
|---|---|---|
| Demographics: | ||
| Age (< or > 80 years) | 2.37 (1.22 – 4.61) | 3.84 (1.70 – 8.17) |
| Gender (Male) | 1.05 (0.77–1.44) | |
| Heart Failure Phenotype: | ||
| Ischemic vs. Non-Ischemic | 1.74 (0.90 – 3.63) | |
| >Moderate Valve Disease | 1.25 (0.60 – 2.60) | |
| Symptoms and Signs of Congestion | ||
| Paroxysmal Nocturnal Dyspnea | 2.94 (0.85–10.09) | |
| JVP/HJ Reflux | 15.19 (1.78–129.61) | |
| Rales | 1.09 (0.57–2.09) | |
| Edema | 0.89 (0.33–2.45) | |
| Radiographic Evidence of Congestion† | 1.74 (0.90–3.33) | |
| Ejection Fraction | ||
| EF – Normal (reference) | - | |
| - Mildly reduced | 0.87 (0.31–2.45) | |
| - Moderately reduced | 0.55 (0.20 – 1.48) | |
| - Severely reduced | 2.84 (1.19 – 6.79) | 4.88 (1.78 – 14.40) |
| Laboratory Tests: | ||
| GFR < 50 ml/min | 3.72 (1.85 – 7.49) | |
| BUN > 45 mg/dl | 2.98 (1.18 – 7.54) | |
| Hemoglobin < 12.0 g/dl | 1.61 (0.84 – 3.10) | |
| Other Medical Problems: | ||
| PVD | 1.95 (1.01 – 3.77) | |
| Diabetes | 1.88 (0.97 – 3.63) | 2.38 (1.10 – 6.68) |
| Preoperative Medications: | ||
| Beta Blocker | 1.03 (0.53 – 2.05) | |
| Ace Inhibitor | 0.96 (0.49 – 1.90) | |
| Digoxin | 1.21 (0.57 – 2.23) | |
| Statin | 0.97 (0.50 – 1.87) | |
| Aldosterone Antagonist | 0.13 (0.02 – 0.98) | |
| Characteristics of Surgery: | ||
| Vascular Surgery | 2.00 (1.04 – 3.85) | |
| Non-elective | 1.97 (0.90 – 4.33) |
Peri-operative events include (Death within 30 days, MI and HF Exacerbation During Index Hospitalization)
Includes pulmonary vascular congestion and/or pleural effusions
Acknowledgements
We are grateful to the statistical guidance provided by Ms. Shing M. Lee, Mr. Jimmy Doung, Dr. William Friedwald, Dr. Roger Vaughan and to Dr. Donald King for his helpful comments and editorial suggestions. This publication was made possible by Grant Number UL1 RR024156 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at the NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from the NIH Roadmap website.
Reference List
- (1).Eagle KA, Berger PB, Calkins H, Chaitman BR, Ewy GA, Fleischmann KE, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) J Am Coll Cardiol. 2002;39:542–53. doi: 10.1016/s0735-1097(01)01788-0. [DOI] [PubMed] [Google Scholar]
- (2).Hernandez AF, Whellan DJ, Stroud S, Sun JL, O'Connor CM, Jollis JG. Outcomes in heart failure patients after major noncardiac surgery. J Am Coll Cardiol. 2004;44:1446–53. doi: 10.1016/j.jacc.2004.06.059. [DOI] [PubMed] [Google Scholar]
- (3).Hernandez AF, Newby LK, O'Connor CM. Preoperative evaluation for major noncardiac surgery: focusing on heart failure. Arch Intern Med. 2004;164:1729–36. doi: 10.1001/archinte.164.16.1729. [DOI] [PubMed] [Google Scholar]
- (4).Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–50. doi: 10.1056/NEJM197710202971601. [DOI] [PubMed] [Google Scholar]
- (5).Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–49. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- (6).Detsky AS, Abrams HB, McLaughlin JR, Drucker DJ, Sasson Z, Johnston N, et al. Predicting cardiac complications in patients undergoing non-cardiac surgery. J Gen Intern Med. 1986;1:211–19. doi: 10.1007/BF02596184. [DOI] [PubMed] [Google Scholar]
- (7).Hammill BG, Curtis LH, nett-Guerrero E, O'Connor CM, Jollis JG, Schulman KA, et al. Impact of heart failure on patients undergoing major noncardiac surgery. Anesthesiology. 2008;108:559–67. doi: 10.1097/ALN.0b013e31816725ef. [DOI] [PubMed] [Google Scholar]
- (8).Xu-Cai YO, Brotman DJ, Phillips CO, Michota FA, Tang WH, Whinney CM, et al. Outcomes of patients with stable heart failure undergoing elective noncardiac surgery. Mayo Clin Proc. 2008;83:280–288. doi: 10.4065/83.3.280. [DOI] [PubMed] [Google Scholar]
- (9).Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol. 2007;50:1707–32. doi: 10.1016/j.jacc.2007.09.001. [DOI] [PubMed] [Google Scholar]
- (10).Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- (11).Newby LK, Alpert JS, Ohman EM, Thygesen K, Califf RM. Changing the diagnosis of acute myocardial infarction: implications for practice and clinical investigations. Am Heart J. 2002;144:957–80. doi: 10.1067/mhj.2002.129778. [DOI] [PubMed] [Google Scholar]
- (12).Apple FS, Wu AH, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: how to use existing assays clinically and for clinical trials. Am Heart J. 2002;144:981–86. doi: 10.1067/mhj.2002.124048. [DOI] [PubMed] [Google Scholar]
- (13).Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–89. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- (14).Croft JB, Giles WH, Pollard RA, Keenan NL, Casper ML, Anda RF. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med. 1999;159:505–10. doi: 10.1001/archinte.159.5.505. [DOI] [PubMed] [Google Scholar]