Abstract
In this paper, we have reported that split ring resonators (SRRs) structures can be used for bioassay applications in order to further improve the assay time and sensitivity. The proof-of-principle demonstration of the ultrafast bioassays was accomplished by using a model biotin-avidin bioassay. While the identical room temperature bioassay (without microwave heating) took 70 min to complete, the identical bioassay took less than 2 min to complete by using SRR structures (with microwave heating). A lower detection limit of 0.01 nM for biotinylated-bovine serum albumin (100-fold lower than the room temperature bioassay) was observed by using SRR structures.
Artificially constructed materials may exhibit different physical characteristics that are not attainable by ordinary materials. One of the most common elements of metamaterials, which was introduced by Pendry et al. in 1999, is the split ring resonator (SRR).1 In recent years, SRRs have received a growing amount of interest since such structures may lead to negative values of permeability. Later experimental and theoretical studies have shown that SRR media, when combined properly with thin wire media, may exhibit left-handed properties.2, 3, 4 An SRR structure consists of concentric rings separated by a gap. Magnetic resonance is induced by the splits at the rings and by the gap between the inner and outer rings.1 The resonance frequency of the SRR depends on its geometrical parameters.5, 6 Since SRR structures are quite undiscovered and are the essential components of left-handed metamaterials, a considerable amount of effort has been made in order to understand the underlying physics of SRRs.6, 7, 8, 9 SRR structures are mainly used to increase antenna performance for obtaining properties such as an electrically small antenna size10, 11, 12, 13 and high directivity.14, 15 In this letter, we proposed the use of SRRs for bioassay (biological assay) applications.
Bioassays are typically conducted to measure the effects of a substance on a living organism. Bioassays are widely used for the detection and determination of a wide variety of proteins, peptides, and small molecules. Currently, the two major limitations encountered in the bioassays are as follows: rapidity of the bioassay and detection sensitivity. Rapidity of the bioassays is controlled by the chemical kinetics involved during the binding of proteins. The rapid detection of target biomolecules, becomes an important issue in the event of an outbreak of an infectious disease or a biological terror attack that has an immediate impact on human health. Sensitivity of the bioassays is affected by the quantum yield of fluorophores and the optical limitations of the detection system.
It was previously shown that it is possible to obtain fast and sensitive bioassays by using low-power microwave heating and silver nanoparticles.16, 17 It was thought that the rapidity of the bioassay was improved by low-power microwave heating of the bioassay components, where a thermal gradient between the assay medium and metal nanoparticles result in the completion of biorecognition events in less than 1 min.16, 17 We refer the reader to the literature16, 17 for the detailed description of the use of microwave heating with metal nanoparticles in a fluorescence-based biosensing scheme. However, in the previous reports the issue of control over the uniform heating of small volume samples was never addressed. In the previous work, additional materials were used to remove the excess microwave energy, which leads to an increase in the duration of microwave heating and in some cases evaporation of the small volume samples. In this regard, one can alleviate these issues using focused microwaves. The focusing of microwaves on a specific region of interest, especially for small volume samples, can be the key solution to this issue. In this regard, it was recently shown the extraction of biological materials from anthrax spores can be achieved by breaking the walls of the spores down by way of focused microwaves using a “bow-tie” structure.18 Despite their potential, these mini-antennas to focus microwaves were never employed in bioassays. In this letter, we report an ultrafast bioassay preparation method that overcomes the above-mentioned limitations using a combination of focused low power microwave heating, SRR structures and silver nanoparticles.
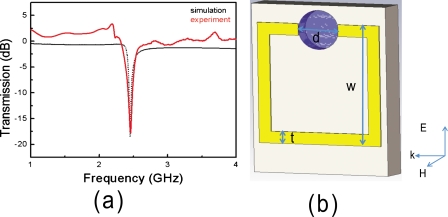
The SRR structures that we used for the present study are patterned on FR4 substrates (2×2 cm2). To employ SRR structures in biosensing applications by using microwave heating, one has to design these structures to function at the desired microwave frequency. Most biosensing applications employ water, which can be efficiently heated at 2.45 GHz using a conventional microwave oven. Subsequently, an SRR structure was designed that can be used in a conventional microwave oven, which eliminates the need for expensive and complicated microwave heating devices. Figure 1b shows the following geometrical parameters of the SRR: d=3 mm, t=0.9 mm, and w=9.4 mm. The FR4 substrates has a thickness of 2.4 mm and a dielectric constant of ε=3.85. A single microcuvette is drilled in the split of the SRR with 10 μl volume capacity.
Figure 1.
(a) Simulated and experimental transmission spectrum of SRR structure. (b) Schematic of the SRR structure printed on a circuit board. The dimensions of the SRR structures are d=3 mm, t=0.9 mm, and w=9.4 mm. A single microcuvette is drilled in the split of the SRR with 10 μl volume capacity.
We calculated the electric field distributions and transmission properties of incident plane electromagnetic (EM) waves through the SRR structure by using a commercial three-dimensional full-wave solver (CST MICROWAVE STUDIO). We also measured the transmission properties of the SRR structure. The transmission properties of a single SRR structure are measured by using an HP 8510C vector network analyzer and two monopole antennas as receiver and transmitter antennas. The measured and calculated transmission spectrum of a single SRR structure is shown in Fig. 1a. The directions of the electric field, magnetic field, and wave vector of the incident EM waves are shown in Fig. 1b. The transmission spectrum exhibits a resonance around 2.45 GHz with a transmission of −20 dB. There is good agreement between the experimental results and numerical simulations.
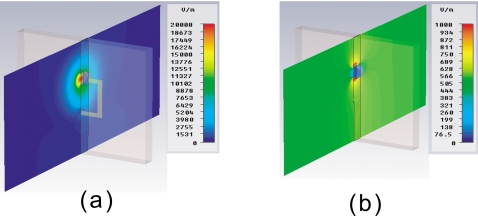
The electric field distribution calculations show that an SRR structure can focus the electric field in the microcuvette (Fig. 2). The electric field inside the cuvette is 20 000 V∕m and around 100 V∕m with and without SRR structure, respectively. Hence, the electric field inside the cuvette is enhanced up to 200-folds as compared to a microcuvette structure without a surrounding SRR. As shown in Fig. 2, the electric field enhancement is predicted to occur uniformly throughout the microcuvette extending into the entire microcuvette. Subsequently, a solution placed in the microcuvette can be heated in rapid and uniform fashion.
Figure 2.
Simulated electric field distribution of (a) microcuvette with SRR structure (b) microcuvette without SRR structure. The scales displayed to the right of the figures represent the magnitude of the electric field intensity distribution.
The microcuvettes of the SRR structures were further modified with thiolated glass beads (20–100 μm diameter) in order to introduce thiol groups to the surface (see Fig. S1, Ref. 19). It is important to note that the glass beads have a larger ratio of surface area∕volume than the flat surface of the microcuvettes and the presence of glass beads increases the available surface for the attachment of silver colloids. Subsequently, a solution of freshly prepared silver colloids was incubated in the microcuvettes for 2 h. This incubation step was repeated 10 times in order to increase the extent of the silver colloids on the surface. After the last incubation step, the microcuvettes were washed off with de-ionized water in order to remove the unbound silver colloids. The silver colloids served as enhancers of luminescence signals and also as a mediator for the creation of a thermal gradient between the bulk and the surface where the bioassay is constructed. In this regard, focused electric fields are able to cause the rapid and selective heating of the solution and the thermal gradient between the solution, and the silver colloids, which in turn result in the rapid assembling of the proteins on the surface of silver colloids without denaturing the proteins.
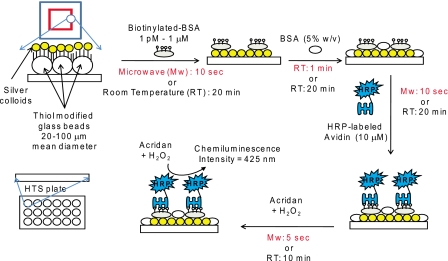
To compare the efficiency of the SRR structures in terms of reduction in total assay time and increased sensitivity, with respect to commonly used enzyme linked immunosorbent assay (ELISA), the detection of a model protein (biotinylated-bovine serum albumin, b-BSA) by using SRR structures and 96-well high throughput screening (HTS) plates (control assay) was carried out by ELISA (Fig. 3). The construction of ELISA was done according to the following procedure: first, a solution of b-BSA (concentration ranging 1 μM to 1 pM) was incubated on SRR structures (10 μl) and HTS plates (100 μl) by using low power microwave heating for 10 s (Emerson Model no: MW8784SB, power input 1050W at 2.45 GHz, duty cycle: 3) or at room temperature for 20 min, respectively. The unbound b-BSA was removed by rinsing with de-ionized water three times. Both surfaces were treated with 5% (w∕v) BSA in order to reduce the nonspecific binding of avidin. Second, a 1 mg∕ml solution of horseradish peroxidase-labeled avidin was incubated on SRR structures (10 s, microwave heating, duty cycle: 3) and HTS plates (20 min, room temperature). The quantification of b-BSA attached to the surfaces was carried out by measuring the chemiluminescence intensity of acridan dye at 425 nm (Fig. 4).
Figure 3.
Schematic depiction of the ELISA for the detection of the model protein (biotinylated-BSA) used in this study.
Figure 4.
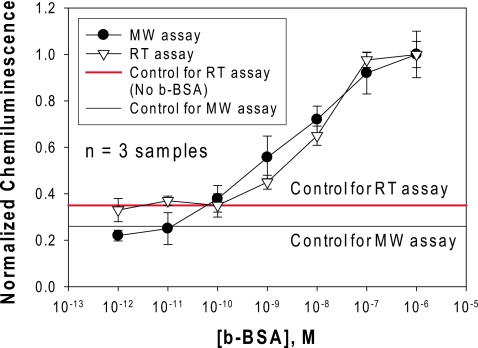
The normalized chemiluminescence intensity of acridan dye at 425 nm after the completion of ELISA for varying concentrations of b-BSA (1 μM to 1 pM) methods. In control experiments, where b-BSA is omitted from the surface, the background signal is measured in order to determine the lower detection limit for ELISA using both the SRR structures and HTS wells.
A typical ELISA test for b-BSA by using HTS plates at room temperature took 70 min to complete. The identical ELISA took less than 2 min to complete by using the SRR structures, low-power microwave heating and silver colloids. In addition, control experiments, where b-BSA is omitted from the surface, were also run to measure the background signal (horizontal lines in Fig. 4) to find out the lower detection limit for ELISA using both the SRR structures and HTS wells. The detectable concentration range for b-BSA was ∼1 nM–1 μM and ∼0.01 nM–1 μM for ELISA run at room temperature (HTS plates) and with microwave heating (SRR structures), respectively. That is, a 100-fold improvement of the lower detection limit for b-BSA using SRR structures over HTS plates is observed. It is important to note that one can consider the effect of silver colloids on the chemiluminescence emission by comparing the emissions from silvered and unsilvered surfaces.20 Since our aim is to compare the total assay time and the detectable concentration range, we did not attempt to investigate the enhancement of chemiluminescence emission directly. We also note that the observed decrease in the lower detection limit is partly attributed to the enhancement of chemiluminescence emission by silver colloids.
In conclusion, we have reported that SRR structures can be used for bioassay applications in order to further improve the assay time and sensitivity. Our proposed bioassay technique is based on (1) the focusing of microwaves by SRR structures on a small volume microcuvette for uniform heating, (2) the creation of a thermal gradient between the assay medium and the silver colloids for the rapid completion of the bioassay steps. The proof-of-principle of the proposed technique was demonstrated for a model protein, b-BSA. The identical ELISA was also carried out on commercially available HTS wells at room temperature incubation instead of microwave heating. While ELISA on HTS wells at room temperature took 70 min to complete, the identical ELISA was completed in less than 2 min by using the proposed technique. In addition, a lower detection limit of 0.01 nM for b-BSA was observed using the SRR structures. Hence, the lower detection limit of the chemiluminescence-based ELISA was improved ∼100-fold by using SRR structures in combination with microwave heating and silver colloids.
Acknowledgments
This work is supported by the European Union under the projects PHOME, ECONAM, N4E, and TUBITAK under Project Nos. 109E301, 107A004, 107A012, and DPT under the project DPT-HAMIT. One of the authors (E.O.) also acknowledges partial support from the Turkish Academy of Sciences. K.A. acknowledges financial support from the NIH-NIBIB (Award No. 7-K25EB007565-03).
References
- Pendry J. B., Holden A. J., Robbins D. J., and Stewart J. W., IEEE Trans. Microwave Theory Tech. 47, 2075 (1999). 10.1109/22.798002 [DOI] [Google Scholar]
- Smith D. R., Padilla W. J., Vier D. C., Nemat-Nasser S. C., and Schultz S., Phys. Rev. Lett. 84, 4184 (2000). 10.1103/PhysRevLett.84.4184 [DOI] [PubMed] [Google Scholar]
- Shelby R. A., Smith D. R., and Schultz S., Science 292, 77 (2001). 10.1126/science.1058847 [DOI] [PubMed] [Google Scholar]
- Aydin K., Guven K., Soukoulis C. M., and Ozbay E., Appl. Phys. Lett. 86, 124102 (2005). 10.1063/1.1888051 [DOI] [Google Scholar]
- Hsu Y., Huang Y., Lih J., and Chern J., J. Appl. Phys. 96, 1979 (2004). 10.1063/1.1767290 [DOI] [Google Scholar]
- Aydin K., Bulu I., Guven K., Kafesaki M., Soukoulis C., and Ozbay E., New J. Phys. 7, 168 (2005). 10.1088/1367-2630/7/1/168 [DOI] [Google Scholar]
- Katsarakis N., Koschny T., Kafesaki M., Economou E., and Soukoulis C., Appl. Phys. Lett. 84, 2943 (2004). 10.1063/1.1695439 [DOI] [Google Scholar]
- Aydin K., Guven K., Katsarakis N., Soukoulis C., and Ozbay E., Opt. Express 12, 5896 (2004). 10.1364/OPEX.12.005896 [DOI] [PubMed] [Google Scholar]
- Smith D., Gollub J., Mock J., Padilla W., and Schurig D., J. Appl. Phys. 100, 024507 (2006). 10.1063/1.2218033 [DOI] [Google Scholar]
- Qureshi F., Antoniades M., and Eleftheriades G., IEEE Antennas Wireless Propag. Lett. 4, 333 (2005). 10.1109/LAWP.2005.857041 [DOI] [Google Scholar]
- Hrabar S., Bartolic J., and Sipus Z., IEEE Trans. Antennas Propag. 53, 110 (2005). 10.1109/TAP.2004.840503 [DOI] [Google Scholar]
- Buell K., Mosallaei H., and Sarabandi K., IEEE Trans. Antennas Propag. 54, 135 (2006). [Google Scholar]
- Alici K. and Ozbay E., J. Appl. Phys. 101, 083104 (2007). 10.1063/1.2722232 [DOI] [Google Scholar]
- Wu B., Wang W., Pacheco J., Chen X., Lu J., Grzegorczyk T., Kong J., Kao P., Theophelakes P., and Hogan M., Microwave Opt. Technol. Lett. 48, 680 (2006). 10.1002/mop.21441 [DOI] [Google Scholar]
- Bulu I., Caglayan H., Aydin K., and Ozbay E., New J. Phys. 7, 223 (2005). 10.1088/1367-2630/7/1/223 [DOI] [Google Scholar]
- Aslan K. and Geddes C., Analyst (Cambridge, U.K.) 133, 1469 (2008). 10.1039/b808292h [DOI] [PubMed] [Google Scholar]
- Aslan K. and Geddes C., Anal. Chem. 77, 8057 (2005). 10.1021/ac0516077 [DOI] [PubMed] [Google Scholar]
- Aslan K., Previte M., Zhang Y., Gallagher T., and Geddes C., Anal. Chem. 80, 4125 (2008). 10.1021/ac800519r [DOI] [PubMed] [Google Scholar]
- See supplementary material at http://dx.doi.org/10.1063/1.3484958 for procedure for the modification of micro-cuvette of the SRR structures.
- Chowdhury M. H., Aslan K., Malyn S. N., Lakowicz J. R., and Geddes C. D., Appl. Phys. Lett. 88, 173104 (2006). 10.1063/1.2195776 [DOI] [PMC free article] [PubMed] [Google Scholar]