Abstract
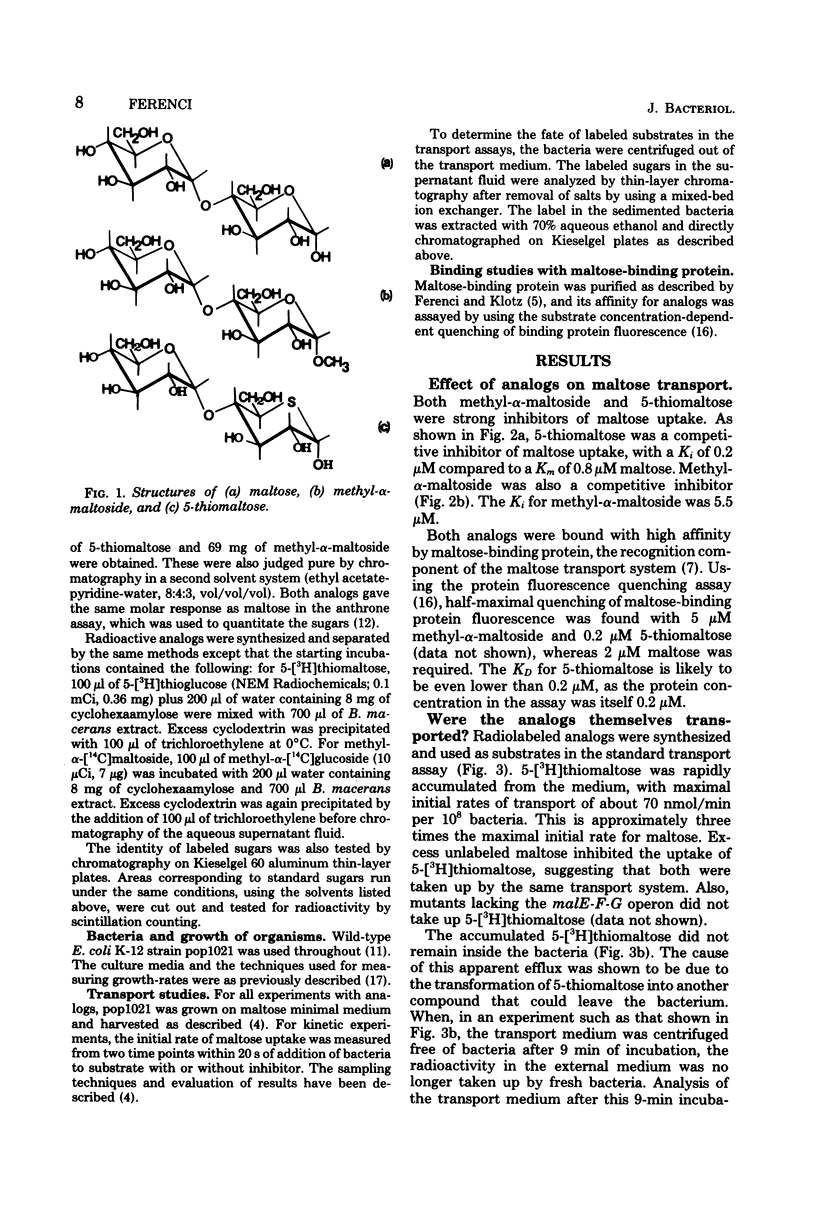
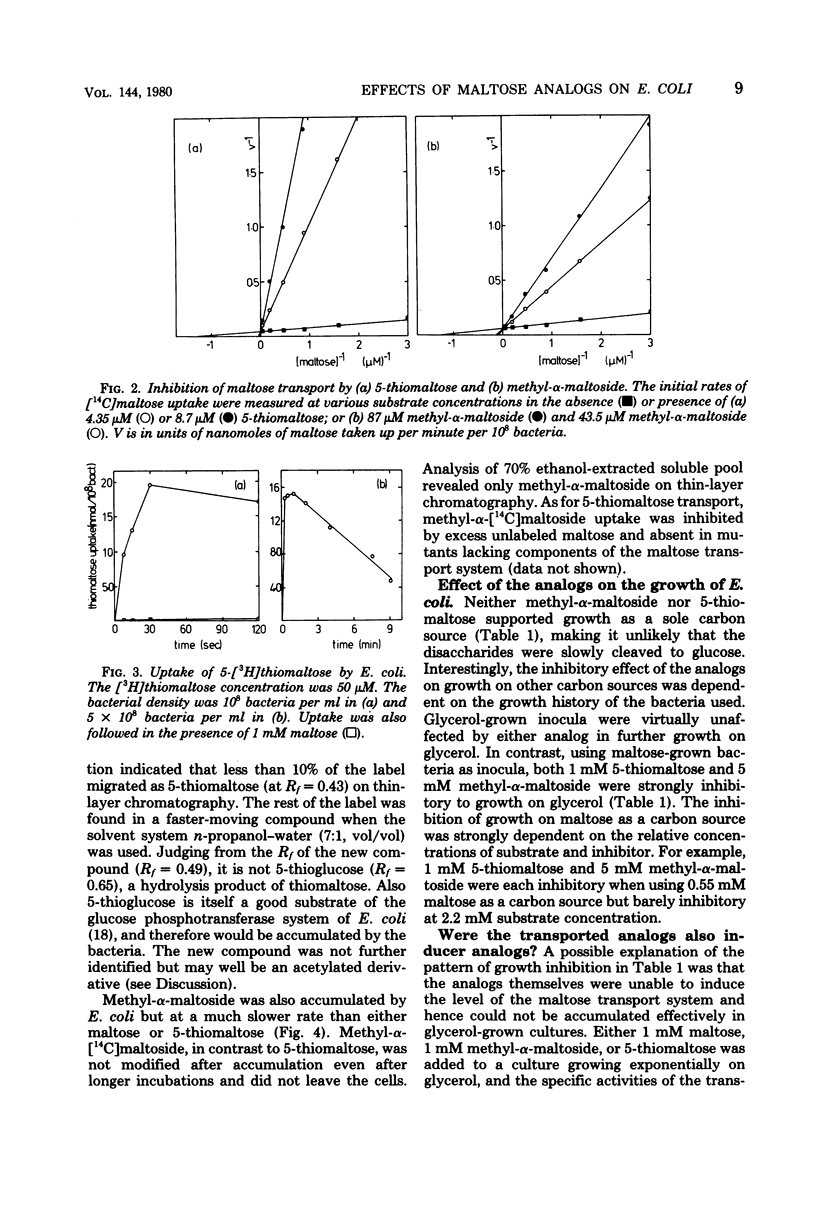
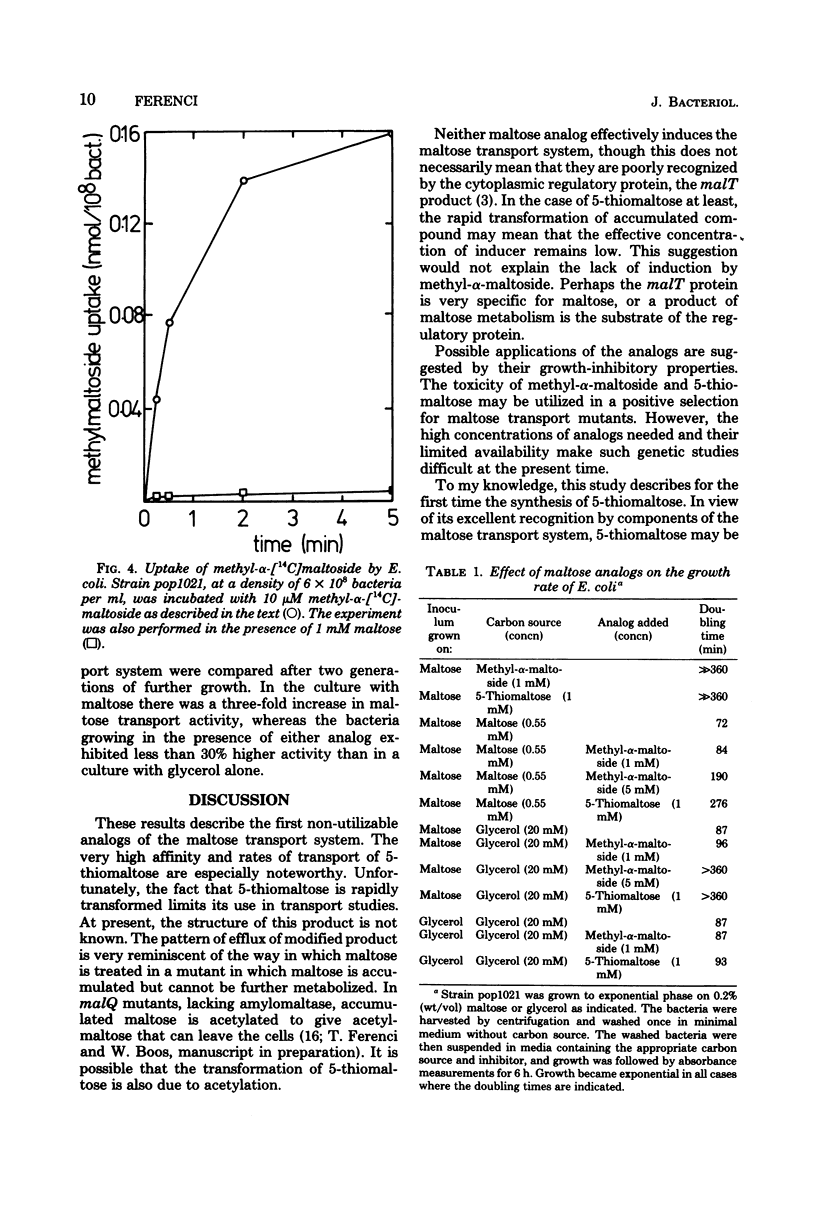
Neither methyl-alpha-maltoside nor 5-thiomaltose is utilized by Escherichia coli as a sole carbon source. Both are, however, effective competitive inhibitors of maltose transport into the bacterium (Km for maltose, 0.8 microM, Ki for methyl-alpha-maltoside, 5.5 microM; Ki for 5-thiomaltose, 0.2 microM). Both analogs are bound by the periplasmic maltose-binding protein. Methyl-alpha-[14C]maltoside and 5-[3H]thiomaltose were both accumulated inside E. coli. Methyl-alpha-maltoside was unchanged after accumulation, but 5-thiomaltose was converted to an unidentified compound that could exit from the bacterium. Both analogs were inhibitory to the growth of E. coli, but only when the bacteria were previously induced for the maltose transport system. The analogs are substrates for but poor inducers of the maltose transport system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Débarbouillé M., Shuman H. A., Silhavy T. J., Schwartz M. Dominant constitutive mutations in malT, the positive regulator gene of the maltose regulon in Escherichia coli. J Mol Biol. 1978 Sep 15;124(2):359–371. doi: 10.1016/0022-2836(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Boos W., Schwartz M., Szmelcman S. Energy-coupling of the transport system of Escherichia coli dependent on maltose-binding protein. Eur J Biochem. 1977 May 2;75(1):187–193. doi: 10.1111/j.1432-1033.1977.tb11516.x. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Klotz U. Affinity chromatographic isolation of the periplasmic maltose binding protein of Escherichia coli. FEBS Lett. 1978 Oct 15;94(2):213–217. doi: 10.1016/0014-5793(78)80940-5. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Schwentorat M., Ullrich S., Vilmart J. Lambda receptor in the outer membrane of Escherichia coli as a binding protein for maltodextrins and starch polysaccharides. J Bacteriol. 1980 May;142(2):521–526. doi: 10.1128/jb.142.2.521-526.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T. The recognition of maltodextrins by Escherichia coli. Eur J Biochem. 1980 Jul;108(2):631–636. doi: 10.1111/j.1432-1033.1980.tb04758.x. [DOI] [PubMed] [Google Scholar]
- Kellermann O., Szmelcman S. Active transport of maltose in Escherichia coli K12. Involvement of a "periplasmic" maltose binding protein. Eur J Biochem. 1974 Aug 15;47(1):139–149. doi: 10.1111/j.1432-1033.1974.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Roa M., Braun-Breton C., Schwartz M. Structure of the malB region in Escherichia coli K12. I. Genetic map of the malK-lamB operon. Mol Gen Genet. 1979 Jul 24;174(3):241–248. doi: 10.1007/BF00267796. [DOI] [PubMed] [Google Scholar]
- Shuman H. A., Silhavy T. J., Beckwith J. R. Labeling of proteins with beta-galactosidase by gene fusion. Identification of a cytoplasmic membrane component of the Escherichia coli maltose transport system. J Biol Chem. 1980 Jan 10;255(1):168–174. [PubMed] [Google Scholar]
- Silhavy T. J., Brickman E., Bassford P. J., Jr, Casadaban M. J., Shuman H. A., Schwartz V., Guarente L., Schwartz M., Beckwith J. R. Structure of the malB region in Escherichia coli K12. II. Genetic map of the malE,F,G operon. Mol Gen Genet. 1979 Jul 24;174(3):249–259. doi: 10.1007/BF00267797. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- WHEELER M., HANKE P., WEILL C. E. UNUSUAL CARBOHYDRATE TRANSFER REACTIONS OF CYCLODEXTRIN TRANSGLUCOSYLASE. Arch Biochem Biophys. 1963 Sep;102:397–399. doi: 10.1016/0003-9861(63)90247-9. [DOI] [PubMed] [Google Scholar]
- Wandersman C., Schwartz M., Ferenci T. Escherichia coli mutants impaired in maltodextrin transport. J Bacteriol. 1979 Oct;140(1):1–13. doi: 10.1128/jb.140.1.1-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



