Abstract
The aim of this study is to clarify the clinical features of Haplorchis taichui infection in humans in Nan Province, Thailand, and to correlate the clinical features with irritable bowel syndrome (IBS)-like symptoms. In this study area, only H. taichui, but neither other minute intestinal flukes nor small liver flukes were endemic. The degree of infection was determined by fecal egg counts and also by collecting adult worms after deworming. The signs and symptoms of individual patients together with their hematological and biochemical laboratory data were gathered to evaluate the relationship between the clinical features and the severity of infection. Special emphasis was made to elucidate the possible similarities of the clinical features of H. taichui infection and IBS-like symptoms. The results showed useful clinical information and the significant (> 50%) proportion of haplorchiasis patients complained of abdominal pain, lassitude, and flatulence, which were the important diagnostic symptoms of IBS. This study has reported a possible link between H. taichui and IBS, and H. taichui might probably play a role in the etiology of these IBS-like symptoms.
Keywords: Haplorchis taichui, clinical feature, irritable bowel syndrome-like symptom
INTRODUCTION
Assessment of the morbidity and mortality due to minute intestinal fluke infections is difficult because of prolonged latent phase, a short acute phase, asymptomatic presentations, and similarity of clinical symptoms to other intestinal helminthiases [1]. In addition, 2 or more species of intestinal flukes often co-exist in the same endemic areas to cause mixed infections in humans, so that it is practically almost impossible to estimate the pathogenesis of a particular species of minute intestinal flukes. More worse, endemic areas of minute intestinal flukes often coincide with those of small liver flukes, Clonorchis sinensis or Opisthorchis viverrini [2-4], of which eggs are difficult to discriminate from those of minute intestinal flukes. Only practical ways of differential diagnosis is, therefore, to collect adult worms for morphological identification.
Among an array of minute intestinal flukes, Haplorchis taichui which belongs to the family Heterophyidae lives in the small intestines of birds and mammals, and are endemic in Southeast Asia, where a high incidence of mixed infections with small liver flukes and/or minute intestinal flukes has been noted [3-7]. Humans become infected with minute intestinal flukes by consuming the metacercariae in infected cyprinoid fish [8-10]. Hence, H. taichui is one of the important pathogens to cause fishborne trematodiases in humans [11]. Recently, we have found by chance a community in Nan Province, northern Thailand where only H. taichui is endemic but no other intestinal flukes nor liver flukes are endemic [12]. Therefore, the pathogenic role of these worms which are still mysterious can be investigated for the cause of a variety of intestinal disorders resembling irritable bowel syndrome (IBS) where relevant data are lacking.
IBS is defined as a functional gastrointestinal disorder in which abdominal pain or discomfort is associated with abnormal defecation as diarrhea or constipation or alterations in bowel habit in the absence of an organic cause [13]. The pathophysiology of IBS remains mysterious and no mechanism is explained uniquely to IBS. There are probably several interrelated factors which occur to varying degrees in patients that account for the clinical symptoms of IBS. However, there are no strong recommendations about the extent and type of testing required to exclude other organic pathology. Investigation of stool for eggs, cysts, and parasites is generally recommended. The parasites associated with IBS-like symptoms are mostly the intestinal protozoa which have not been fully investigated [14].
In the present study, we aimed to clarify the clinical features of haplorchiasis, by analyzing the signs and symptoms and laboratory data of the patients in relation to the severity of infection determined by fecal egg count or by the worm burden. While a link between severe haplorchiasis and IBS-like symptoms has been suggested, clear evidence of the association could not be obtained.
MATERIALS AND METHODS
Coproparasitological examinations
This study was approved by the Ethics Committee of Faculty of Tropical Medicine, Mahidol University. Most of the residents in the study area, Chalerm Phrakiet District, Nan Province, in northern Thailand along the border with Lao PDR, were hilltribe people, particularly Hmong and Lahu, whose houses are located in mountainous areas in tropical zones. Fecal samples of the residents were collected and examined by the modified cellophane thick-smear method [15]. At the same time, the participants were requested to complete a questionnaire and underwent physical examinations. Details of the results of epidemiological survey for intestinal helminthiases in general have been reported elsewhere [12].
Among 2,540 participants, 1,418 were identified to have intestinal helminthic infections by fecal egg examinations. The majority of fecal egg-positives were infected with soil-transmitted nematodes: 368 cases of Ascaris lumbricoides, 648 hookworms, and 185 Trichuris trichiura, 20 Strongyloides stercoralis, 24 Enterobius vermicularis, 47 Taenia spp., and 1 case of Paragonimus heterotremus. In addition, we found 593 participants whose stools were positive for minute intestinal fluke eggs. Among them, we selected 210 subjects, who were defined to have minute intestinal fluke infection alone by fecal egg examinations. All those minute intestinal fluke egg-positive cases were treated with a single dose of praziquantel (40 mg/kg), especially those having heavy infections (> 1,000 EPG, n = 16) were given 60 ml saturated magnesium sulfate solution 1 hr post-praziquantel administration to collect adult worms. After deworming, all fecal samples were collected 4-5 times and the sediments were washed extensively with tap water until the supernatant became clear. All of the expelled worms were collected, identified, and counted under a stereomicroscope. Some randomly selected worms were stained for morphological identification.
Physical examination and clinical laboratory investigation
The symptoms and signs together with the laboratory data of each patient were collected from the personal records. Routine hematological tests consisted of hemoglobin, hematocrit, total and differential white blood cell counts, particularly for eosinophils. Biochemical tests included albumin and total protein. Clinical features of patients with heavy infection (EPG ≥ 1,000) were analyzed further using selected parameters, such as lassitude, complaints of abdominal pain, flatulence, loose fecal excretion and eosinophil counts.
RESULTS
Fecal egg examination and worm collection
The intensity of infection with minute intestinal flukes by fecal egg output varied from 1 to ≥ 1,000 EPG. Among 210 participants, 151 (71.9%) had moderate infection with the egg-count range of 100-999. Heavy infection defined by EPG ≥ 1,000 were 16 (7.6%), while light or low infections with the EPG ranges 10-99 and 1-9 were 29 and 14, respectively. H. taichui single infection cases consisted of 137 males and 73 females, with a male: female ratio of about 2 : 1. Their ages ranged from 12 to 68 years. About 30% of intestinal fluke egg positive cases were at the ages of 31-40 years.
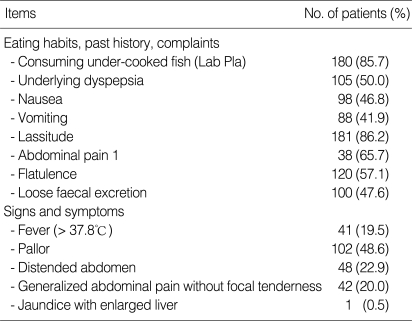
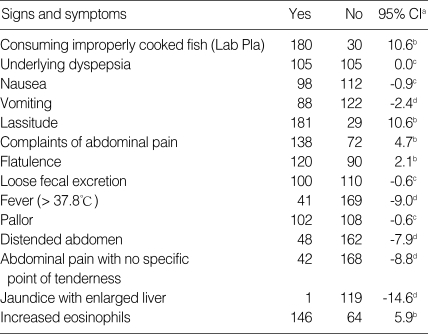
Clinical features of 210 intestinal fluke egg-positive patients are summarized in Table 1. The correlation of the signs and symptoms with H. taichui infection was calculated by means of the patient proportion with statistical significance of 95% confidence interval (CI) (Table 2). The data of consuming undercooked fish, past history of underlying dyspepsia, nausea, lassitude, complaints of abdominal pain, flatulence, loose fecal excretion, pallor and increased eosinophils are seen in ≥ 50.0% of the patient proportion, while fever, vomiting, distended abdomen with generalized abdominal pain are seen in patient proportion < 50.0%.
Table 1.
Clinical features of haplorchiasis patients
Table 2.
Correlation of the signs and symptoms with infection
aConfidence interval; bPatient proportion > 50.0%; cPatient proportion = 50.0%; dPatient proportion < 50.0%.
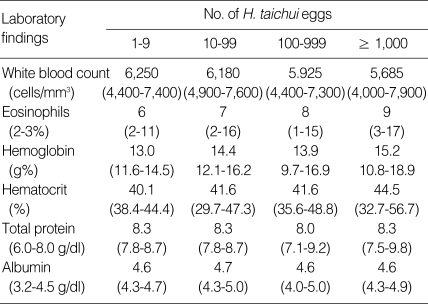
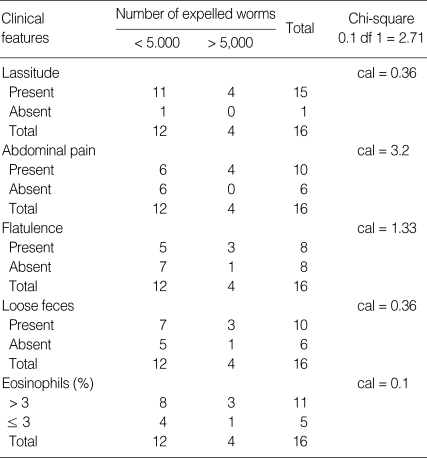
Next, the correlation between the severity of infection and the laboratory data of the patients was examined (Table 3). Except for the significant eosinophilia, which was proportional with EPG, all hematological and biochemical data, including the white blood cell count, hemoglobin, hematocrit, total protein, and albumin, were similar regardless of the intensities of fecal egg output. For the 16 patients with heavy infection (> 1,000 EPG), they were further divided into 2 groups depending on the number of H. taichui worms expelled by deworming. The correlation of the number of expelled worms with the lassitude, complaints of abdominal pain, flatulence, loose fecal excretion, or the number of eosinophils were examined. As shown in Table 4, the parameters revealed no correlation with the number of worms (P < 0.1), in other words, the severity of infection, may be due to insufficient sample numbers. However, the complaints of abdominal pain seemed to be correlated with the number of worms (P < 0.1).
Table 3.
Correlation between the laboratory data and the severity of infection
Table 4.
Correlation of expelled worms with clinical features
DISCUSSION
The prevalence of H. taichui infection in the studied area was quite high due to their habit of consuming undercooked fish, such as Lab Pla which can lead the infection [16]. Severe haplorchiasis, with fecal egg outputs ≥ 1,000 in EPG, was common among patients aged > 36 years, and was predominant in males than females (14 males and 2 females). These patients are supposed to have acquired infection very early in their life. Although the life span of individual worm is not long, the accumulation of flukes by repeated infections may have contributed towards the developments of the disease. In our study, some symptomatic complaints were commonly (≥ 50.0% of the study population) found in haplorchiasis taichui patients, which might be related to the disease; particularly pallor, abdominal pain, discomfort with excessive gas, and loose feces. Such patients were at a higher risk of suffering symptoms suggestive of a functional gastrointestinal disorder [13,14]. Other clinical features, such as distended abdomen, generalized pain in the epigastric area, and fever, were not clearly associated with the infection, in < 50.0% of the patients (Table 3). Except for the eosinophil counts, the laboratory data were not proportional to the severity of the infections (Table 4). The elevation of eosinophils is a suggestive marker for helminthic disease. In our study, total protein and albumin levels were within normal range, suggesting that the nutritional status of the residents in this area are basically good and infection did not cause nutritional problems.
Since there are no biological markers for IBS, diagnosis is based on the new symptom-based Rome III criterion which is more clinical oriented [17]. The symptomatic criteria have been proven to be useful in population studies, especially where the prevalence of IBS greatly exceeds that of organic gastrointestinal diseases [16]. Several interconnected factors probably occur to varying degrees in patients, which account for the clinical symptoms of IBS, such as abdominal pain or discomfort, with associated changes in the bowel frequency or fecal form, and anorexia. In addition, persistent low-grade inflammation may play a role in IBS, which revealed normal mucosa but lymphocytic infiltration in the region of the myenteric plexus [17]. Symptoms suggesting functional gastrointestinal disorders are frequent in developing countries, and are often related to intestinal parasites. Therefore, the investigation of a possible link between IBS and haplorchiasis, by determining expelled worms in patients, will help to elucidate the possible role of H. taichui as a pathogen in IBS. A case report by Ryang et al. [18] mentioned about the symptoms presented by the infection with Heterophyes nocens which is in the Family Heterophyidae and close to H. taichui. The symptoms were presented, including epigastric pain, indigestion, abdominal discomfort for 3 months with severe diarrhea, abdominal pain, and vomiting for about 1 month. Recently, the incidence of H. taichui infection was reported as the pathogenic parasite in 3 patients with the presence of mucosal ulceration or hemorrhages, fusion and shortening villi, chronic inflammation and fibrosis of the submucosa of small intestine [19]. Moreover, the increase in the number of eosinophils in haplorchiasis patients was related to the intensity of fecal egg output. Disappointedly, a low correlation (P < 0.1) was revealed between the number of eosinophils and the intensity of expelled worms. A further study should comprise a larger size and include all patients, not just severe cases.
Although the survey for intestinal parasite infections is primarily based on fecal egg examinations, patients might be misdiagnosed as having O. viverrini infections, since minute intestinal fluke eggs are highly similar to O. viverrini eggs in morphology [20,21]. Thus, over- and under-estimation would occur for the prevalence of O. viverrini and/or minute intestinal fluke infections in areas where small liver fluke and minute intestinal flukes are co-endemic. In the present study, we found that H. taichui is heavily endemic in the study area of Nan Province, but we did not find O. viverrini-infected cases. Even by the continuous survey of O. viverrini infection by the Ministry of Health, Thailand [22], Opisthorchis cases were not reported in Nan Province, although all surrounding provinces are known endemic areas of opisthorchiasis. Since O. viverrini and H. taichui can share snail and fish intermediate hosts, extensive surveys for O. viverrini infection in various hosts are necessary in this and other study areas of Nan Province.
It can be concluded that the symptoms attributed to H. taichui infection are non-specific, IBS-like symptoms, including complaints of abdominal pain or discomfort, with excessive gas or flatulence, lassitude, and loose feces, were seen in patients with statistically significant proportion of > 50%. In the severe group of patients, the risk of functional gastrointestinal problems causing complaints of abdominal pain was significantly higher among subjects with heavy Haplorchis infections. White cell count, hemoglobin, hematocrit, total protein, and albumin levels, yielded no useful diagnostic data in this study. However, it seems to be specifically indicated from the past history of underlying dyspepsia, a habit of consuming improperly cooked fish, and physical examination. Hence, clinicians be reminded that H. taichui is reported here as a possible etiologic agent for IBS-like symptoms.
ACKNOWLEDGEMENTS
This study was partially supported by a grant from Faculty of Tropical Medicine, Mahidol University. We would like to thank all participants and health volunteers for their help with stool collection. Thanks also to Prof. Prayong Radomyos, Dr. Karunee Kwanbunjan, Dr. Tippayarat Yoonuan, Mr. Chatree Muennoo and Mrs. Panida Muangkhum for their assistance in technical help and laboratory support.
References
- 1.Chai JY, Lee SH. Intestinal trematodes of humans in Korea: Metagonimus, heterophyids and echinostomes. Korean J Parasitol. 1990;28(suppl):103–122. doi: 10.3347/kjp.1990.28.suppl.103. [DOI] [PubMed] [Google Scholar]
- 2.Waikagul J. Intestinal fluke infections in Southeast Asia. Southeast Asian J Trop Med Public Health. 1991;22:158–162. [PubMed] [Google Scholar]
- 3.Chai JY, Park JH, Han ET, Guk SM, Shin EH, Lin A, Kim JL, Sohn WM, Young TS, Eom KS, Min DY, Hwang EH, Phommasack B, Insisiengmay B, Rim JH. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane Municipality and Saravane Province in Laos. J Helminthol. 2005;79:283–289. doi: 10.1079/joh2005302. [DOI] [PubMed] [Google Scholar]
- 4.Chai JY, Han ET, Shin EH, Sohn WM, Yong TS, Eom KS, Min DY, Um JY, Park MS, Hoang EH, Phommasack B, Insisiengmay B, Lee SH, Rim HJ. High prevalence of Haplorchis taichui, Prosthodendrium molenkampi and other helminth infections among people in Khammouane province, Lao PDR. Korean J Parasitol. 2009;47:243–247. doi: 10.3347/kjp.2009.47.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thu ND, Dalsgaard A, Loan LT, Murrell KD. Survey for zoonotic liver and intestinal trematode metacercariae in cultured and wild fish in An Giang Province, Vietnam. Korean J Parasitol. 2007;45:45–54. doi: 10.3347/kjp.2007.45.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovis L, Mak TK, Phongluxa K, Soukhathammavong P, Sayasone S, Akkhavong K, Odermatt P, Keiser J, Felger I. PCR diagnosis of Opisthorchis viverrini and Haplorchis taichui infections in a Lao community in an area of endemicity and comparison of diagnostic methods for parasitological field surveys. J Clin Microbiol. 2009;47:1517–1523. doi: 10.1128/JCM.02011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayasone S, Vonghajack Y, Vanmany M, Rasphone O, Tesana S, Utzinger J, Akkhavong K, Odermatt P. Diversity of human intestinal helminthiasis in Lao PDR. Trans R Soc Trop Med Hyg. 2009;103:247–254. doi: 10.1016/j.trstmh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Kumchoo K, Wongsawad C, Chai JY, Vanittanakorn P, Rojanapaibul A. High prevalence of Haplorchis taichui metacercariae in cyprinoid fish from Chiang Mai Province, Thailand. Southeast Asian J Trop Med Public Health. 2005;36:451–455. [PubMed] [Google Scholar]
- 9.Thien PC, Dalsgaard A, Thanh BN, Olsen A, Murrell KD. Prevalence of fishborne zoonotic parasites in important cultured fish species in the Mekong Delta, Vietnam. Parasitol Res. 2007;101:1277–1284. doi: 10.1007/s00436-007-0633-5. [DOI] [PubMed] [Google Scholar]
- 10.Rim HJ, Sohn WM, Yong TS, Eom KS, Chai JY, Min DY, Lee SH, Hoang EH, Phommasack B, Insisengmay S. Fishborne trematode metacercariae detected in freshwater fish from Vientiane Municipality and Savannakhet Province, Lao PDR. Korean J Parasitol. 2008;46:253–260. doi: 10.3347/kjp.2008.46.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keiser J, Utzinger J. Food-borne trematodiases. Clin Microbiol Rev. 2009;22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maipanich W, Waikagul J, Watthanakulpanich D, Muennoo C, Sanguankiat S, Pubampen S, Anantaphruti MT, Nuamtanong S, Yoonuan T, Visetsuk K. Intestinal parasitic infections among inhabitants of the North, West-central and Eastern border areas of Thailand. J Trop Med Parasitol. 2004;27:51–58. [Google Scholar]
- 13.Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol. 2010;25:691–699. doi: 10.1111/j.1440-1746.2009.06120.x. [DOI] [PubMed] [Google Scholar]
- 14.Stark D, van Hal S, Marriott D, Ellis J, Harkness J. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol. 2007;37:11–20. doi: 10.1016/j.ijpara.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 16.Chuboon S, Wongsawad C, Ruamsuk A, Nithikathkul C. Survival of Haplorchis taichui metacercariae in Lab-Pla, Thai traditional food preparation. Southeast Asian J Trop Med Public Health. 2005;36(suppl 4):110–111. [PubMed] [Google Scholar]
- 17.Whitehead WE, Drossman DA. Validation of symptom-based diagnostic criteria for irritable bowel syndrome: a critical review. Am J Gastroenterol. 2010;105:814–820. doi: 10.1038/ajg.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryang YS, Lee CY, Lee KJ, Lee SH, Chai JY. An incidental case of human Heterophyes nocens infection diagnosed by sectional morphology in a biopsy specimen of the small intestine. Korean J Parasitol. 1999;37:189–194. doi: 10.3347/kjp.1999.37.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tornblom H, Lindberg G, Nyberg B, Veress B. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972–1979. doi: 10.1053/gast.2002.37059. [DOI] [PubMed] [Google Scholar]
- 20.Sukontason K, Unpunyo P, Sukontason KL, Piangjai S. Evidence of Haplorchis taichui infection as pathogenic parasite: three case reports. Scand J Infect Dis. 2005;37:388–390. doi: 10.1080/00365540510034473. [DOI] [PubMed] [Google Scholar]
- 21.Kaewkes S, Elkins DB, Sithithaworn P, Haswell-Elkins MR. Comparative studies on the morphology of the eggs of Opisthorchis viverrini and lecithodendriid trematodes. Southeast Asian J Trop Med Public Health. 1991;22:623–630. [PubMed] [Google Scholar]
- 22.Kaewpitoon N, Kaewpitoon SJ, Pengsaa P. Opisthorchiasis in Thailand: review and current status. World J Gastroenterol. 2008;14:2297–2302. doi: 10.3748/wjg.14.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]