Abstract
Feline intestinal tritrichomoniasis by Tritrichomonas foetus was first recognized in USA in 1999 and has so far been reported from UK, Norway, Switzerland, and Australia, but not from the Far East Asian countries. In November 2008, 2 female and male littermate Siamese cats, 6-month old, raised in a household in Korea were referred from a local veterinary clinic with a history of chronic persistent diarrhea. A direct smear examination of fecal specimens revealed numerous trichomonad trophozoites which were isolated by the fecal culture in InPouch™ TF-Feline medium. A PCR testing of the isolate based on the amplification of a conserved portion of the T. foetus internal transcribed spacer (ITS) regions (ITS1 and ITS2) and the 5.8S rRNA gene, and the molecular sequencing of the PCR amplicons confirmed infection with T. foetus. This is the first clinical case of feline intestinal trichomoniasis caused by T. foetus in Korea.
Keywords: Tritrichomonas foetus, cat, diarrhea, clinical case
INTRODUCTION
Tritrichomonas foetus is a pear-shaped, approximately 10-25 µm long and 3-15 µm wide flagellate and has a single nucleus and 4 flagella. Three of the flagella are free anteriorly, while the 4th extends backwards to form an undulating membrane along the length of the organism and then continues posteriorly as a free flagellum [1]. The organism has long been considered as a venereal pathogen of cattle causing infertility, vaginitis, placentitis, abortion, anoestrus, and endometritis [2]. It has a worldwide distribution, and significant economic loss has been recognized in the past when artificial insemination of cattle was not widely used [3]. It is generally accepted that the bovine venereal T. foetus and the porcine intestinal T. suis are considered to be the same species based on morphology and sequence identity of rRNA [3].
Recently, T. foetus has also been identified as a cause of prolonged and intractable large bowel diarrhea in cats [4-9], although recent investigation on the internal transcribed region of the ribosomal DNA unit and the TR7/TR8 variable-length repeat of T. foetus isolated from cats and cattle indicate that the 2 isolates are genetically distinct and at least 2 genotypes are recognized [10]. The organism colonizes the ileum, cecum, and colon where it resides in close contact with the epithelium, and is often associated with either transient or chronic diarrhea in cats [9,11-13]. In general, however, clinical symptoms of affected cats are likely to resolve spontaneously within 2 years of onset [14]. Although a few drugs, including ronidazole, have shown efficacious against feline tritrichomoniasis, currently there are no commercially-available drugs against the intestinal T. foetus infection in cats [15].
According to previous reports, the prevalence of feline intestinal T. foetus infection was 31% (36 out of 117) in USA, 14.4% (16 out of 111) in UK, 32% (24 out of 74) in Italy, and 24.4% (11 out of 45) in Switzerland [5-7,9]. However, no clinical case of T. foetus infection in cats has been reported in the Far Eastern countries. In the present study, we describe the first clinical case of feline intestinal trichomoniasis caused by T. foetus in Korea, as evidenced by a successful isolation and proliferation of T. foetus trophozoites from rectal swab sample in culture, and identification of the organism by detection of T. foetus-specific ribosomal DNA using PCR and sequencing.
CASE DESCRIPTION
Two 6-month-old littermate Siamese cats, 1 female and 1 male (Fig. 1), were referred from a local practitioner with a history of chronic persistent diarrhea that lasted for several months. The cats were purchased from a local pet shop at 2 months old. The owner reported that the cats had loose to semiformed mucoid diarrhea shortly after their residence at her house which lasted for several weeks when she finally consulted her veterinarian. Cats were raised indoor with no contact with other animals, and were fed a combination of commercially-available canned and dry food. Upon microscopy, numerous motile trichomonal trophozoites were observed in the feces. In a period of 2 months, the 2 cats did not respond to the treatment with a combination treatment of metronidazole and fenbendazole. Trophozoites collected by the rectal swab were stained with Giemsa and were also successfully cultured in the InPouch™, TF-Feline medium (BioMed Diagnotics, White City, Oregon, USA). After the culture was stabilized, 3 consecutive series of limiting dilution was performed to establish a homogenous isolate. Scanning electron microscopy was used to morphologically identify the organism according to a previously described method by Pereira-Neves and Benchimol [16]. Briefly, culture samples were allowed to adhere on to a cover glass coated with poly L-lysine (Iwaki, Tokyo, Japan) and were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) for 2 hr at room temperature. Trophozoites were post-fixed for 15 min in 1% OsO4, serially dehydrated in ethanol, and were critical-point dried [7]. They were then sputter-coated with gold and inspected on a Hitachi S-2400 scanning electron microscope.
Fig. 1.
Two 6-month-old female and male littermate Siamese cats from Korea with uncontrolled diarrhea by medication with metronidazole.
DNA was extracted using MasterPure™, DNA Purification Kit (EPICENTRE® Biotechnologies, Madison, Wisconsin, USA) from trophozoites in culture media or directly from rectal swab samples. A single-tube nested PCR with primer pairs TFITS-F (5'-CTG CCG TTG GAT CAG TTT CG-3')/TFITS-R (5'-GCA ATG TGC ATT CAA AGA TCG-3') and TFR-3 (5'-CGG GTC TTC CTA TAT GAG ACA GAA CC-3')/TFR-4 (5'-CCT GCC GTT GGA TCA GTT TCG TTA A-3') was performed as designed by Gookin et al. [17]. The TFR-3/TFR-4 primers have been demonstrated to specifically amplify a 347-bp fragment of the T. foetus internal transcribed spacer region 1 (ITS1), 5.8S, and ITS2 ribosomal DNAs from bovine preputial or vaginal samples. The TFITS-F/TFITS-R primers were designed to amplify a 208-bp fragment of T. foetus ITS1 and 5.8S ribosomal DNAs [17]. The 208-bp product is nested within the sequence amplified by the TFR-3/TFR-4 primers. The ITS1/5.8S/ ITS2 genomic region was amplified with primers TFR-3/TFR-4 and ITS1/5.8S genomic region was amplified with primers TFITS-F/TFITS-R.
The reaction for the single-tube nested PCR of T. foetus DNA was performed in a total volume of 20 µl using genomic DNA, 1 unit HiPi™, thermostable DNA polymerase in 250 Mm Tris-HCl (pH 9.0), 80 Mm (NH4)2SO, 10% DMSO, 8.75 mM MgCl, 0.05% bromophenol blue, 12% glycerol and stabilizer, 1.25 pmol each of primers TFR-3 and TFR-4, 25 pmol each of primers TFITS-F and TFITS-R.
DNA amplification was performed using the Palm Cycler (Corbett Research, Sydney, Australia) under the following condition: initial denaturation at 95℃ for 5 min; denaturation at 95℃ for 30 sec, and annealing and extension at 72℃ for 1 min for 30 cycles; denaturation at 95℃ for 30 sec and annealing at 57℃ for 30 sec, and extension at 72℃ for 30 sec for 30 cycles; and a final extension at 72℃ for 5 min. Agarose gel electrophoresis was carried out, followed by staining of gel with ethidium bromide, and DNA was extracted from the agarose gel using QIAEX II® Gel Extraction Kit (Qiagen, Valencia, California, USA) according to the manufacturer's instructions. DNA sequencing was performed using an ABI system 3700 automated DNA sequencer (Applied Biosystem, Foster City, California, USA).
Using the DNA Basic module (DNAsis MAX, Alameda, California, USA), the nucleotide sequences were compared with those selected from other known Tritrichomonadidae. Following sequences obtained from GenBank™ were used in phylogenetic and sequence similarity analyses: AF466751, AF339736 and TFU85967 (T. foetus), TSU85966 (T. suis), TMU86612 (T. mobilensis), AY886846 (T. muris), AY245140 (T. nonconforma), AY055802 (T. augusta), FJ357444 (T. vaginalis) and M86482 (T. vaginalis). Phylogenetic and bootstrap (1,000 replicates) analyses based on the nucleotide alignments were constructed using the neighbor-joining method and the unweighted-pair group method of Molecular Evolutionary Genetics analysis (MEGA version 4.0) with a pairwise distance [18]. A sequence similarity search was performed for the Tritrichomonadidae using the DNAMAN, version 5.1 software (Lynnon Biosoft, Quebec, Canada).
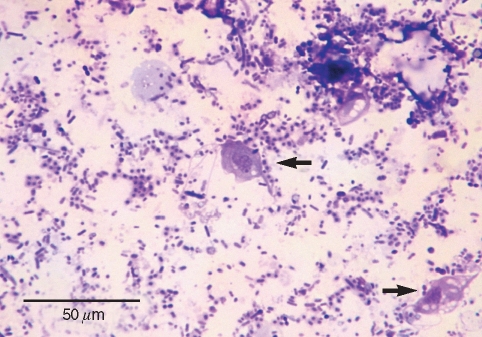
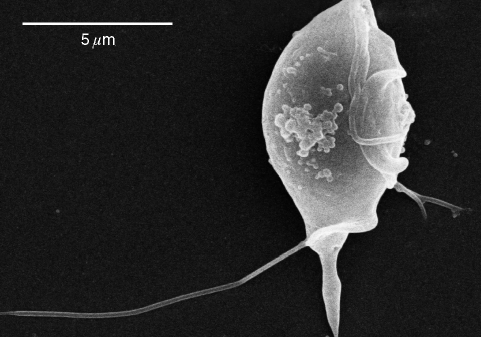
A direct microscopic examination of rectal swab samples from the infected cats revealed numerous motile trophozoites of trichomonads, featuring a pyriform body with an undulating membrane and 3 free anterior flagella which were confirmed by the Giemsa staining of fresh rectal swab samples (Fig. 2). The in vitro culture of the rectal swab in the InPouch™, TF-Feline medium successfully isolated trichomonal trophozoites. The scanning electron microscopy of cultured trophozoites distinctively visualized 3 anterior flagella and a posterior flagellum extended backward along the undulating membrane (Fig. 3).
Fig. 2.
Light microscopic image of Tritrichomonas foetus trophozoites from the feces of a Siamese cat in Giemsa stain.
Fig. 3.
Scanning electron micrograph of a Tritrichomonas foetus trophozoite isolated from a Siamese cat in Korea.
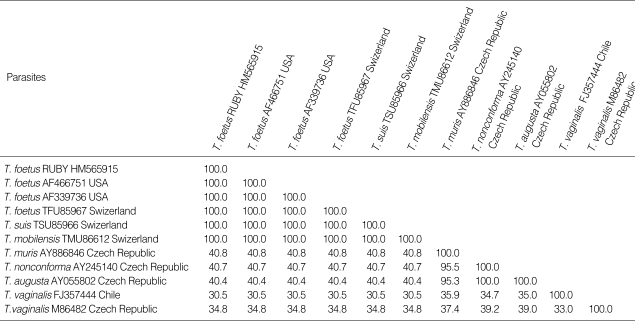
Primer pairs TFR-3/TFR-4 and TFITS-F/TFITS-R used together in a single-tube nested PCR confirmed the presence of T. foetus organism in rectal swab samples of littermate Siamese cats by the amplification of the 208-bp product (Fig. 4). The sequence and phylogenetic tree analysis of the 208-bp PCR product revealed 100% sequence identity with T. foetus, T. suis and T. mobilensis reported previously from USA and Swizerland (Table 1; Fig. 5).
Fig. 4.
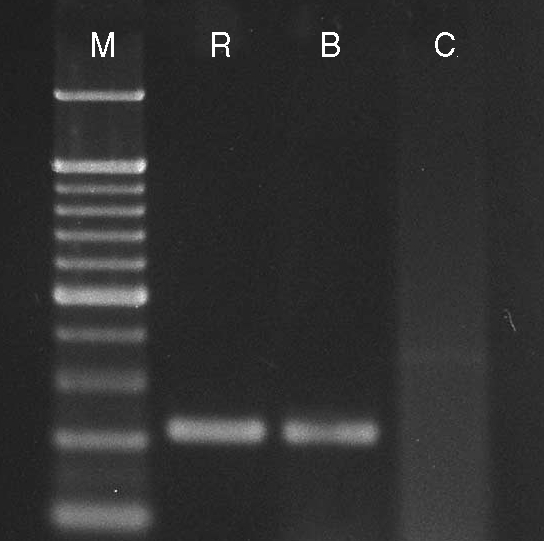
Single-tube nested PCR products with primer pairs TFR-3/TFR-4 and TFITS-F/TFITS-R confirming the presence of Tritrichomonas foetus organism in rectal swab samples of the 2 female and male littermate Siamese cats by amplification of the 208-bp product. M, molecular weight marker; R, female Siamese cat; B, male Siamese littermate cat; C, culture medium.
Table 1.
The sequence homology analysis of the 208-bp PCR products of Tritrichomonas foetus from a Siamese cat from Korea
T. foetus isolate from the female Siamese cat in this study.
Fig. 5.
Sequence of the 208-bp PCR product of Tritrichomonas foetus isolated from the rectal swab feces of a Siamese cat from Korea. The ITS1/5.8S genomic region was amplified with primer pairs TFR-3/TFR-4 and TFITS-F TFITS-R.
DISCUSSION
The present study demonstrated T. foetus infection in 2 Siamese cats from Korea with a history of chronic diarrhea by Giemsa staining, scanning electron microscopy, in vitro culture, PCR assay, and DNA sequence analysis. This is the first case of feline intestinal trichomoniasis in Korea. No clinical case of T. foetus infection in cats has so far been reported in the Far Eastern countries.
In USA, the prevalence rate of intestinal T. foetus infection was 31% among cats (36 out of 117), while a survey of 111 cats with diarrhea performed in UK found 16 T. foetus-positive fecal samples by PCR (14.4%) [5,9]. In Italy, 24 of 74 cats with chronic large bowel diarrhea (32%) were found to be infected with T. foetus in an animal shelter [6]. There was a report in Australia of feline trichomoniasis in a 10-week-old female cat which had chronic diarrhea [4]. In Switzerland, 45 cats suffering from chronic diarrhea were investigated for intestinal infection, including a search for T. foetus using bovine InPouch™ TF-Feline medium and PCR assay [7] from which 11 cats (24.4%) were culture- positive. In Norway, T. foetus was reported in the uterus of a cat with pyometra and in the feces of 3 other cats in the same household, 1 of which had chronic diarrhea [8].
Feline gastrointestinal T. foetus infection in this case did not respond to the chemotherapy with metronidazole and fendendazole. A previous report indicated that when ronidazole [1-methy-5-nitroimidazole-2-yl)-methyl carbamate; > 99% pure, Sigma-Aldrich], currently not approved by FDA, was administered at 30-50 mg/kg twice a day for 14 days, 10/10 cats were negative for T. foetus infection for follow-up duration of 21 to 30 weeks after treatment [15]. Therefore, after T. foetus was identified as the causative agent of prolonged large bowel diarrhea in this case, we attempted to treat the two cats infected with T. foetus using ronidazole at 30 mg/kg every 12 hr for 14 days. The medication, however, was discontinued intermittently according to the owner, and the diarrhea relapsed with T. foetus trophozoites reappeared in the rectal swab samples after 1 month. Further studies are needed to obtain the infection status of cats raised in Korea with this protozoan parasite, and identify an effective chemotherapeutic regimen against the Korean isolate of T. foetus.
References
- 1.Taylor MA, Coop RL, Wall R. Veterinary Parasitology. 3rd ed. Oxford, UK. Ames, Iowa: Blackwell; 2007. pp. 119–120. [Google Scholar]
- 2.Cobo ER, Cano D, Campero CM. Experimental infection with Tritrichomonas suis in heifers. Vet Parasitol. 2001;99:73–78. doi: 10.1016/s0304-4017(01)00444-7. [DOI] [PubMed] [Google Scholar]
- 3.Lun ZR, Chen XG, Zhu XQ, Li XR, Xie MQ. Are Tritrichomonas foetus and Tritrichomonas suis synonyms? Trends Parasitol. 2005;21:122–125. doi: 10.1016/j.pt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Bissett SA, Gowan RA, O'Brien CR, Stone MR, Gookin JL. Feline diarrhoea associated with Tritrichomonas cf. foetus and Giardia coinfection in an Australian cattery. Aust Vet J. 2008;86:440–443. doi: 10.1111/j.1751-0813.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- 5.Gunn-Moore DA, McCann TM, Reed N, Simpson KE, Tennant B. Prevalence of Tritrichomonas foetus infection in cats with diarrhoea in the UK. J Feline Med Surg. 2007;9:214–218. doi: 10.1016/j.jfms.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holliday M, Deni D, Gunn-Moore DA. Tritrichomonas foetus infection in cats with diarrhoea in a rescue colony in Italy. J Feline Med Surg. 2009;11:131–134. doi: 10.1016/j.jfms.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey CF, Schild M, Hemphill A, Stunzi P, Muller N, Gottstein B, Burgener IA. Intestinal Tritrichomonas foetus infection in cats in Switzerland detected by in vitro cultivation and PCR. Parasitol Res. 2009;104:783–788. doi: 10.1007/s00436-008-1255-2. [DOI] [PubMed] [Google Scholar]
- 8.Dahlgren SS, Gjerde B, Pettersen HY. First record of natural Tritrichomonas foetus infection of the feline uterus. J Small Anim Pract. 2007;48:654–657. doi: 10.1111/j.1748-5827.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- 9.Gookin JL, Stebbins ME, Hunt E, Burlone K, Fulton M, Hochel R, Talaat M, Poore M, Levy MG. Prevalence of and risk factors for feline Tritrichomonas foetus and Giardia infection. J Clin Microbiol. 2004;42:2707–2710. doi: 10.1128/JCM.42.6.2707-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slapeta J, Craig S, McDonell D, Emery D. Tritrichomonas foetus from domestic cats and cattle are genetically distinct. Exp Parasitol. 2010;126:209–213. doi: 10.1016/j.exppara.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Yaeger MJ, Gookin JL. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet Pathol. 2005;42:797–804. doi: 10.1354/vp.42-6-797. [DOI] [PubMed] [Google Scholar]
- 12.Gookin JL, Breitschwerdt EB, Levy MG, Gager RB, Benrud JG. Diarrhea associated with trichomonosis in cats. J Am Vet Med Assoc. 1999;215:1450–1454. [PubMed] [Google Scholar]
- 13.Gookin JL, Levy MG, Law JM, Papich MG, Poore MF, Breitschwerdt EB. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res. 2001;62:1690–1697. doi: 10.2460/ajvr.2001.62.1690. [DOI] [PubMed] [Google Scholar]
- 14.Foster DM, Gookin JL, Poore MF, Stebbins ME, Levy MG. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc. 2004;225:888–892. doi: 10.2460/javma.2004.225.888. [DOI] [PubMed] [Google Scholar]
- 15.Gookin JL, Copple CN, Papich MG, Poore MF, Stauffer SH, Birkenheuer AJ, Twedt DC, Levy MG. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med. 2006;20:536–543. doi: 10.1892/0891-6640(2006)20[536:eorfto]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Pereira-Neves A, Benchimol M. Phagocytosis by Trichomonas vaginalis: new insights. Biol Cell. 2007;99:87–101. doi: 10.1042/BC20060084. [DOI] [PubMed] [Google Scholar]
- 17.Gookin JL, Birkenheuer AJ, Breitschwerdt EB, Levy MG. Single-tube nested PCR for detection of Tritrichomonas foetus in feline feces. J Clin Microbiol. 2002;40:4126–4130. doi: 10.1128/JCM.40.11.4126-4130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]