Abstract
The link between diabetes mellitus and tuberculosis has been recognised for centuries. In recent decades, tuberculosis incidence has declined in high-income countries, but incidence remains high in countries that have high rates of infection with HIV, high prevalence of malnutrition and crowded living conditions, or poor tuberculosis control infrastructure. At the same time, diabetes mellitus prevalence is soaring globally, fuelled by obesity. There is growing evidence that diabetes mellitus is an important risk factor for tuberculosis and might affect disease presentation and treatment response. Furthermore, tuberculosis might induce glucose intolerance and worsen glycaemic control in people with diabetes. We review the epidemiology of the tuberculosis and diabetes epidemics, and provide a synopsis of the evidence for the role of diabetes mellitus in susceptibility to, clinical presentation of, and response to treatment for tuberculosis. In addition, we review potential mechanisms by which diabetes mellitus can cause tuberculosis, the effects of tuberculosis on diabetic control, and pharmacokinetic issues related to the co-management of diabetes and tuberculosis.
Introduction
The association between diabetes mellitus and tuberculosis and their synergistic role in causing human disease has been recognised for centuries. Ancient works by Yugimahamuni, an Indian siddhar, describe the symptoms of patients with “meganoikal” (urinary disorders), which progressed from obesity to impotence, thirst, and glycosuria, and ultimately, to unconsciousness or tuberculosis.1 The introduction of insulin in the 1920s, the discovery of streptomycin in the 1940s, and the subsequent development of other antibiotics substantially lowered case fatality rates for individuals with diabetes mellitus or tuberculosis. Improved sanitation, better nutrition, and less crowding led to markedly diminished tuberculosis incidence. In recent decades, tuberculosis has increasingly become a problem in low-income countries, particularly those with HIV epidemics, and non-insulin-dependent diabetes mellitus (NIDDM) has emerged as a growing worldwide chronic health condition, as a consequence of increases in obesity, changing patterns of diet and physical activity, and aging populations.2–5 The effect of diabetes on the development and severity of tuberculosis, and the complex interrelations between nutrition, obesity, diabetes, and tuberculosis remain provocative issues in public health and clinical medicine.6–8 In the setting of the increasing overlap of populations at risk for both diseases, the combination of tuberculosis and diabetes mellitus represents a worldwide health threat.
Our aim was to evaluate the published work and synthesise a concise Review of the following topics: the epidemiology of diabetes mellitus and tuberculosis disease; the effect of diabetes mellitus on tuberculosis incidence, radiographic presentation, severity, and outcomes; the potential mechanisms by which diabetes mellitus increases tuberculosis incidence; the cause–effect relation of tuberculosis on incident diabetes mellitus; and the pharmacological issues in cotreatment of tuberculosis and diabetes mellitus.
Double burden of tuberculosis and diabetes
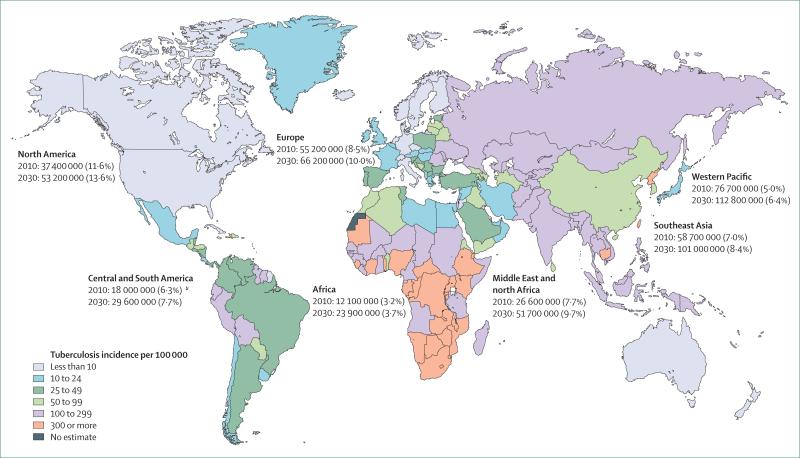
The burden of communicable diseases is concentrated in low-income countries. However, non-communicable diseases, which represented 47% of the disease burden in 1990 in low-income countries, have been predicted to rise to 69% by 2020.9 Increasing industrialisation and urbanisation leads to higher rates of obesity and diabetes. The number of people with diabetes, which was 171 million in 2000, is expected to grow to 366 million–440 million by 2030, with three-quarters of patients with diabetes living in low-income countries (figure).10,12,13 Diabetes poses a large financial burden in countries with limited resources. For example, in Africa, where mean per capita expenditures on health are US$30–800, the mean annual cost for diabetes care ranges between $2144 and $11 430 (direct costs $876–1220).14 In many countries, insulin is expensive or availability is poor: a 1-month supply of insulin can cost up to 20 days’ wages.15 Thus, social and economic conditions heavily influence treatment options.16
Figure. Projected prevalent diabetes cases and current worldwide tuberculosis incidence.
Estimated number and percent of individuals with diabetes mellitus in 2010 compared with 2030 projections are shown. Tuberculosis incidence per 100 000 population data for 2007 are shown. Data from International Diabetes Foundation and WHO.10,11
In these resource-limited settings, tuberculosis continues to be have high mortality. Whereas the most common causes of death in low-income and middle-income countries are ischaemic heart disease and cerebrovascular disease, HIV and tuberculosis are in the top five causes of death.17 Tuberculosis, poverty, and poor access to health services are closely linked, complicating provision of tuberculosis care.18 Comorbidities such as diabetes mellitus complicate tuberculosis care further. Several studies show that coaffliction with tuberculosis and diabetes mellitus is common, both in low-income and high-income countries.19–22 How will overburdened public health services manage the costs of chronic non-infectious diseases as the overlap between those with communicable and non-communicable diseases increases?
Effect of diabetes on tuberculosis risk and severity
Historically, the incidence of tuberculosis in patients with diabetes has been high.23,24 In 1934, a treatise on the association between diabetes and tuberculosis was written by Howard Root (a physician at the Deaconess Hospital, Boston, MA, USA), before the availability of antimycobacterial drugs.24 His lengthy tome described the epidemiology, pathology, and clinical course of dually affected patients. In his studies, tuberculosis in adults with diabetes was more common than expected, and risk was particularly high in schoolchildren and adolescents with diabetes. In his autopsy series of 126 patients, no pathological findings unique to “the tubercular diabetic” were discovered. Among a total of 245 tubercul osis cases in diabetic patients, he found “no special insidiousness” of signs and symptoms, and similar radiographic findings to those of non-diabetic patients. Tuberculosis developed most frequently in patients with poor diabetic control. In the Philadelphia Diabetic Survey, Boucot and colleagues25 found a two-fold increase in prevalent tuberculosis by chest radiograph in 3106 diabetic patients compared with 70767 controls of similar demographics. Furthermore, they found that diabetic patients who needed more than 40 units of insulin per day were twice as likely to develop tuberculosis as those using lower doses, thus linking severity of diabetes mellitus with risk of tuberculosis.
In the past 20 years, the debate over whether diabetes mellitus causes increased susceptibility to tuberculosis, as well as differences in presentation, severity, and response to therapy, has been rekindled. We summarise the research addressing these issues.
Tuberculosis incidence in patients with diabetes
The risk of developing active tuberculosis is a two-step process, beginning with initial exposure to and infection by Mycobacterium tuberculosis followed by subsequent progression to disease. Studies of diabetes mellitus and tuberculosis generally focus on active tuberculosis disease. However, in one study in a general medicine clinic in Spain, 69 (42%) of 163 diabetic patients had a positive tuberculin skin test, suggesting a high rate of latent tuberculosis in diabetic patients, although this could have been confounded by age and there was no control group.26 Several case–control studies have shown that the relative odds of developing tuberculosis in diabetic patients ranges from 2·44 to 8·33 compared with non-diabetic patients (table 1).27–30 Several large-scale longitudinal cohort studies have shown similar findings.19,33,35,39,40 In Korea, a 3-year longitudinal study involving 800 000 civil servants showed that the risk ratio of tuberculosis in diabetic patients versus non-diabetic controls was 3·47 (95% CI 2·98–4·03).33 In a study of the UK General Practice Research Dtabase, which includes records from over 2 million patients, Jick and colleagues37 identified all cases of tuberculosis reported between 1990 and 2001 and compared them with controls, and found that the adjusted odds ratio (adjusted for age, sex, and practice) for tuberculosis was 3·8 (95% CI 2·3–6·1) for diabetic patients compared with those without diabetes. In Hong Kong, in a 5-year study of 42 000 elderly individuals, the adjusted hazard of active tuberculosis was higher in diabetic patients than in individuals without diabetes (1·77; 95% CI 1·41– 2·24), but this increased risk was only present in those with haemoglobin A1c concentrations greater than 7%.40 These large studies involving thousands of participants provide convincing data that diabetes mellitus is a moderate-to-strong risk factor for the development of active tuberculosis. Indeed, a recent large meta-analysis showed that diabetic patients were 3·1 times (95% CI 2·27–4·26) more likely to have tuberculosis than controls, with higher effect sizes in non-North American populations.41 Several studies suggest that the risk of developing active tuberculosis among diabetic patients is particularly high among Hispanic people, perhaps because latent tuberculosis infection is more common in these populations.34,36,38 Among Hispanic people aged 25–54 years, the tuberculosis risk attributable to diabetes was 25%, equivalent to that of HIV.34
Table 1.
Studies on diabetes mellitus (DM) as a risk factor for the development of tuberculosis (TB)
| Year | Location | Setting | Type of study | Participants (n) | Outcome variable and findings | |
|---|---|---|---|---|---|---|
| Boucot et al25 | 1952 | Philadelphia, USA | .. | Chest radiograph survey comparing DM patients with healthy industrial workers | 73 873 | Prevalent TB by chest radiograph: 8·4% in DM (2 times that of controls) |
| Olmos et al31 | 1989 | Chile | Teaching hospital | Retrospective cohort of DM patients followed 10 years | 1529 | 10-year risk of TB in IDDM or NIDDM vs general population: 24% IDDM, 4% NIDDM, 0·8% general population |
| Swai et al32 | 1990 | Tanzania | Inpatient and outpatient clinics | Prospective cohort of DM patients followed 1–7 years | 1250 | Risk of pulmonary TB: 9·0% in IDDM, 2·7% in NIDDM |
| Bermejo et al26 | 1995 | Spain | General medicine clinics | Cross-sectional study of TST results in DM patients | 163 | TST positivity at 3 days: 42·2% |
| Kim et al33 | 1995 | Korea | Civil servants | Longitudinal cohort study using insurance claims | 8015 cases, 806 698 controls | RR of developing pulmonary TB: 3·47 (DM vs non-DM); 95% CI 1·19–1·45 |
| Pablos-Mendez et al34 | 1997 | California, USA | Inpatient hospitals | Case–control study using discharge diagnoses | 5290 cases, 37 366 controls | OR of DM comparing tuberculosis patients with patients with deep venous thrombosis, pulmonary embolus, or appendicitis: 2·95 for Hispanic people (95% CI 2·61–3·33), 1·31 for white people (1·19–1·45) |
| Mboussa et al27 | 2003 | Congo | University hospital | Case–control study using chart review | 32 cases, 100 controls | OR for TB: 8·33 (DM vs non-DM) |
| Shah and Hux35 | 2003 | Ontario, Canada | Inpatient and outpatient | Retrospective cohort study using province-wide administrative database | 513 749 in each group | OR for TB: 1·12 (DM vs non-DM); 95% CI 1·03–1·23 |
| Ponce-De-León et al36 | 2004 | Mexico | Inpatient and outpatient clinics | Population-based cohort linked to statewide cluster household survey | 1915 | IRR of TB: 6·8 (DM vs non-DM); 95% CI 5·7–8·2 |
| Coker et al29 | 2006 | Russia | TB clinics in urban setting | Case–control study with controls sampled from general population | 334 cases, 334 controls | AOR for TB: 7·83 (DM vs non-DM), controlling for assets, overcrowding, employment, and financial security; 95% CI 2·37–25·9 |
| Jabbar et al30 | 2006 | Pakistan | Teaching hospital | Case–control study using discharge diagnoses | 1458 cases, 40 900 controls | OR for TB: 7·83 (DM vs non-DM); 95% CI 6·55–9·37 |
| Jick et al37 | 2006 | UK | General practices | Case–control study using large countrywide database | 497 cases, 1966 controls | AOR for TB: 3·8 (DM vs non-DM), adjusting for steroid use, smoking, body–mass index, pulmonary diseases, immunosuppressive use; 95% CI 2·3–6·1 |
| Perez et al38 | 2006 | Texas, USA | Inpatient clinic | Case–control study using hospital discharge database | 4915 cases, 70 808 controls | AOR for TB (DM vs non-DM), adjusting for sex, age, and race/ethnicity: 1·51 in non-border Texas (95% CI 1·36–1·67), 1·82 in counties bordering Mexico (95% CI 1·57–2·12) |
| Shetty et al28 | 2006 | India | Outpatient clinic | Matched case–control study using chart review; controls were relatives of cases. | 189 cases, 189 controls | OR for TB: 2·44 (patients with diabetes, hypertension, or heart disease vs those without), matched for age and sex; 95% CI 1·17–5·09 |
| Dyck et al39 | 2007 | Saskatchewan, Canada | Inpatient and outpatient clinics | Retrospective cohort study using large health database | 2122 cases* | IRR for TB: 1·53 (DM vs non-DM); 95% CI 1·25–1·87 |
| Leung et al40 | 2008 | Hong Kong | Elderly health service | Prospective population-based cohort | 42 116 | AHR for TB: 1·77 (95% CI 1·41–2·24), DM vs non-DM; 3·11 (95% CI 1·63–5·92) in diabetics with HbA1C>7% vs HbA1C<7% |
Numbers of controls not reported. AHR=adjusted hazard ratio. AOR=adjusted odds ratio (OR). IDDM=insulin-dependent diabetes mellitus. IRR=incidence rate ratio. NIDDM=non-insulin-dependent diabetes mellitus. TST=tuberculin skin test. RR=risk ratio.
If diabetes is associated with tuberculosis, one might ask whether severity of diabetes is related to the magnitude of risk. Two studies have compared the incidence of active tuberculosis between insulin-dependent diabetes mellitus (IDDM) and NIDDM. In a cohort of 1529 diabetic individuals in Chile, who were followed prospectively from 1959 to 1982, the 10-year actuarial probability of developing tuberculosis was 24% in IDDM and 4·8% in NIDDM.31 In a prospective study of diabetic patients followed for 1–7 years in Tanzania, 9·0% of patients with IDDM and 2·7% of patients with NIDDM developed pulmonary tuberculosis.32 These two studies provide evidence that insulin dependence, as a marker for severity of disease, predicts increased tuberculosis risk. In a recent study of 4690 elderly diabetic patients in Hong Kong, those with haemoglobin A1c greater than 7% had a three times increased hazard of active tuberculosis compared with those with haemoglobin A1c less than 7% (hazard ratio 3·11; 95% CI 1·63–5·92).40 These data suggest that poor glycaemic control is a risk factor for tuberculosis.
Although there is no reason, a priori, to expect an association with diabetes mellitus and drug resistance, two studies have shown that diabetic patients are more likely to develop multidrug-resistant tuberculosis than those without diabetes.42,43 However, four studies in disparate settings showed no significant increased risk.44–47 The scientific mechanism by which diabetes mellitus would lead to preferential acquisition of multidrug-resistant tuberculosis is unclear.
Radiographic findings in tuberculous diabetic patients
The radiographic presentation of tuberculosis depends on many factors, including duration of illness and host immune status. In 1927, Sosman and Steidl48 reported that a large proportion of diabetic patients with tuberculosis had lower-lung involvement, whereas non-diabetic patients usually had upper-lobe infiltrates. Subsequent studies in the 1970s and 1980s corroborated this finding,49,50 and it was widely believed that pulmonary tuberculosis in diabetic patients presented with an atypical radiographic pattern and distribution, particularly lower-lung involvement. Clinically, this is important because lower-lobe tuberculosis might be misdiagnosed as community-acquired pneumonia or cancer. Also, patients with pulmonary tuberculosis that do not have upper-lobe involvement are less likely to have positive sputum smears and cultures.51 Whereas in one series, 20% of patients with diabetes mellitus presented with lower-lobe involvement,49 in other studies, lower-lobe involvement was only seen in 1·8% (8 of 438 patients) and 8·3% (1 of 12 patients).52,53 Subsequent studies have yielded mixed results (table 2).
Table 2.
Studies assessing chest radiographic findings* in patients with tuberculosis, comparing diabetic to non-diabetic patients
| Year | Study location | Participants (n) |
Lower lung more commonly involved? | More cavitary lesions? | More diffuse involvement? | ||
|---|---|---|---|---|---|---|---|
| With diabetes | Without diabetes | ||||||
| Weaver49 | 1974 | USA | 20 | 182 | Yes | .. | .. |
| Marais50 | 1980 | South Africa | 9 | 427 | Yes | .. | .. |
| Ikezoe et al54 | 1992 | Japan | 31† | 71 | No | Yes | Yes |
| Morris et al55 | 1992 | Texas, USA | 20 | 20 | No | No | No |
| Umut et al56 | 1994 | Turkey | 37 | 37 | No | Yes | Yes |
| Kuaban et al57 | 1996 | Cameroon | .. | 273‡ | Yes | .. | .. |
| al-Wabel et al58 | 1997 | Saudi Arabia | 28 | 38 | No | .. | .. |
| Bacakoglu et al59 | 2001 | Turkey | 92 | 92 | No§ | No§ | No |
| Perez-Guzman et al60,61 | 2000–01 | Mexico | 192 | 130 | Yes | Yes | Yes |
| Shaikh et al62 | 2003 | Saudi Arabia | 187 | 505 | Yes | .. | .. |
| Wang et al63 | 2005 | Taiwan | 99 | 362 | No | Yes | .. |
| Wang et al64 | 2008 | Taiwan | 74 | 143 | Yes | Yes | .. |
| Al-Tawfiq et al51 | 2009 | Saudi Arabia | 57 | 78 | .. | No | .. |
Apart from the study by Ikezoe et al,54 in which computed tomography was used.
Patients with diabetes mellitus or who were immunocompromised.
Patients with tuberculosis, of whom 28 had lower-lung disease.
Insulin-dependent diabetes mellitus had more cavitary disease than non-insulin-dependent diabetes mellitus; in subgroup analysis, diabetes mellitus was a risk factor for lower-lung disease in patients aged >40 years.
=not reported.
Of note, older individuals are more likely to have lower-lobe involvement, and preferential changes in lower-lobe alveolar oxygen tension related to age or diabetes mellitus has been suggested to favour lower-lobe disease in these groups.51,61 In most series, multilobar disease or the presence of multiple cavities was more common in diabetic patients, but lower-lung disease was rarely more common in diabetic patients than in controls, except, perhaps, in patients aged over 40 years.54,55,59,60,62 Results vary substantially between studies, and the frequency of unusual radiographic findings in diabetic patients has probably been overstated.
Severity of disease and outcomes in diabetic patients with tuberculosis
Mycobacterial burden, culture conversion, and relapse
If diabetes alters immunity to tuberculosis, leading to higher baseline mycobacterial burdens and longer times to culture conversion with treatment, a higher rate of relapse might result. Three small retrospective studies suggest that baseline mycobacterial burdens might be higher in diabetic patients than in controls.27,64,65 However, results of studies assessing sputum-culture conversion show mixed results depending on the outcome variable used (table 3). In studies that assessed sputum-culture conversion after at least 2 months of treatment (a common surrogate marker used to predict tuberculosis relapse), conversion proportions were similar in diabetic patients and controls.20,68 For example, in a study in Indonesia, diabetes was not a risk factor for sputum-smear or sputum-culture positivity at 2 months after adjustment for age, sex, body–mass index, study site, chest radiographic findings, and baseline sputum mycobacterial load.67 Similarly, among 692 smear-positive tuberculosis patients in Saudi Arabia, 98·9% of diabetic patients and 94·7% of controls had negative sputum cultures at 3 months.66 However, in studies assessing time to sputum-culture conversion, diabetic patients seem to take longer to achieve culture negativity. In one study in Turkey, patients with diabetes who received tuberculosis treatment had longer sputum-culture conversion times than non-diabetic patients (67 vs 55 days; p=0·02).69 In a study that used survival analysis to measure time to culture conversion, median time to culture negativity was significantly longer in diabetic patients than in controls (42 vs 37 days; p=0·03).70 Using similar techniques, a third study also found a trend toward increased median time to culture conversion in diabetic patients (49 vs 39 days; p=0·09).20 Together, these data suggest that although bacillary burden might be higher at presentation in diabetic patients, leading to modestly longer times to sputum-culture conversion, rates of sputum-culture conversion are similar to those of non-diabetic patients by 2–3 months of treatment. Whether increased time to culture conversion in diabetic patients leads to higher risk of relapse has not been adequately studied.
Table 3.
Studies assessing the effect of diabetes mellitus on conversion of sputum smear or culture from positive to negative in patients treated for tuberculosis (TB)
| Year | Location | Setting | Type of study | Participants (n) | Outcome variables and findings (diabetes vs non-diabetes) | |
|---|---|---|---|---|---|---|
| Singla et al66 | 2006 | Saudi Arabia | TB referral hospital | Retrospective study of smear-positive pulmonary TB patients | 692 | 2-month sputum smear conversion: 83·8% vs 90·7% (p=0·011); 3-month sputum smear conversion: 98·9% vs 94·7% (p=0·013) |
| Alisjahbana et al67 | 2007 | Indonesia | Outpatient clinics | Prospective cohort study of new pulmonary TB patients | 634 | Proportion with positive microscopic examination of sputum after 2 months of treatment: 18·1% vs 10%. AOR 1·90 (0·82–4·42), adjusting for age, sex, BMI, study site, radiographic findings, and sputum mycobacterial load at treatment initiation |
| Banu Rekha et al68 | 2007 | India | TB research centres | Retrospective analysis of new smear-positive TB patients enrolled in clinical trials | 190 | Conversion to negative after completion of intensive-phase TB treatment: sputum smear, 58% vs 61%; sputum culture, 86% vs 88% |
| Guler et al69 | 2007 | Turkey | Referral hospital | Retrospective study of hospitalised pulmonary TB patients | 737 | Time to culture conversion: 67 days vs 55 days (p=0·02), AOR 5·25 (1·84–14·99) of sputum-culture conversion to negative after 2 months of treatment, adjusted for sex, smoking, radiographic findings, and baseline AFB positivity |
| Restrepo et al70 | 2008 | Texas, USA | TB programmes | Retrospective study of culture-positive TB patients in large database | 469 | Time to culture conversion: 42 days vs 37 days (p=0·03; Kaplan-Meier, log-rank test), AHR 0·75 (0·59–0·96) for culture conversion |
| Dooley et al20 | 2009 | Maryland, USA | TB patients in three counties | Retrospective cohort study of culture-positive pulmonary TB patients | 207 | Median time to sputum culture conversion: 49 days vs 39 days (p=0·09, log-rank test), Proportion converting culture to negative by 2 months: 70% vs 69% (p=0·94) |
| Maalej et al71 | 2009 | Tunisia | Hospital | Retrospective case–control study of smear-positive or culture-positive pulmonary TB patients | 142 | Time to culture conversion: 43 (SD 27) days vs 28 (SD 20) days (p=0·03) |
AFB=acid-fast bacillus. AHR=adjusted hazards ratio. AOR=adjusted odds ratio. BMI=body mass index.
Treatment failure and death
Does diabetes mellitus predispose a patient to treatment failure or death? In one study in Egypt, which compared 119 patients with treatment failure to 119 controls, diabetes conferred a 3·9 times increased risk of treatment failure in patients receiving directly observed short-course therapy (table 4).72 In a study in Indonesia in patients with high adherence to treatment, 6-month sputum cultures were positive in 22·2% of patients with diabetes mellitus and in 6·9% of controls; these differences remained after adjustment for age, sex, body mass index, and other factors.67 Importantly, drug resistance was lower, and medication adherence was higher in diabetic patients, so increased failure was not due to resistance or non-adherence to treatment. In a descriptive case–control study by Mboussa and colleagues,27 treatment failure or death was seen in 41% of the patients with tuberculosis and diabetes mellitus, but in only 13% of those with tuberculosis alone. Of the eight patients who died in the tuberculosis and diabetes group, seven patients died of respiratory failure related to tuberculosis whereas one patient died of diabetic coma.
Table 4.
Studies assessing the effect of diabetes mellitus on treatment failure and death in patients treated for tuberculosis (TB)
| Year | Location | Setting | Type of study | Participants (n) | Outcome variables and findings (diabetes vs non-diabetes)* | |
|---|---|---|---|---|---|---|
| Treatment failure | ||||||
| Morsy et al72 | 2003 | Egypt | TB treatment centres | Case–control study assessing risk factors for treatment failure, matched for sex and centre | 119 cases, 119 controls | Crude OR 3·91 (1·65–9·5) for sputum smear positivity after 5 months of treatment; AOR 9·32 (2·7–31·7) adjusted for factors including age, sex, distance to tuberculosis centre, health education, and disease knowledge |
| Alisjahbana et al67 | 2007 | Indonesia | Outpatient clinics | Prospective cohort study of new pulmonary TB patients | 634 | Proportion with positive sputum culture at 6 months: 22·2% vs 6·9%. AOR 7·65 (1·89–30·95), adjusted for age, sex, BMI, radiographic findings, 2-month sputum results, non-compliance, and drug resistance |
| Mortality | ||||||
| Oursler et al73 | 2002 | Maryland, USA | Outpatient clinic | Retrospective cohort study of culture-confirmed TB patients | 139 | HR 4·8 (2·0–11·6), AHR 6·7 (1·6–29·3), adjusted for renal disease, COPD, HIV infection, and age |
| Mboussa et al27 | 2003 | Congo | University hospital | Case–control study using chart review | 32 cases, 100 controls | 25·1% vs 8% |
| Lindoso et al21 | 2008 | Sao Paulo, Brazil | Urban | Retrospective study of all TB-related deaths using death certificates, surveillance data, hospital records | 416 | Proportion of patients with TB-related death who had diabetes mellitus: 16% |
| Dooley et al20 | 2009 | Maryland, USA | TB patients in three counties | Retrospective cohort study of culture-positive TB patients | 297 | OR 2·0 (0·74–5·2), AOR 6·5 (1·1–38·0), adjusted for HIV status, age, weight, and foreign birth |
| Wang et al64 | 2009 | Taiwan | Teaching hospital | Retrospective study of culture-positive pulmonary TB patients | 217 | OR 2·56 (1·08–6·03), AOR 5·5 (2·27–13·5), adjusting for age and sex |
Unless otherwise indicated. AHR=adjusted hazards ratio (HR). AOR=adjusted odds ratio (OR). BMI=body mass index. COPD=chronic obstructive pulmonary disease.
Two retrospective cohort studies of patients with pulmonary tuberculosis in Maryland, USA, have shown a 6·5–6·7 times increased risk of death in diabetic patients compared to non-diabetic controls after adjustment for important cofactors.20,73 In a recent study by Wang and colleagues,64 1-year all-cause mortality was 17·6% in diabetic patients versus 7·7% in non-diabetic controls, and death specifically attributable to pulmonary tuberculosis was significantly more common in diabetic patients (12·2% vs 4·2%). Among 416 tuberculosis-related deaths in Sao Paulo, Brazil in 2002, diabetes was a common co-morbidity, present in 16%.21
These studies suggest that treatment failure and death are more frequent in diabetic patients. However, whether aggressive management of diabetes mellitus would improve treatment response remains unclear. Furthermore, because causes of death are not reported in most studies, we do not know whether excess mortality is explained by increased severity of tuberculosis in diabetic patients or by the existence of comorbidities attributable to diabetes mellitus compounded by more advanced age.
How might diabetes mellitus lead to tuberculosis?
Poorly controlled diabetes can lead to multiple complications, including vascular disease, neuropathy, and increased susceptibility to infection.74 Diabetes might also lead to increased susceptibility to disease caused by M tuberculosis via multiple mechanisms. The mechanisms include those directly related to hyperglycaemia and cellular insulinopenia, as well as indirect effects on macrophage and lymphocyte function, leading to diminished ability to contain the organism.
The most important effector cells for containment of tuberculosis are phagocytes (alveolar macrophages and their precursor monocytes) and lymphocytes. Diabetes is known to affect chemotaxis, phagocytosis, activation, and antigen presentation by phagocytes in response to M tuberculosis. In diabetic patients, chemotaxis of monocytes is impaired, and this defect does not improve with insulin.75 In mice with streptozotocin-induced persistent diabetes mellitus (streptozotocin is an islet-cell toxin), macrophages had a tenth of the phagocytic activity of control mice but similar intracellular killing.76 In these experiments, 90% of mice died after challenge with tuberculosis compared with 10% of normal mice. In a study of patients with tuberculosis, alveolar macrophages were less activated and had decreased hydrogen peroxide production in those with diabetes.77 In their role as antigen-presenting cells for the initiation of lymphocyte activation, phagocytes bind and then internalise antigen for processing and presentation via their Fc receptors; once activated, they produce interleukin 2, enhancing T-cell proliferation. Insulin deficiency can cause impaired internalisation of Fc-receptor-bound material.78 Pancreatectomised rats have poor Fc-receptor-mediated phagocytosis.75 In patients with NIDDM, one study showed normal interleukin-2 production by monocytes with normal numbers of Fc receptors but decreased populations of monocytes bearing complement receptor 3, which could lead to diminished adherence and phagocytosis.79
Diabetes might adversely affect T-cell production of interferon γ, and T-cell growth, function, and proliferation. Interferon γ potentiates the nitric-oxide-dependent intracellular killing activity of macrophages. In experiments involving mice with streptozotocin-induced diabetes that were challenged with M tuberculosis, concentrations of interferon γ were diminished, and production of inducible nitric-oxide synthase by macrophages was low;80 bacterial burden was also higher than in control mice.81 Interferon-γ production was further impaired in high glucose conditions.80 In addition, concentrations of interleukin 12, a T-cell-stimulating factor produced by macrophages, were lower in the lungs and spleen of diabetic animals. Similarly, in the Goto Kakizaki rat model of NIDDM, interferon-γ, interleukin-12, and nitric-oxide production were diminished in response to M tuberculosis.82 Lymphocyte proliferation in response to phytohaemagglutinin is weak in patients with poorly controlled IDDM.83 In a study of patients with NIDDM, a change in glucose concentration or addition of interleukin 12 did not increase T-lymphocyte proliferation or expression of interleukin-2 receptor.79
These studies and others point to depressed immunological function in IDDM and NIDDM that might predispose a patient to infections for which cell-mediated immunity has a pivotal role, such as tuberculosis. Decreased phagocyte and T-cell function are likely contributors. The implications of diabetes-related differences in the immune response to tuberculosis are being investigated.84 The relative contribution of genetics, vitamin deficiencies, and other factors to increased risk of tuberculosis in diabetic patients remains to be established.61,75
Does tuberculosis lead to diabetes?
If diabetes can predispose a patient to tuberculosis, can infection with tuberculosis lead to diabetes mellitus? Infections, including tuberculosis, often worsen glycaemic control in diabetic patients, and poorly controlled diabetes might in turn augment the severity of infections.85 Some studies suggest that tuberculosis can even cause diabetes in those not previously known to be diabetic. Many studies have used oral glucose tolerance testing to show that patients with tuberculosis have higher rates of glucose intolerance than community controls.78,86,87 Whereas the high incidence of abnormal oral glucose tolerance found in tuberculosis patients is of concern, it is unclear whether glucose intolerance or diabetes mellitus was truly incident, or whether prevalent diabetes mellitus was being newly diagnosed in patients receiving expanded medical services related to tuberculosis treatment. Also, the implications of these findings depend on whether diabetes mellitus persists in these patients, and whether its presence is substantially more common with tuberculosis than with other infectious diseases.
In a study in Nigeria, tuberculosis patients with impaired glucose tolerance had normal tests after 3 months of tuberculosis treatment.88 In Turkey, oral glucose tolerance tests were given to 58 patients with active tuberculosis and 23 patients with community-acquired pneumonia.89 Of those with tuberculosis, 10% were glucose intolerant and 9% had diabetes; of patients with community-acquired pneumonia, none had glucose intolerance and 17% were diabetic. All patients had normal tests 3 months and 2 years after the start of treatment. The latter two studies suggest that infection causes reversible glucose intolerance and that this effect is not specific to tuberculosis. In Indonesia, 13% (60 of 454) of patients with tuberculosis had diabetes, compared with 3·2% (18 of 556) of age-matched and sex-matched controls from the same residential unit; for 60% of these patients, diabetes was a new diagnosis.90 Whereas impairment of glucose metabolism probably preceded tuberculosis in these patients rather than the reverse, these data underscore the importance of screening tuberculosis patients for diabetes.
Pharmacological issues in the co-management of diabetes mellitus and tuberculosis
Infections are known to worsen diabetic control, and tuberculosis is no exception. Although tuberculosis can cause glucose intolerance and might predispose patients to diabetes mellitus, the drugs used to treat tuberculosis might also worsen glycaemic control in patients with diabetes. Overlapping toxicities must also be considered when co-managing tuberculosis and diabetes, such as peripheral neuropathy caused by treatmetn with isoniazid. Given the risk of peripheral neuropathy, pyridoxine should be given with isoniazid during tuberculosis treatment in diabetic patients.91 In addition, treatment with rifampicin can cause hyperglycaemia directly or indirectly via interactions with oral hypoglycaemic drugs.92,93
Rifampicin is a powerful inducer of a host of metabolising enzymes, including cytochrome P450 system enzymes and phase II enzymes.94 Induction of these enzymes can lead to accelerated metabolism of drugs given with rifampicin and reduced treatment effect. The sulfonyl ureas are among the most commonly used oral hypoglycaemic drugs for patients with NIDDM. Glyburide and glipizide are both substrates of cytochrome P450 isoenzyme 2C9 (CYP2C9), and pharmacokinetic studies show that serum concentrations of these drugs are decreased by 39% and 22%, respectively, when given with rifampicin.92 Pharmacodynamic data further show that glyburide's hypoglycaemic effect is reduced when given with rifampicin. Thiazolidinediones are often used as substrates for the cytochrome P450 enzymes. Rosiglitazone is metabolised largely by CYP2C8, and rifampicin decreases concentrations of rosiglitazone by 54–65% and of the related drug pioglitazone by 54%.95–97 Nateglinide, a short-acting insulin secretagogue given to prevent postprandial hyperglycaemia, is metabolised by oxidative bio transformation, with involvement from CYP2C9 and CYP3A4; its area under the curve is reduced by only 24% with no appreciable glycaemic effect when given with rifampicin.98 Repaglinide, another related drug, had an area under the curve that was decreased by 31–57% when given with rifampicin, although its glucose-lowering effect was reduced in one study and unchanged in another.99,100 In patients with IDDM, insulin requirements might increase when on rifampicin.99 Rifampicin has been shown to cause early-phase hyperglycaemia with associated hyperinsulinaemia even in non-diabetic patients.101,102 Rifampicin's direct and indirect effects on glycaemic control make careful monitoring with appropriate dose adjustment of diabetic agents essential in diabetic patients with tuberculosis.
Just as tuberculosis drug treatment affects diabetes treatment, diabetes might alter the pharmacokinetics of antituberculosis drugs. In one study in Indonesia, diabetic patients with tuberculosis had rifampicin serum concentrations that were 53% lower than in non-diabetic patients with tuberculosis, and there was an indirect relation between fasting glucose and rifampicin concentrations.103 Given that low concentrations of anti-tuberculosis drugs have been linked to treatment failure or resistance, this finding is of particular concern. Diabetes can also cause changes in oral absorption, decreased protein binding of drugs, and renal insufficiency or fatty liver with impaired drug clearance.104 Its effect on tuberculosis drug concentrations has not been formally studied; in cases of poor response to treatment in diabetic patients with tuberculosis, therapeutic drug monitoring might be considered.105
Search strategy and selection criteria.
We searched the PubMed database on three occasions over 2 years by use of the following search terms: (“tuberculosis”[MeSH Terms] OR “tuberculosis”[All Fields]) AND (“diabetes mellitus”[MeSH Terms] OR “diabetes mellitus”[All Fields] OR “diabetes”[All Fields] OR “NIDDM”[All Fields] OR “IDDM”[All Fields]). The date reange of the search was from June, 2007, to August, 2009. We searched EMBASE by use of a similar search strategy. A hand search of references in included articles as well as recent reviews of diabetes mellitus and tuberculosis was also done. We included preclinical, cross-sectional, retrospective and prospective cohort, case–control, and pharmacokinetic studies written in English, French, Spanish, or Portuguese. Clinical studies that compared any of the following tuberculosis endpoints in diabetic versus non-diabetic patients and included a point estimate were included: incidence, radiographic presentation, severity of disease, or outcomes (failure, relapse, mortality, etc). Quantitative analysis was not done due to the scope of this Review and the paucity of high-quality studies.
Future research
In reviewing and summarising the published work on the complex relation between tuberculosis and diabetes mellitus and their respective treatments, we have found that many important topics have been poorly studied or not studied at all. Although tuberculosis is clearly more common in diabetic patients, several questions remain unanswered that would greatly affect the clinical management of the two diseases and, thus, merit increased attention: does diabetes mellitus lead to increased susceptibility to initial tuberculosis infection, or, rather, does diabetes mellitus lead to increased progression from latent tuberculosis to active tuberculosis? Would screening for and treatment of latent tuberculosis in diabetic patients be appropriate and cost-effective; if so, in which populations? Which tuberculosis patients should we screen for diabetes mellitus? Does diabetes substantially prolong sputum smear and culture positivity; if so, are diabetic patients at higher risk of relapse than non-diabetic patients, and might this affect appropriate treatment duration? Does aggressive management of diabetes mellitus in patients with tuberculosis affect treatment outcomes? If mortality is higher in tuberculosis patients with diabetes, what are the most common preventable causes of death in coaffected individuals? Is there a relation between low rifampicin concentrations and tuberculosis treatment failure or acquisition of resistance in diabetic patients; if so, what might be the role of therapeutic drug monitoring?
With increasing rates of obesity and diabetes worldwide and continued high rates of tuberculosis in low-income countries, we can expect that the number of individuals who have both tuberculosis and diabetes mellitus will increase markedly in the coming decades. More research in this largely neglected area would therefore be beneficial.
Acknowledgments
KED is supported by US National Institutes of Health grant K23AI080842. REC is supported by NIH grant AI01607.
Footnotes
Conflict of interests
We declare that we have no conflicts of interests.
References
- 1.Rajalakshmi S, Veluchamy G. Yugi's pramegam and diebetes mellitus: an analogue. Bull Indian Inst Hist Med Hyderabad. 1999;29:83–87. [PubMed] [Google Scholar]
- 2.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–97. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 3.van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2002;136:201–09. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 4.Popkin BM. Will China's nutrition transition overwhelm its health care system and slow economic growth? Health Aff (Millwood) 2008;27:1064–76. doi: 10.1377/hlthaff.27.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Kamineni A, Carnethon M, Djousse L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169:798–807. doi: 10.1001/archinternmed.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevenson CR, Critchley JA, Forouhi NG, et al. Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn. 2007;3:228–45. doi: 10.1177/1742395307081502. [DOI] [PubMed] [Google Scholar]
- 7.Harries AD, Billo N, Kapur A. Links between diabetes mellitus and tuberculosis: should we integrate screening and care? Trans R Soc Trop Med Hyg. 2009;103:1–2. doi: 10.1016/j.trstmh.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Leung CC, Lam TH, Chan WM, et al. Lower risk of tuberculosis in obesity. Arch Intern Med. 2007;167:1297–304. doi: 10.1001/archinte.167.12.1297. [DOI] [PubMed] [Google Scholar]
- 9.Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg. 2006;100:191–99. doi: 10.1016/j.trstmh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 10.International Diabetes Foundation . Diabetes atlas. 4th edn International Diabetes Foundation; Brussels: 2009. [Google Scholar]
- 11.WHO [Oct 25, 2009];WHO report 2009: Global tuberculosis control—annex 3: the Stop TB Strategy, case reports, treatment outcomes and estimates of TB burden. http://www.who.int/tb/publications/global_report/2009/ annex_3/en/
- 12.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 13.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 14.Kirigia JM, Sambo HB, Sambo LG, Barry SP. Economic burden of diabetes mellitus in the WHO African region. BMC Int Health Hum Rights. 2009;9:6. doi: 10.1186/1472-698X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendis S, Fukino K, Cameron A, et al. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull World Health Organ. 2007;85:279–88. doi: 10.2471/BLT.06.033647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidibe EH. Main complications of diabetes mellitus in Africa. Ann Med Interne (Paris) 2000;151:624–28. [PubMed] [Google Scholar]
- 17.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 18.Keshavjee S, Gelmanova IY, Pasechnikov AD, et al. Treating multidrug-resistant tuberculosis in Tomsk, Russia: developing programs that address the linkage between poverty and disease. Ann NY Acad Sci. 2008;1136:1–11. doi: 10.1196/annals.1425.009. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson CR, Forouhi NG, Roglic G, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg. 2009;80:634–39. [PMC free article] [PubMed] [Google Scholar]
- 21.Lindoso AA, Waldman EA, Komatsu NK, Figueiredo SM, Taniguchi M, Rodrigues LC. Profile of tuberculosis patients progressing to death, city of Sao Paulo, Brazil, 2002. Rev Saude Publica. 2008;42:805–12. doi: 10.1590/s0034-89102008000500004. [DOI] [PubMed] [Google Scholar]
- 22.Restrepo BI, Fisher-Hoch SP, Crespo JG, et al. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135:483–91. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barach J. Historical facts in diabetes mellitus. Ann Med Hist. 1928;10:387. [PMC free article] [PubMed] [Google Scholar]
- 24.Root H. The association of diabetes and tuberculosis. N Engl J Med. 1934;210:1, 78, 127. [Google Scholar]
- 25.Boucot KR, Dillon ES, Cooper DA, Meier P, Richardson R. Tuberculosis among diabetics: the Philadelphia survey. Am Rev Tuberc. 1952;65:1–50. [PubMed] [Google Scholar]
- 26.Bermejo M, Gil S, Velasco M, Prado A, Garcia C, Guijarro M. Tuberculin test in diabetic patients in a health center. Aten Primaria. 1995;16:154–57. [PubMed] [Google Scholar]
- 27.Mboussa J, Monabeka H, Kombo M, Yokolo D, Yoka-Mbio A, Yala F. Course of pulmonary tuberculosis in diabetics. Rev Pneumol Clin. 2003;59:39–44. [PubMed] [Google Scholar]
- 28.Shetty N, Shemko M, Vaz M, D'souza G. An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int J Tuberc Lung Dis. 2006;10:80–86. [PubMed] [Google Scholar]
- 29.Coker R, McKee M, Atun R, et al. Risk factors for pulmonary tuberculosis in Russia: case-control study. BMJ. 2006;332:85–87. doi: 10.1136/bmj.38684.687940.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jabbar A, Hussain SF, Khan AA. Clinical characteristics of pulmonary tuberculosis in adult Pakistani patients with coexisting diabetes mellitus. East Mediterr Health J. 2006;12:522–27. [PubMed] [Google Scholar]
- 31.Olmos P, Donoso J, Rojas N, et al. Tuberculosis and diabetes mellitus: a longitudinal-retrospective study in a teaching hospital. Rev Med Chil. 1989;117:979–83. [PubMed] [Google Scholar]
- 32.Swai AB, McLarty DG, Mugusi F. Tuberculosis in diabetic patients in Tanzania. Trop Doct. 1990;20:147–50. doi: 10.1177/004947559002000402. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Hong YP, Lew WJ, Yang SC, Lee EG. Incidence of pulmonary tuberculosis among diabetics. Tuber Lung Dis. 1995;76:529–33. doi: 10.1016/0962-8479(95)90529-4. [DOI] [PubMed] [Google Scholar]
- 34.Pablos-Mendez A, Blustein J, Knirsch CA. The role of diabetes mellitus in the higher prevalence of tuberculosis among Hispanics. Am J Public Health. 1997;87:574–79. doi: 10.2105/ajph.87.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–13. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 36.Ponce-De-Leén A, García-García ML, García-Sancho MC, et al. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584–90. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 37.Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55:19–26. doi: 10.1002/art.21705. [DOI] [PubMed] [Google Scholar]
- 38.Perez A, Brown HS, 3rd, Restrepo BI. Association between tuberculosis and diabetes in the Mexican border and non-border regions of Texas. Am J Trop Med Hyg. 2006;74:604–11. [PMC free article] [PubMed] [Google Scholar]
- 39.Dyck RF, Klomp H, Marciniuk DD, et al. The relationship between diabetes and tuberculosis in Saskatchewan: comparison of registered Indians and other Saskatchewan people. Can J Public Health. 2007;98:55–59. doi: 10.1007/BF03405386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung CC, Lam TH, Chan WM, et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol. 2008;167:1486–94. doi: 10.1093/aje/kwn075. [DOI] [PubMed] [Google Scholar]
- 41.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bashar M, Alcabes P, Rom WN, Condos R. Increased incidence of multidrug-resistant tuberculosis in diabetic patients on the Bellevue Chest Service, 1987 to 1997. Chest. 2001;120:1514–19. doi: 10.1378/chest.120.5.1514. [DOI] [PubMed] [Google Scholar]
- 43.Fisher-Hoch SP, Whitney E, McCormick JB, et al. Type 2 diabetes and multidrug-resistant tuberculosis. Scand J Infect Dis. 2008;40:888–93. doi: 10.1080/00365540802342372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singla R, Khan N. Does diabetes predispose to the development of multidrug-resistant tuberculosis? Chest. 2003;123:308–09. [PubMed] [Google Scholar]
- 45.Subhash HS, Ashwin I, Mukundan U, et al. Drug resistant tuberculosis in diabetes mellitus: a retrospective study from south India. Trop Doct. 2003;33:154–56. doi: 10.1177/004947550303300311. [DOI] [PubMed] [Google Scholar]
- 46.Suárez-García I, Rodríguez-Blanco A, Vidal-Pérez JL, et al. Risk factors for multidrug-resistant tuberculosis in a tuberculosis unit in Madrid, Spain. Eur J Clin Microbiol Infect Dis. 2009;28:325–30. doi: 10.1007/s10096-008-0627-y. [DOI] [PubMed] [Google Scholar]
- 47.Tanrikulu AC, Hosoglu S, Ozekinci T, Abakay A, Gurkan F. Risk factors for drug resistant tuberculosis in southeast Turkey. Trop Doct. 2008;38:91–93. doi: 10.1258/td.2007.070131. [DOI] [PubMed] [Google Scholar]
- 48.Sosman M, Steidl J. Diabetic tuberculosis. Am J Roentgenol. 1927;17:625. [Google Scholar]
- 49.Weaver RA. Unusual radiographic presentation of pulmonary tuberculosis in diabetic patients. Am Rev Respir Dis. 1974;109:162–63. doi: 10.1164/arrd.1974.109.1.162. [DOI] [PubMed] [Google Scholar]
- 50.Marais RM. Diabetes mellitus in black and coloured tuberculosis patients. S Afr Med J. 1980;57:483–84. [PubMed] [Google Scholar]
- 51.Al-Tawfiq JA, Saadeh BM. Radiographic manifestations of culture-positive pulmonary tuberculosis: cavitary or non-cavitary? Int J Tuberc Lung Dis. 2009;13:367–70. [PubMed] [Google Scholar]
- 52.Segarra F, Sherman DS, Rodriguez-Aguero J. Lower lung field tuberculosis. Am Rev Respir Dis. 1963;87:37–40. doi: 10.1164/arrd.1963.87.1.37. [DOI] [PubMed] [Google Scholar]
- 53.Hadlock FP, Park SK, Awe RJ, Rivera M. Unusual radiographic findings in adult pulmonary tuberculosis. AJR Am J Roentgenol. 1980;134:1015–18. doi: 10.2214/ajr.134.5.1015. [DOI] [PubMed] [Google Scholar]
- 54.Ikezoe J, Takeuchi N, Johkoh T, et al. CT appearance of pulmonary tuberculosis in diabetic and immunocompromised patients: comparison with patients who had no underlying disease. AJR Am J Roentgenol. 1992;159:1175–79. doi: 10.2214/ajr.159.6.1442377. [DOI] [PubMed] [Google Scholar]
- 55.Morris JT, Seaworth BJ, McAllister CK. Pulmonary tuberculosis in diabetics. Chest. 1992;102:539–41. doi: 10.1378/chest.102.2.539. [DOI] [PubMed] [Google Scholar]
- 56.Umut S, Tosun GA, Yildirim N. Radiographic location of pulmonary tuberculosis in diabetic patients. Chest. 1994;106:326. [PubMed] [Google Scholar]
- 57.Kuaban C, Fotsin JG, Koulla-Shiro S, Ekono MR, Hagbe P. Lower lung field tuberculosis in Yaounde, Cameroon. Cent Afr J Med. 1996;42:62–65. [PubMed] [Google Scholar]
- 58.al-Wabel AH, Teklu B, Mahfouz AA, al-Ghamdi AS, el-Amin OB, Khan AS. Symptomatology and chest roentgenographic changes of pulmonary tuberculosis among diabetics. East Afr Med J. 1997;74:62–64. [PubMed] [Google Scholar]
- 59.Bacakoglu F, Basoglu OK, Cok G, Sayiner A, Ates M. Pulmonary tuberculosis in patients with diabetes mellitus. Respiration. 2001;68:595–600. doi: 10.1159/000050578. [DOI] [PubMed] [Google Scholar]
- 60.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Salazar-Lezama MA, Vargas MH. Atypical radiological images of pulmonary tuberculosis in 192 diabetic patients: a comparative study. Int J Tuberc Lung Dis. 2001;5:455–61. [PubMed] [Google Scholar]
- 61.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Vargas MH. Progressive age-related changes in pulmonary tuberculosis images and the effect of diabetes. Am J Respir Crit Care Med. 2000;162:1738–40. doi: 10.1164/ajrccm.162.5.2001040. [DOI] [PubMed] [Google Scholar]
- 62.Shaikh MA, Singla R, Khan NB, Sharif NS, Saigh MO. Does diabetes alter the radiological presentation of pulmonary tuberculosis. Saudi Med J. 2003;24:278–81. [PubMed] [Google Scholar]
- 63.Wang JY, Lee LN, Hsueh PR. Factors changing the manifestation of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2005;9:777–83. [PubMed] [Google Scholar]
- 64.Wang CS, Yang CJ, Chen HC, et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect. 2009;137:203–10. doi: 10.1017/S0950268808000782. [DOI] [PubMed] [Google Scholar]
- 65.Hendy M, Stableforth D. The effect of established diabetes mellitus on the presentation of infiltrative pulmonary tuberculosis in the immigrant Asian community of an inner city area of the United Kingdom. Br J Dis Chest. 1983;77:87–90. [PubMed] [Google Scholar]
- 66.Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis. 2006;10:74–79. [PubMed] [Google Scholar]
- 67.Alisjahbana B, Sahiratmadja E, Nelwan EJ, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–35. doi: 10.1086/519841. [DOI] [PubMed] [Google Scholar]
- 68.Banu Rekha VV, Balasubramanian R, Swaminathan S, et al. Sputum conversion at the end of intensive phase of category-1 regimen in the treatment of pulmonary tuberculosis patients with diabetes mellitus or HIV infection: an analysis of risk factors. Indian J Med Res. 2007;126:452–58. [PubMed] [Google Scholar]
- 69.Guler M, Unsal E, Dursun B, Aydln O, Capan N. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract. 2007;61:231–35. doi: 10.1111/j.1742-1241.2006.01131.x. [DOI] [PubMed] [Google Scholar]
- 70.Restrepo BI, Fisher-Hoch SP, Smith B, et al. Mycobacterial clearance from sputum is delayed during the first phase of treatment in patients with diabetes. Am J Trop Med Hyg. 2008;79:541–44. [PMC free article] [PubMed] [Google Scholar]
- 71.Maalej S, Belhaoui N, Bourguiba M, et al. Pulmonary tuberculosis and diabetes. A retrospective study of 60 patients in Tunisia. Presse Med. 2009;38:20–24. doi: 10.1016/j.lpm.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 72.Morsy AM, Zaher HH, Hassan MH, Shouman A. Predictors of treatment failure among tuberculosis patients under DOTS strategy in Egypt. East Mediterr Health J. 2003;9:689–701. [PubMed] [Google Scholar]
- 73.Oursler KK, Moore RD, Bishai WR, Harrington SM, Pope DS, Chaisson RE. Survival of patients with pulmonary tuberculosis: clinical and molecular epidemiologic factors. Clin Infect Dis. 2002;34:752–59. doi: 10.1086/338784. [DOI] [PubMed] [Google Scholar]
- 74.Bloomgarden ZT. Inflammation and insulin resistance. Diabetes Care. 2003;26:1922–26. doi: 10.2337/diacare.26.6.1922. [DOI] [PubMed] [Google Scholar]
- 75.Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabetes Metab. 1992;18:187–201. [PubMed] [Google Scholar]
- 76.Saiki O, Negoro S, Tsuyuguchi I, Yamamura Y. Depressed immunological defence mechanisms in mice with experimentally induced diabetes. Infect Immun. 1980;28:127–31. doi: 10.1128/iai.28.1.127-131.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang CH, Yu CT, Lin HC, Liu CY, Kuo HP. Hypodense alveolar macrophages in patients with diabetes mellitus and active pulmonary tuberculosis. Tuber Lung Dis. 1999;79:235–42. doi: 10.1054/tuld.1998.0167. [DOI] [PubMed] [Google Scholar]
- 78.Abbras CK. Fc receptor-mediated phagocytosis: abnormalities associated with diabetesmellitus. Clin Immunol Immunopathol. 1991;58:1–17. doi: 10.1016/0090-1229(91)90144-y. [DOI] [PubMed] [Google Scholar]
- 79.Chang FY, Shaio MF. Decreased cell-mediated immunity in patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;28:137–46. doi: 10.1016/0168-8227(95)00168-8. [DOI] [PubMed] [Google Scholar]
- 80.Yamashiro S, Kawakami K, Uezu K, et al. Lower expression of Th1-related cytokines and inducible nitric oxide synthase in mice with streptozotocin-induced diabetes mellitus infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2005;139:57–64. doi: 10.1111/j.1365-2249.2005.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol. 2007;37:518–24. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sugawara I, Yamada H, Mizuno S. Pulmonary tuberculosis in spontaneously diabetic goto kakizaki rats. Tohoku J Exp Med. 2004;204:135–45. doi: 10.1620/tjem.204.135. [DOI] [PubMed] [Google Scholar]
- 83.MacCuish AC, Urbaniak SJ, Campbell CJ, Duncan LJ, Irvine WJ. Phytohemagglutinin transformation and circulating lymphocyte subpopulations in insulin-dependent diabetic patients. Diabetes. 1974;23:708–12. doi: 10.2337/diab.23.8.708. [DOI] [PubMed] [Google Scholar]
- 84.Restrepo BI, Fisher-Hoch SP, Pino PA, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–41. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams’ textbook of endocrinology. 10th edn WB Saunders Company; Philadelphia: 2003. [Google Scholar]
- 86.Nichols GP. Diabetes among young tuberculous patients; a review of the association of the two diseases. Am Rev Tuberc. 1957;76:1016–30. doi: 10.1164/artpd.1957.76.6.1016. [DOI] [PubMed] [Google Scholar]
- 87.Zack MB, Fulkerson LL, Stein E. Glucose intolerance in pulmonary tuberculosis. Am Rev Respir Dis. 1973;108:1164–69. doi: 10.1164/arrd.1973.108.5.1164. [DOI] [PubMed] [Google Scholar]
- 88.Oluboyo PO, Erasmus RT. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle. 1990;71:135–38. doi: 10.1016/0041-3879(90)90010-6. [DOI] [PubMed] [Google Scholar]
- 89.Basoglu OK, Bacakoglu F, Cok G, Sayiner A, Ates M. The oral glucose tolerance test in patients with respiratory infections. Monaldi Arch Chest Dis. 1999;54:307–10. [PubMed] [Google Scholar]
- 90.Alisjahbana B, van Crevel R, Sahiratmadja E, et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis. 2006;10:696–700. [PubMed] [Google Scholar]
- 91.American Thoracic Society, CDC, Infectious Diseases Society of America Treatment of tuberculosis. MMWR Recomm Rep. 2003;52:1–77. [PubMed] [Google Scholar]
- 92.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivisto KT. Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide. Clin Pharmacol Ther. 2001;69:400–06. doi: 10.1067/mcp.2001.115822. [DOI] [PubMed] [Google Scholar]
- 93.Atkin S, Masson E, Bodmer C, Walker B, White M. Increased insulin requirement in a patient with type 1 diabetes on rifampicin. Diab Med. 1992;10:202. doi: 10.1111/j.1464-5491.1993.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 94.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 95.Niemi M, Backman JT, Neuvonen PJ. Effects of trimethoprim and rifampin on the pharmacokinetics of the cytochrome P450 2C8 substrate rosiglitazone. Clin Pharmacol Ther. 2004;76:239–49. doi: 10.1016/j.clpt.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 96.Park JY, Kim KA, Kang MH, Kim SL, Shin JG. Effect of rifampin on the pharmacokinetics of rosiglitazone in healthy subjects. Clin Pharmacol Ther. 2004;75:157–62. doi: 10.1016/j.clpt.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 97.Jaakkola T, Backman JT, Neuvonen M, Laitila J, Neuvonen PJ. Effect of rifampicin on the pharmacokinetics of pioglitazone. Br J Clin Pharmacol. 2006;61:70–78. doi: 10.1111/j.1365-2125.2005.02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ. Effect of rifampicin on the pharmacokinetics and pharmacodynamics of nateglinide in healthy subjects. Br J Clin Pharmacol. 2003;56:427–32. doi: 10.1046/j.1365-2125.2003.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hatorp V, Hansen KT, Thomsen MS. Influence of drugs interacting with CYP3A4 on the pharmacokinetics, pharmacodynamics, and safety of the prandial glucose regulator repaglinide. J Clin Pharmacol. 2003;43:649–60. [PubMed] [Google Scholar]
- 100.Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivisto KT. Rifampin decreases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2000;68:495–500. doi: 10.1067/mcp.2000.111183. [DOI] [PubMed] [Google Scholar]
- 101.Takasu N, Yamada T, Miura H, et al. Rifampicin-induced early phase hyperglycemia in humans. Am Rev Respir Dis. 1982;125:23–27. doi: 10.1164/arrd.1982.125.1.23. [DOI] [PubMed] [Google Scholar]
- 102.Waterhouse M, Wilson C, White VL, Chowdhury TA. Resolution of insulin-requiring diabetes after cessation of chemotherapy for tuberculosis. J R Soc Med. 2005;98:270–71. doi: 10.1258/jrsm.98.6.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nijland HM, Ruslami R, Stalenhoef JE, et al. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis. 2006;43:848–54. doi: 10.1086/507543. [DOI] [PubMed] [Google Scholar]
- 104.Gwilt PR, Nahhas RR, Tracewell WG. The effects of diabetes mellitus on pharmacokinetics and pharmacodynamics in humans. Clin Pharmacokinet. 1991;20:477–90. doi: 10.2165/00003088-199120060-00004. [DOI] [PubMed] [Google Scholar]
- 105.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62:2169–83. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]