Abstract
Culture of chicken nodose neurons with CNTF but not BDNF causes a significant increase in T-type Ca2+ channel expression. CNTF-induced channel expression requires 12 hr stimulation in order to reach maximal expression and is not affected by inhibition of protein synthesis, suggesting the involvement of a posttranslational mechanism. In this study we have investigated the biochemical mechanism responsible for the CNTF-dependent stimulation of T-type channel expression in nodose neurons. Stimulation of nodose neurons with CNTF evoked a considerable increase in STAT3 and ERK phosphorylation. CNTF-evoked ERK phosphorylation was transient whereas BDNF-evoked activation of ERK was sustained. Pre-treatment of nodose neurons with the JAK inhibitor P6 blocked STAT3 and ERK phosphorylation, whereas the ERK inhibitor U0126 prevented ERK activation but not STAT3 phosphorylation. Both P6 and U0126 inhibited the stimulatory effect of CNTF on T-type channel expression. Inhibition of STAT3 activation by the selective blocker stattic has no effect on ERK phosphorylation and T-type channel expression. These results indicate that CNTF-evoked stimulation of T-type Ca2+ channel expression in chicken nodose neurons requires JAK-dependent ERK signaling. A cardiac tissue extract derived from E20 chicken heart was also effective in promoting T-type Ca2+ channel expression and STAT3 and ERK phosphorylation. The ability of the heart extract to stimulate JAK/STAT and ERK activation was developmentally regulated. These findings provide further support to the idea that CNTF or a CNTF-like factor mediates normal expression of T-type channels.
Keywords: nodose neuron, expression, T-type Ca2+ channels, cytokine, signaling
INTRODUCTION
Ca2+ influx through voltage-gated Ca2+ channels (VGCC) constitutes one main source of intracellular Ca2+ in neurons. Biophysical, pharmacological and molecular analyses have revealed the presence of two families of voltage-gated Ca2+ channels (reviewed by Catterall, 1998). The family of low voltage-activated (LVA) Ca2+ channels (also known as T-type channels because of their tiny conductance and transient opening) generates fast-inactivating currents at relatively hyperpolarized potentials. The family of high voltage-activated (HVA) Ca2+ channels requires stronger depolarizations for opening and can be further divided into several subfamilies including L, N, P and Q-type Ca2+ channels. Presently, there is little understanding about the cellular mechanisms that regulate LVA and HVA Ca2+ channel expression during neuronal development and differentiation.
The functional expression of T-type Ca2+ channels is developmentally regulated in chicken nodose neurons (Pachuau & Martin-Caraballo, 2007a). T-type Ca2+ currents are restricted to a few nodose neurons between embryonic day (E) 7 and E10 but are present in approximately 60% of nodose neurons by E17. Although the functional expression of T-type Ca2+ channels occurs late in development, transcripts of the T-type Ca2+ channel pore-forming α1H subunit are already present by E7. T-type Ca2+ channel expression can be evoked in vitro by culture of E7 nodose neurons with a heart-derived extract suggesting that extrinsic factors present in the culture medium regulate the functional expression of T-type Ca2+ channels in chicken nodose neurons. Chicken CNTF and leukemia inhibitory factor (LIF) but not BDNF mimic the stimulatory effect of cardiac tissue on T-type Ca2+ channel expression (Pachuau & Martin-Caraballo, 2007b). Interestingly, T-type Ca2+ channel expression reaches a maximum after 12 hr-exposure to CNTF or heart extract and persists in the presence of protein synthesis inhibitors (Pachuau & Martin-Caraballo, 2007b). The lack of effect of protein synthesis inhibitors combined with our findings that T-type Ca2+ channel transcripts are already present at E7 suggest that the functional expression of T-type Ca2+ channels is regulated by a posttranslational mechanism in chicken nodose neurons. The time course of CNTF-evoked expression of T-type Ca2+ channels by a protein synthesis-independent mechanism raises a question as to what signaling mechanism mediates calcium channel expression in nodose neurons.
In many systems, CNTF (and related neuropoietic cytokines) stimulates neuronal survival and differentiation through the activation of a heteromeric receptor complex composed of a ligand-specific glycosyl-phosphatidylinositol (GPI)-anchored receptor (CNTFRα1), a leukemia inhibitory factor receptor β (LIFRβ) molecule, and the signaling protein gp130 (reviewed by Inoue et al., 1996). Binding of CNTF to CNTFRα1 leads to heterodimerization of gp130 and LIFRβ [reviewed by Inoue et al., 1996]. CNTF-induced dimerization of the gp130-LIFRβ complex results in the phosphorylation of Janus tyrosine kinases (JAKs) (Dziennis & Habecker, 2003; Jiao et al., 2003; Rhee et al., 2004; reviewed by Heinrich et al., 2003). Once activated, JAKs phosphorylate various tyrosine residues on the cytoplasmic tail of gp130, which then becomes a docking site for several signal-transducing proteins such as signal transducer and transducer of transcription (STAT) transcription factors and proteins containing a src homology 2 (SH2) domain. CNTF-evoked activation of the JAK/STAT signaling cascade is often associated with long-term changes in gene expression through dimerization of STAT transcription factors followed by nuclear translocation (Symes et al., 1997). Activation of gp130-LIFRβ receptor complex can also lead to stimulation of other signaling molecules including mitogen-activated protein (MAP) and PI3 kinase (Boulton et al., 1994; Alonzi et al., 2001). Therefore, it is not obvious by which mechanism JAK/STAT activation could regulate T-type Ca2+ channel expression in chicken nodose neurons, in a way that does not require changes in gene expression and can occur in a relatively short period of time.
In this study we have examined the signaling mechanism involved in the CNTF-dependent upregulation of T-type Ca2+ channel expression in chicken nodose neurons. Our data indicate that CNTF and heart extract evoke activation of JAK/STAT and ERK signaling pathways, which results in increased functional expression of T-type Ca2+ channels. JAK-dependent, transient activation of the ERK signaling pathway appears to mediate the stimulatory effect of CNTF on T-type channel expression.
METHODS
Cell cultures
Nodose ganglia were isolated from chicken embryos at embryonic day 7 (E7) as described previously (Pachuau & Martin-Caraballo, 2007a, b). Briefly, nodose ganglia were excised into a HEPES-based, Ca2+- and Mg2+-free solution, mildly trypsinized (0.05% trypsin for 12 min), dissociated by trituration, and plated onto poly-D-lysine-coated glass coverslips (for whole cell recordings) or poly-D-lysine-coated 35 mm-tissue culture plastic dishes (for immunoblotting). For western blot analysis, nodose ganglia were isolated from at least 40 embryos in order to obtain sufficient number of cells. Nodose neurons were allowed to attach to the tissue culture plastic dishes for 1.5 hr in basal culture medium. Basal culture medium consisted of Eagle's minimal essential medium (EMEM, BioWhittaker, Walkersville, MA) supplemented with 10% heat-inactivated horse serum, 2 mM glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin. After cell attachment, cell cultures were washed with serum-free culture media and maintained for 2 hr in a 5% CO2 humidified incubator. Following 2 hr incubation in serum-free media, cell cultures were stimulated as described in the results section. For whole cell recordings, nodose neurons were allowed to attach to round glass coverlips (12 mm) for 1.5 hr in supplemented culture medium. Cell cultures were stimulated with chicken CNTF overnight in order to promote T-type channel expression. Unless indicated otherwise, the culture medium was also supplemented with BDNF (50 ng/mL) in order to promote neuronal survival in cell cultures used for electrophysiological recordings. Cell cultures were maintained in a 5% CO2 incubator at 37°C for up to 24 hr.
The BE(2)-C cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in a 1:1 mixture of Ham's F12 and EMEM supplemented with 10% heat-inactivated horse serum, 2 mM glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin. BE(2)-C cells were stimulated after reaching 70% confluency.
Electrophysiology
Dissociated nodose neurons were visualized using an Olympus X71 inverted microscope equipped with Hoffman optics. Recordings were performed at room temperature (22–24°C). Recording electrodes were made from thin wall borosilicate glass (3–4 MΩ) and filled with a solution consisting of (in mM) 120 CsCl, 2 MgCl2, 10 HEPES-KOH, 10 EGTA, 1 ATP, and 0.1GTP (pH 7.4 with CsOH). Normal external saline for measurements of Ca2+ currents contained (in mM) 145 tetraethylammonium chloride (TEACl), 10 CaCl2, and 10 HEPES (pH 7.4 with CsOH). To measure Ca2+ currents, a 200 ms-depolarizing step to various potentials was applied from a holding potential of −100 mV in normal external saline. Voltage commands, data acquisition and analysis were performed with a MULTICLAMP 700A amplifier and PCLAMP software (Axon Instruments, Foster City, CA). Pipette offset, whole cell capacitance, and series resistance (usually <10 MΩ) were compensated automatically with the MultiClamp 700B Commander. For quantitative analyses, we normalized for cell size by dividing current amplitudes by cell capacitance, determined by integration of the transient current evoked by a 10-mV voltage step from a holding potential of −60 mV (Pachuau and Martin-Caraballo, 2007b).
Western blot analysis
Immunoblot analysis of ERK and STAT3 phosphorylation was performed on cell cultures obtained from E7 nodose ganglia or BE(2)-C cells. Cell cultures were maintained in serum-free EMEM culture media for 2 hr prior to stimulation. Controls consisted of non-stimulated samples. Immediately following stimulation, cultures were returned to the incubator for varying lengths of time as indicated. Cultures were then washed twice with ice-cold PBS and lysed in 2X-Laemmli sample buffer. Samples were resolved by sodium dodecyl sulfate-polyacrylamide (10 %) gel electrophoresis (SDS-PAGE). Proteins were transferred to nitrocellulose membranes. After transfer, membranes were blocked with Aquablock blocking buffer (EastCoastBio, North Berwick, ME) overnight at 4°C. Membranes were incubated with 1:1000 dilutions of primary antibodies (dissolved in blocking buffer) for 4 hr [rabbit anti-phospho-STAT3 (Cell Signaling, Danvers, MA)/mouse anti-STAT 3 (BD Biosciences Pharmingen, San Diego, CA) or mouse anti-phospho-ERK (Sigma, St. Louis, MO)/rabbit anti-ERK (Santa Cruz, Santa Cruz, CA)]. Each membrane was probed with the phosphorylated and non-phosphorylated antibodies simultaneously. After four washes, membranes were incubated with 1:10 000 fluorescent secondary antibodies in the dark. The following secondary antibodies were used: Alexa Fluor 700 goat anti-mouse IgG (Invitrogen, Carlsbad, CA) and IRDye 800 anti-rabbit IgG (Rockland, Gilbertsville, PA). After washing the membranes three times, images of Alexa Fluor 700 and IRDye 800 were acquired using the 700 and 800 nm channels of the Odyssey infrared imaging system (LICOR Biosciences GmbH, Lincoln, NE). Each experiment was repeated at least three times.
RNA isolation and real time PCR
Total RNA was extracted from homogenized nodose ganglia by TRIzol reagent according to the manufacturer's instructions (Invitrogen). Total RNA was used as a template to synthesize single-stranded cDNA using the RT Script kit (USB, Cleveland, OH) and 12–18 oligo-dT primers (Invitrogen). Amplification of CNTFRα, LIFRβ, and gp130 was performed with a SYBR green amplification kit (Applied Biosystems, Foster City, CA) using the following set of specific primers: CNTFRα, forward primer 5'GCCAAAGACAATGACATCGG-3', reverse primer 5'GCTCGTCGTCTCGGTGATCT-3'; LIFRβ, forward primer 5'TAAGCGGTATTGAGTTTAGA-3', reverse primer 5'ATCTTCAAGCGAGCATA -3'; and gp130, forward primer 5'CAAATGGAACTTACGAAGTCA-3', reverse primer 5' TTGCCATCTTTAGGTAGTGTTCTA-3'. PCR reactions were carried out as follows: one cycle of 95°C for 10 min followed by forty cycles of amplification (95°C/15 sec, 65°C/1 min). CNTFRα, LIFRβ, and gp130 expression was normalized to the housekeeping gene GAPDH. Quantification of GAPDH cDNA was performed with the powerSYBR master mix (Applied Biosystems) using the following set of primers: forward primer (5' CATCCAAGGAGTGAGCCA -3'), reverse primer (5' TGGAGGAAGAAATTGGAGGA -3'). PCR reactions consisted of one cycle of 95°C for 10 min followed by forty cycles of amplification (95°C/15 sec, 57°C/1 min). Each PCR reaction also included a non-template negative control. All PCR reactions were run simultaneously in duplicates. At the completion of the PCR reactions, the amount of target message in each sample was estimated from a threshold cycle number (CT), which is inversely correlated with the abundance of its initial mRNA. CNTFRα, LIFRβ, and gp130 expression was normalized to GAPDH to correct for differences in RNA concentration according to the delta-delta CT method (Livak & Schmittgen, 2001). After completion of PCR reactions, products were separated on 1.5% low melting point agarose gels. Bands were excised and submitted for sequencing at the Vermont Cancer Center DNA facility on an Applied Biosystems DNA sequencer.
Data Analysis
Averaged data values are presented as mean ± SEM where indicated. Statistical analyses consisted of Student's unpaired t-test when single comparisons were made, or one-way ANOVA followed by post hoc analysis using Tukey's honest significant difference test for unequal n for comparisons between multiple groups (STATISTICA software, Tulsa, OK). Throughout, p ≤ 0.05 was regarded as significant. For electrophysiological experiments, data were collected from a minimum of two platings (i.e. from multiple cultures).
Chemicals and drugs
Trypsin was from Sigma (St. Louis, MO). BDNF was obtained from R&D Systems (Minneapolis, MN), whereas chicken CNTF (also known as growth promoting activity) was kindly provided by Dr. Rae Nishi (University of Vermont). U0126 and P6 were obtained from Calbiochem (Gibbstown, NJ). Stattic was purchased from Tocris (Ellisville, MO). Culture medium and supplements including serum were from BioWhittaker (Walkersville, MA).
RESULTS
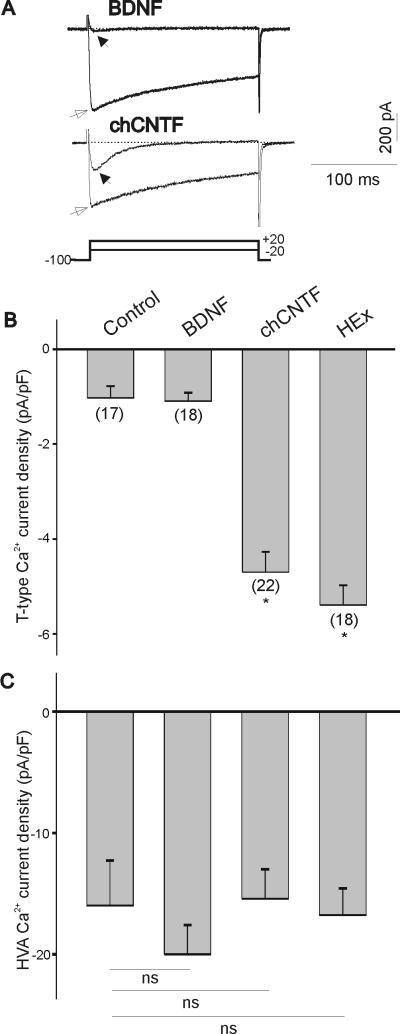
Ca2+ currents were isolated by substitution of Na+ ions with external tetraethylammonium (TEA) and by blocking outward K+ currents with Cs+ ions in the pipette solution. Maximal T-type Ca2+ currents were generated by a voltage step to −20 mV from a holding potential of −100 mV (Fig. 1A, stimulation protocol is represented in lower trace). HVA Ca2+ currents were evoked by a voltage step to +20 mV from a holding potential of − 100 mV (Fig. 1A). The culture of E7 nodose neurons with chicken CNTF (50 ng/mL) for 24 hr caused a significant increase in T-type Ca2+ current amplitude compared with nodose neurons maintained in the presence of the survival-promoting factor BDNF (Fig. 1A). To compensate for changes in cell size that may occur under different culture conditions, whole-cell currents were normalized to cell size by dividing current amplitudes by cell capacitance (see Methods, Pachuau and Martin-Caraballo, 2007b). As represented in Fig. 1B, in acutely isolated E7 (control) and BDNF treated neurons, T-type Ca2+ current density was relatively low. Culture of nodose neurons with chCNTF or heart extract for 24 hr caused a >3-fold increase in T-type Ca2+ current densities. The stimulatory effect of chicken CNTF on T-type Ca2+ current density was not significantly different from that caused by heart extract (200 μg/mL, p>0.05). We have previously shown that the increase in T-type Ca2+ current density does not involve a change in the voltage-dependent activation and inactivation parameters of the currents and is likely mediated by an increase in the number of functional channels in the membrane (Pachuau & Martin-Caraballo, 2007b). HVA Ca2+ current densities did not change significantly in the presence of CNTF or heart extract when compared with acutely isolated E7 or BDNF-treated nodose neurons (Fig. 1C). This suggests that the stimulatory effect of CNTF and heart extract is specific and only affects the functional expression of T-type but not HVA Ca2+ channels.
Figure 1.
Effect of BDNF, chick CNTF and heart extract on T-type Ca2+ channel expression in vitro. A) Representative traces of inward Ca2+ currents of E7 nodose neurons cultured for 24 hr with BDNF (50 ng/mL) or chCNTF (50 ng/mL). T-type Ca2+ currents were generated by a 200 ms depolarizing pulses to −20 mV from a holding potential of −100 mV (filled arrows). HVA Ca2+ currents were generated by a 200 ms depolarizing pulse to +20 mV from a holding potential of −100 mV (empty arrows). Stimulation protocol for both T-type and HVA Ca2+ currents is shown in bottom trace. B) Mean T-type Ca2+ current densities after 24 hr treatment with BDNF, chCNTF and heart extract as compared with acutely isolated E7 nodose neurons (control). Current densities were obtained by dividing current amplitude by cell capacitance. Note little differences in T-type Ca2+ current densities between acutely isolated and BDNF-treated neurons. Culture of nodose neurons with chCNTF or heart extract evokes a significant increase in T-type Ca2+ current densities (* denotes p ≤ 0.05 vs. BDNF). C) Culture of E7 nodose neurons with BDNF, chick CNTF or heart extract does not alter mean HVA Ca2+ current densities (ns=not significant).
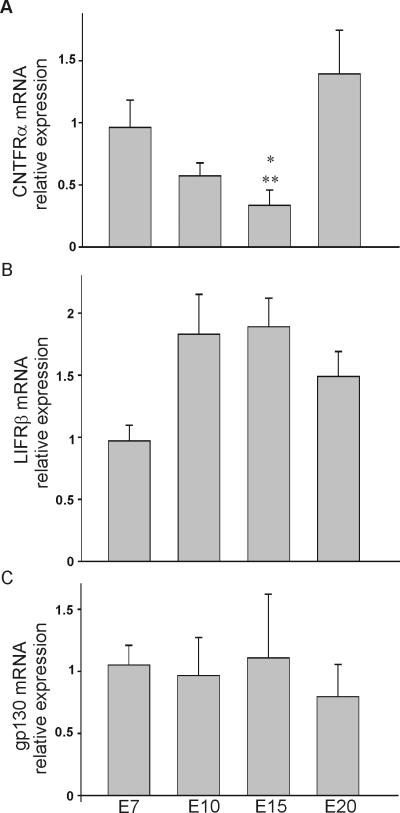
CNTF (and related neuropoietic cytokines) stimulate neuronal survival and differentiation through the activation of heteromeric receptor complexes composed of a ligand-specific glycosyl-phosphatidylinositol (GPI)-anchored receptor (CNTFRα1), a LIFRβ receptor molecule, and the signaling protein gp130 (reviewed by Inoue et al., 1996). Since the expression of these CNTF signaling components has not been previously demonstrated in chicken nodose neurons, our first aim was to determine the expression pattern of CNTFRα1, LIFRβ, and gp130. To quantify age-dependent changes in the level of expression of CNTFRα1, LIFRβ, and gp130 transcripts we performed real time PCR analysis using specific primers against each transcript (Fig. 2A, B & C). Age-dependent changes in the expression pattern of CNTFRα1, LIFRβ, and gp130 mRNA were quantified in samples obtained from E7, E10, E15 and E20 embryos. In each sample, the level of expression of CNTFRα1, LIFRβ, and gp130 mRNA was normalized to that of the housekeeping gene GAPDH. There were no significant age-dependent changes in the pattern of expression of LIFRβ and gp130 mRNA with age (Fig. 2B and C). However, there was a biphasic change in CNTFRα1 mRNA expression between E7 and E20 (Fig. 2A). Although CNTFRα1 mRNA was highly expressed at E7 and E20, we detected a noticeable reduction of CNTFRα1 expression by E15.
Figure 2.
Expression pattern of CNTFRα, LIFRβ and gp130 transcripts in chick nodose neurons. A, B, C) Relative expression of CNTFRα, LIFRβ and gp130 transcripts in chick nodose neurons as determined by real time PCR. Plots of the relative expression of CNTFRα, LIFRβ and gp130 mRNA as a function of age. * and ** denote p ≤ 0.05 vs. E7 and E20, respectively.
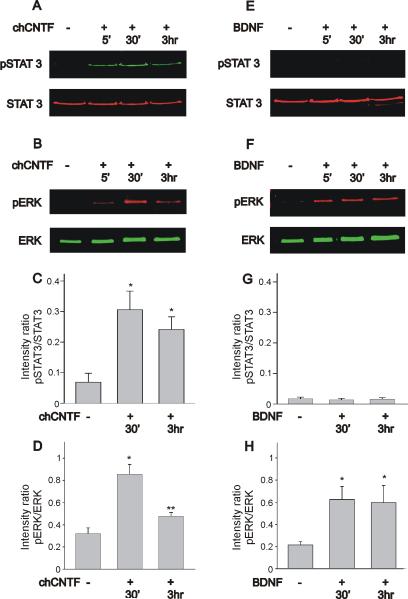
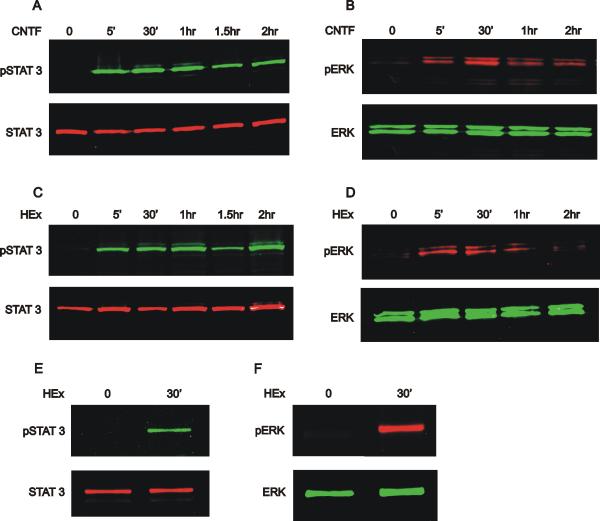
CNTF-induced dimerization of the gp130-LIFRβ receptor complex leads to phosphorylation of JAKs and activation of STAT transcription factors (Jiao et al., 2003; Rhee et al., 2004; reviewed by Heinrich et al., 2003). In this study we used STAT3 phosphorylation as a reliable biochemical marker of JAK activation, since no specific antibody is currently available against the chicken isoforms of JAK (unpublished results, Jiao et al., 2003). Beside activation of the JAK/STAT signaling cascade, CNTF can also stimulate other signaling molecules such as the mitogen-activated protein (MAP) kinase ERK in a cell-specific manner (Winston & Hunter, 1995; Dziennis & Habecker, 2003; Jiao et al., 2003). Initially, we investigated whether stimulation of nodose neurons with chCNTF leads to activation of STAT3 and ERK signaling. Activated STAT3 was probed with an antibody that recognized Tyr705, whereas phosphorylated ERK was assessed with an antibody that recognizes Thr183 and Tyr185. Acutely isolated nodose neurons were treated with 50 ng/mL chicken CNTF for varying lengths of time (5 min, 30 min and 3 hr). Following incubation with CNTF, cells were lysed and assayed for STAT3 and ERK phosphorylation using two-color western blot detection with the Odyssey infrared imaging system. Treatment of nodose neurons with CNTF caused increased phosphorylation of STAT3 and ERK (Fig. 3A & B). Both STAT3 and ERK phosphorylation reached a peak after 30 min stimulation (Fig. 3A & B). Plots of the intensity ratio of phosphorylated STAT3 to total STAT3 indicate that stimulation of nodose neurons with chCNTF evoked a statistically significant increase in STAT3 activation (Fig. 3C). ERK phosphorylation was transient and underwent a considerable reduction after 3 hr continuous stimulation with CNTF (Fig. 3B & D). The ratio of phosphorylated ERK as a function of total ERK was used to quantify temporal changes in ERK activation. As represented in Fig. 3D, CNTF stimulation of nodose neurons caused an ~3-fold increase in the ratio of pERK to total ERK. However, after 3 hr stimulation with chCNTF, the ratio of pERK to total ERK returns to near normal values. Treatment of nodose neurons with BDNF caused increased phosphorylation of ERK but not STAT3 activation (Fig. 3E & F). When compared with the levels of STAT3 activation evoked by chCNTF, the pSTAT3/total STAT3 intensity ratio evoked by BDNF stimulation of nodose neurons was minimal (Fig. 3G). ERK phosphorylation was sustained for the duration of the stimulation with BDNF (Fig. 3H). As represented in Fig. 3H, BDNF stimulation of nodose neurons caused a ~3-fold increase in the ratio of pERK to total ERK. That level of activation was maintained after 3 hr continuous stimulation with BDNF. We should point out that immunoblot analysis of ERK in chicken nodose neurons only reveals one single band since differently from mammals; chicks only express one isoform of the kinase (Sanada et al., 2000).
Figure 3.
Time course of STAT3 and ERK activation in nodose sensory neurons following stimulation with chCNTF or BDNF. A, B) Stimulation of nodose cell cultures with chCNTF generates a considerable increase in STAT3 and ERK phosphorylation. C) Phosphorylation pattern of STAT3 as determined by the intensity ratio of pSTAT3 to total STAT3. Stimulation of nodose neurons with chCNTF for 30 min causes a significant increase in the pSTAT3/STAT3 ratio. D) Phosphorylation pattern of ERK as determined by the intensity ratio of pERK to total ERK. Note that stimulation with chCNTF for 30 min causes a significant increase in the pERK/ERK ratio. The pERK/ERK intensity ratio decreases significantly after 3 hr stimulation with CNTF (n=8). E, F) Stimulation of nodose cell cultures with BDNF results in a significant increase in ERK activation without evoking STAT3 activation. G) The intensity ratio of pSTAT3 to total STAT3 was very low following stimulation of nodose neurons with BDNF. Notice that the scale of the Y-axis in figures C and G are the same in order to visualize differences in the pattern of STAT3 activation evoked by chCNTF and BDNF. H) As determined by the pERK/total ERK intensity ratio, BDNF stimulation of nodose neurons causes a significant increase in ERK phosphorylation, that was maintained for up to 3 hr (n=7). Cell cultures of nodose neurons were treated with chCNTF (50 ng/mL) or BDNF (50 ng/mL) for various lengths of time (5 min, 30 min and 3 hr). Cell lysates were collected and subjected to immunoblot analysis using a two-color western blot detection with the Odyssey infrared imaging system. * denotes p ≤ 0.05 vs. control (no treatment), ** denotes p ≤ 0.05 vs. CNTF treatment for 30 min.
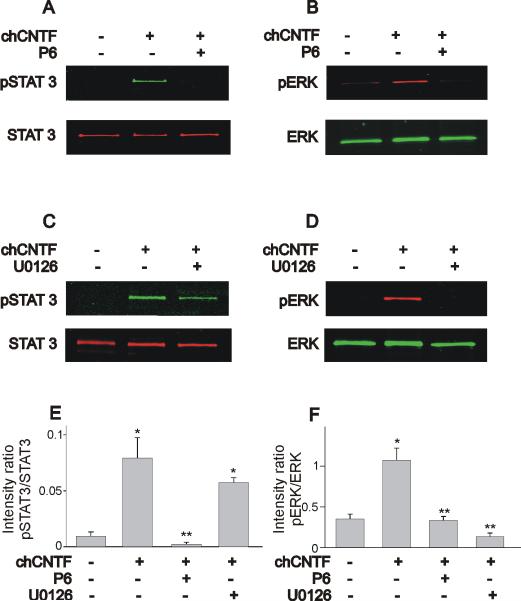
Chicken CNTF-evoked activation of STAT3 and ERK was prevented by pretreatment of nodose neurons with the JAK inhibitor P6 (10 μM). This compound selectively targets kinases of the JAK family and has been widely used to study cytokine-evoked signaling (Lucet et al., 2006; Pedranzini et al., 2006). As described earlier, stimulation of nodose neurons with chCNTF causes a significant increase in STAT3 and ERK activation (Fig. 4A & B). Pre-treatment of nodose neurons with P6 for 1 hr prior to stimulation with chCNTF (for 30 min) prevents STAT3 and ERK phosphorylation (Fig. 4A & B). To investigate whether CNTF-evoked activation of STAT3 proceeds in parallel with ERK activation we used the selective blocker of ERK phosphorylation U0126 (Schonhoff et al., 2001; Chae & Dryer, 2005). Pre-treatment of nodose neurons with the ERK inhibitor U0126 (50 μM) did not affect STAT3 phosphorylation, suggesting that STAT3 activation runs parallel to ERK activation (Fig. 4C). However, the stimulatory effect of chCNTF on ERK phosphorylation was inhibited by U0126 (Fig. 4D). As represented in Fig. 4E, the ratio of pSTAT3 to total STAT3 was only affected by JAK inhibition with P6. U0126 did not alter the ratio of pSTAT3 to total STAT3 when compared with CNTF treated neurons (Fig. 4E). Stimulation of nodose neurons with chCNTF caused a significant increase in the ratio of pERK to total ERK (Fig. 4F). However, both P6 and U0126 caused a significant reduction in the ratio of ERK phosphorylation (Fig. 4F). We should point out that in some blots for total STAT3 (Fig. 4C but also Fig. 6A and Fig. 8A), we observed a second lower band, which could represent some non-specific binding of the antibody and the high sensitivity of the two color western blot.
Figure 4.
Effect of the JAK inhibitor P6 and the ERK inhibitor U0126 on STAT3 and ERK phosphorylation in nodose neurons. A, B) Chick CNTF evoked activation of STAT3 and ERK is blocked by the inhibitor of JAK kinases P6 (10 μM). C, D) The ERK inhibitor U0126 (50 μM) only blocks ERK phosphorylation but does not affect STAT3 activation. E, F) Effect of P6 and U0126 on the pSTAT3/STAT3 and pERK/total ERK intensity ratio. Stimulation of nodose neurons with chCNTF caused a significant increase in the pSTAT3/total STAT3 ratio. Notice that only P6 but not U0126 caused a significant reduction in the pSTAT3/STAT3 intensity ratio. Stimulation of nodose neurons with chCNTF also caused a 3-fold increase in the pERK/total ERK ratio. Treatment with either P6 or U0126 inhibits the stimulatory effect of chCNTF on ERK phosphorylation (n= 3–5). In these experiments, nodose neurons were isolated at E7 and pre-treated with P6 (or U0126) for 1 hr prior exposure to chCNTF for 30 min. * denotes p ≤ 0.05 vs. control (no treatment), ** denotes p ≤ 0.05 vs. CNTF treatment for 30 min.
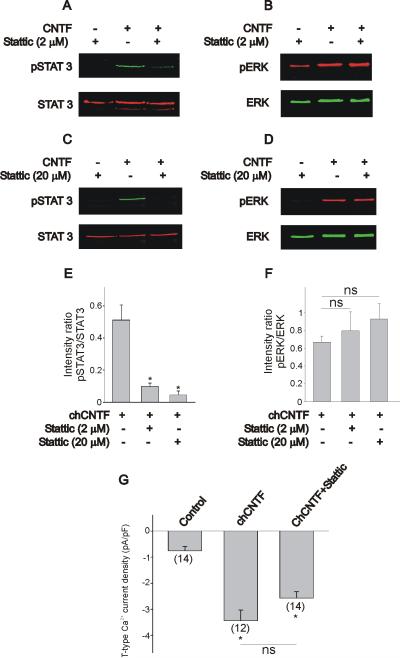
Figure 6.
Effect of the STAT3 inhibitor stattic on the CNTF-evoked stimulation of T-type Ca2+ channel expression and activation of JAK/STAT3 and ERK. A, B) At 2 μM stattic caused a significant reduction of STAT3 phosphorylation without affecting ERK activation. C, D) At higher concentrations (20 μM), stattic eliminated the CNTF-induced STAT3 phosphorylation without any noticeable effect on ERK activation. Notice that when applied alone, stattic does not cause any noticeable effect on the basal levels of STAT3 and ERK phosphorylation. E–F) Effect of stattic on the pSTAT3/STAT3 and pERK/total ERK intensity ratios. Notice that only pSTAT3/STAT3 but not pERK/total ERK intensity ratio is affected by stattic. Nodose neurons were isolated at E7 and pre-treated with stattic for 1 hr prior exposure to chCNTF for 30 min. G) Inhibition of STAT3 phosphorylation by stattic did not alter T-type Ca2+ channel expression evoked by chCNTF (* denotes p ≤ 0.05 vs. control). In these experiments, nodose neurons were isolated at E7 and maintained in culture for 12 hr in the presence of CNTF (50 ng/mL). The culture medium was also supplemented with BDNF (50 ng/mL) to promote neuronal survival. Controls were exposed to BDNF alone. Cultures were pre-treated with stattic for 1 hr prior stimulation with chCNTF. * denotes p ≤ 0.05 vs. chCNTF (E) or control (G), whereas ns indicates that no significant differences were detected between groups.
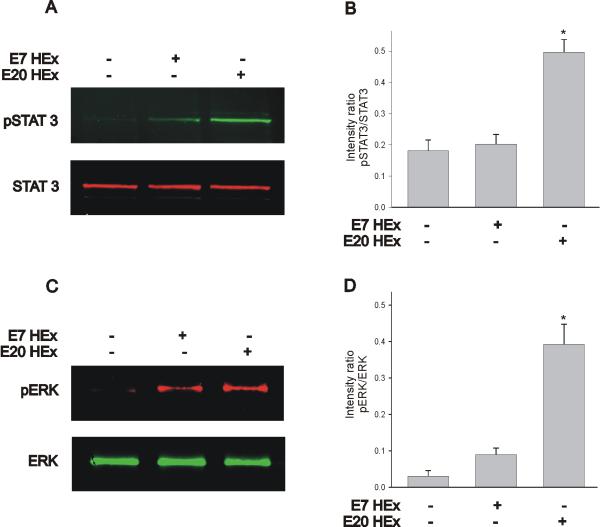
Figure 8.
The ability of the heart extract to evoke STAT3 and ERK phosphorylation is developmentally regulated. A, B) The E7 heart extract was less effective in eliciting STAT3 phosphorylation when compared with nodose neurons stimulated with an E20 heart extract. C, D) The stimulatory effect of the E7 heart extract on ERK phosphorylation was considerably lower than that generated by the E20 heart extract. Cell cultures of nodose neurons were treated with an E7 or E20 heart extract (at 200 μg/mL) for 30 min. Cell lysates were collected and subjected to immunoblot analysis of STAT3 and ERK phosphorylation. * denotes p ≤ 0.05 vs. non-stimulated samples.
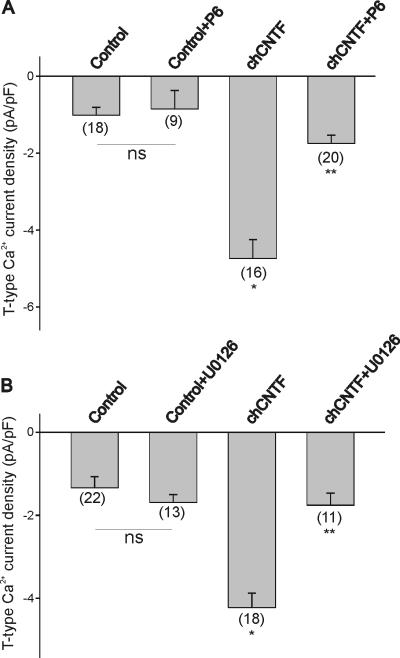
Is JAK-dependent ERK activation required for the stimulatory effect of CNTF on T-type Ca2+ channel expression? To test whether JAK/STAT3 activation is required for the stimulatory effect of chCNTF on T-type Ca2+ channel expression, we tested the effect of the JAK inhibitor P6 on whole cell Ca2+ currents. Nodose neurons were isolated at E7 and cultured overnight in the presence of CNTF with or without P6 (10 μM). As previously stated, stimulation of nodose neurons with chCNTF evoked a 3-fold increase in T-type Ca2+ current density when compared with BDNF-treated neurons (control, Fig. 5A). Treatment of nodose neurons with P6 inhibited the stimulatory effect of CNTF on T-type Ca2+ current density (Fig. 5A). Treatment of nodose cell cultures with P6 alone did not have any significant effect on current expression when compared with non-treated controls. We should also point out that P6 did not have any effect on HVA current densities (chCNTF= 26.3±4.3 pA/pF, n=18; chCNTF+P6=30.7±3.5 pA/pF, n=29; p >0.05 vs. chCNTF). Similarly, inhibition of ERK activation with U0126 also prevented the stimulatory effect of chCNTF on T-type Ca2+ current density (Fig. 5B). Treatment of nodose cell cultures with U0126 alone did not have any significant effect on current expression when compared with non-treated controls (Fig. 5B). These results demonstrate that JAK-dependent ERK activation is required for the CNTF-evoked expression of T-type Ca2+ channels in nodose neurons.
Figure 5.
Effect of JAK/STAT and ERK signaling inhibitors on the CNTF-evoked stimulation of T-type Ca2+ channel expression. A) The JAK inhibitor P6 blocked the stimulatory effect of chCNTF on T-type Ca2+ channel expression. B) Inhibition of ERK activation with U0126 blocked T-type Ca2+ channel expression evoked by CNTF. In these experiments, nodose neurons were isolated at E7 and maintained in culture for 12 hr in the presence of CNTF (50 ng/mL). The culture medium was also supplemented with BDNF (50 ng/mL) to promote neuronal survival. Controls represent BDNF-treated cultures. Cultures were pre-treated with P6 or U0126 for 1 hr prior stimulation with CNTF. * denotes p ≤ 0.05 vs. control; ** denotes p ≤ 0.05 vs. chCNTF.
In this work we used STAT3 activation as a convenient biochemical marker for CNTF-evoked activation of JAK. Thus, the question arises: is STAT3 activation necessary for the CNTF-evoked stimulation of T-type Ca2+ channel expression in chicken nodose neurons? To investigate this question we tested the effect of the specific STAT3 inhibitor stattic. This compound selectively inhibits the SH2 domain of STAT3 (Schust et al., 2006). Biochemical analysis of STAT3 activation indicated that indeed, pretreatment of nodose neurons with stattic (2 μM) caused a significant reduction in STAT3 phosphorylation (Fig. 6A). Inhibition of STAT3 with stattic did not affect ERK phosphorylation (Fig. 6B), suggesting that ERK activation is not downstream of STAT3 activation. A higher dose of stattic (20 μM) caused a complete inhibition of STAT3 phosphorylation without any effect on ERK activation as well (Fig. 6 C& D). The overall effect of stattic on the pSTAT3/STAT3 and pERK/ERK ratios is represented in Fig. 6E and F. At both 2 and 20 μM, stattic caused a significant reduction in the pSTAT3/STAT3 ratio (Fig. 6E), whereas these concentrations did not have any significant effect on the pERK/ERK ratio (Fig. 6F). Incubation of nodose neurons with stattic (>2 μM) for over 12 hr caused extensive cell death. Therefore, for whole cell recordings, we used a 2 μM concentration of stattic to investigate whether inhibition of STAT3 has any effect on T-type Ca2+ channel expression. In cell cultures treated with 2 μM stattic overnight, whole cell recordings of calcium currents still revealed the presence of a transient Ca2+ current. CNTF-generated T-type Ca2+ current densities in the presence or absence of stattic were not statistically different (Fig. 6G).
Does the cardiac tissue extract also evoke stimulation of JAK/STAT3 and ERK signaling cascades? Since biochemical experiments are difficult to carry out with the limited number of neurons available in nodose ganglia, we used the neuroblastoma cell line BE(2)-C. CNTF activation of the gp130 receptor complex has been well characterized in BE(2)-C cells (Jiao et al., 2003). Initially, we investigated whether stimulation of BE(2)-C cells with CNTF and heart extract leads to activation of STAT and ERK signaling (Fig. 7). BE(2)-C cells were treated with 10 ng/mL chicken CNTF or 200 μg/mL heart extract for varying amounts of time. Following incubation with chCNTF or heart extract, cells were lysed and assayed for STAT3 and ERK phosphorylation using two-color western blot. As previously reported (Jiao et al., 2003), treatment of BE(2)-C cells with chCNTF caused increased phosphorylation of STAT3 and ERK (Fig. 7A, B). STAT3 phosphorylation appeared to peak earlier (5 min stimulation) than ERK phosphorylation, which reached its peak after 30 min stimulation (Fig. 7A, B). STAT3 phosphorylation was sustained for up to 2 hr in the presence of CNTF (Fig. 7A). On the contrary, ERK phosphorylation was transient and underwent a considerable reduction after 1hr continuous stimulation with CNTF (Fig. 7B). Does stimulation of BE(2)-C cells with heart extract also cause a similar pattern of protein phosphorylation? Indeed, treatment of BE(2)-C cells with heart extract for 5 min caused a significant increase in STAT3 phosphorylation whereas ERK activation reached a peak after 30 min stimulation (Fig. 7C, D). Like the activation pattern generated with chCNTF, heart extract evoked a sustained phosphorylation of STAT3 but a transient activation of ERK. Since 30 min-stimulation with CNTF and heart extract appears to cause maximal phosphorylation of STAT3 and ERK in BE(2)-C cells, we investigated whether stimulation of cultured nodose neurons with heart extract (200 μg/mL) also results in STAT3 and ERK phosphorylation. A 30 min-stimulation of nodose neurons with heart extract also evoked a significant increase in STAT3 and ERK phosphorylation. The ability of the heart extract to stimulate JAK/STAT and ERK activation was developmentally regulated. As represented in Fig. 8, phosphorylation of STAT3 and ERK by heart extract from E7 chicken embryos was low when compared with the stimulation elicited by the E20 heart extract (Fig. 8A–D).
Figure 7.
Time course of STAT3 and ERK activation in BE(2)-C cells following stimulation with chCNTF (A, B) or heart extract (C, D). E, F) Stimulation of nodose cell cultures with heart extract for 30 min also generated a considerable increase in STAT3 and ERK phosphorylation. Cell cultures of BE(2)-C cells or nodose neurons were treated with CNTF (50 ng/mL) for various lengths of time. Cell lysates were collected and subjected to immunoblot analysis using a two-color western blot detection with the Odyssey infrared imaging system.
DISCUSSION
Previous work from our laboratory has demonstrated that in chicken nodose neurons, CNTF or a cardiac-derived tissue extract stimulate the functional expression of T-type Ca2+ channels (Pachuau & Martin-Caraballo, 2007b). CNTF and heart extract-induced currents require 12 hr stimulation in order to reach maximal expression and are not affected by inhibition of protein synthesis, suggesting the involvement of a posttranslational mechanism. In this study we have examined the signaling mechanism involved in the CNTF- and heart extract-evoked stimulation of T-type Ca2+ channel expression in chicken nodose neurons. Three main conclusions can be drawn from these experiments. First, transcripts of the CNTF receptor signaling molecules CNTFRα1, LIFRβ, and gp130 are present in chicken nodose neurons at early stages of development. Second, CNTF- and heart extract-evoked signaling involves activation of the JAK/STAT3 and ERK signaling pathways. Third, the functional expression of T-type Ca2+ channel expression evoked by CNTF in nodose neurons requires JAK-dependent, transient activation of ERK signaling.
Cytokine-evoked activation of the JAK/STAT signaling cascade is often associated with long-term changes in gene expression through dimerization of STAT transcription factors followed by nuclear translocation and regulation of gene transcription (Symes et al., 1997). Our present results demonstrate that stimulation of nodose neurons with CNTF or heart extract results in a significant increase in STAT3 phosphorylation. Since STAT3 activation can be used as a biochemical marker for cytokine-evoked JAK activation, these results indicate that the stimulatory effect of CNTF on channel expression involves activation of JAK/STAT3 signaling. Activation of JAK signaling is essential for the stimulation of T-type channel expression induced by CNTF as revealed by experiments with the JAK inhibitor P6. Treatment of nodose neurons with P6 prevented the CNTF-evoked phosphorylation of STAT3 and blocked the stimulatory effect of CNTF on T-type Ca2+ channel expression. We should point out however, that CNTF-evoked T-type Ca2+ channel expression is regulated by a STAT3-independent mechanism. This conclusion is based on our evidence showing that T-type channel expression was not affected by the STAT3 inhibitor stattic at a concentration that greatly diminished STAT3 activation. The nature of the cellular processes regulated by STAT3 activation in nodose neurons are yet to be fully characterized but they may be associated with long-term regulation of neuronal survival and neurotransmitter expression, which require changes in gene expression (Schweizer et al., 2002; Dziennis & Habecker, 2003).
Our previous results indicate that the stimulatory effect of CNTF and heart extract on T-type Ca2+ channel expression involves a protein synthesis-independent mechanism (Pachuau & Martin-Caraballo, 2007a, b). Thus, it is not obvious by which mechanism JAK/STAT3 activation could regulate T-type Ca2+ channel expression in chicken nodose neurons. One possibility is that JAK activation leads to phosphorylation of other signaling molecules (Boulton et al., 1994; Xia et al., 1996; Takahashi-Tezuka et al., 1997), which could ultimately regulate channel expression. Our present results demonstrate that indeed, activation of JAK/STAT3 also led to activation of ERK signaling. Inhibition of JAK activation with P6 eliminated CNTF-evoked ERK phosphorylation and blocked the stimulatory effect of CNTF on T-type Ca2+ channel expression, suggesting that JAK-dependent ERK activation is required for the stimulatory effect of CNTF on channel expression. Blockade of ERK activation with the specific MAP kinase blocker U0126 also blocked the stimulatory effect of CNTF and heart extract on T-type Ca2+ channel expression (present work, see also Pachuau & Martin-Caraballo, 2007b). These results demonstrate that ERK activation plays an essential role in the stimulation of T-type Ca2+ channel expression by CNTF. JAK-dependent ERK activation likely involves phosphorylation of tyrosine groups in the gp130 cytoplasmic tail, which then act as docking sites for SH2 domain-containing adaptor proteins (Schiemann et al., 1997; Lelièvre et al., 2001). These events ultimately lead to ERK phosphorylation via activation of the small GTPase Ras and its downstream effect Raf (Winston & Hunter, 1995; Xia et al., 1996; Schiemann et al., 1997). Cytokine-evoked activation of ERK can initiate a variety of intracellular signaling processes through phosphorylation of cytosolic proteins, including intracellular signaling molecules that can potentially affect the processing and trafficking of membrane proteins (Robertson et al., 2005; Huang et al., 2007). Several of these proteins, including the small GTPases Rab and ADP-ribosylation factors, are downstream targets of activated ERK (Shirane & Nakayama, 2006; Robertson et al., 2006). Although the ERK-dependent trafficking mechanism of T-type Ca2+ channels in nodose neurons is not yet fully understood, previous studies on ciliary neurons indicate that ERK-dependent signaling regulates the trafficking of large conductance K+ channels (Lhuillier & Dryer, 2000).
As demonstrated by the present results, the duration of ERK activation appears to have a significant effect on T-type Ca2+ channel expression. Although BDNF caused a sustained activation of ERK, it did not evoke any significant change in T-type Ca2+ channel expression above baseline. On the contrary, CNTF and heart extract activation of ERK was transient, which resulted in a significant increase in the T-type Ca2+ channel expression. These results are consistent with the idea that the pattern of ERK activation plays a critical role in regulating cellular function. For example, epidermal growth factor (EGF) induces transient ERK activation in PC12 cells, resulting in cell proliferation. On the contrary, sustained ERK activation induced by nerve growth factor (NGF) leads to cell differentiation into a neuronal phenotype (York et al., 1998; reviewed by Grewal et al., 1999). Several factors could underlie the temporal differences of ERK activation by CNTF and BDNF in nodose neurons. First, ERK phosphorylation may activate a host of different signaling molecules, which in turn could terminate ERK activation by a negative feedback mechanism. For example, cytokine signaling is negatively controlled by suppressors of cytokine signaling (SOCS) molecules. Activation of SOCS molecules controls cellular responses via downregulation of JAK/STAT activation either by acting directly as kinase inhibitors of JAK proteins or by competing with STAT binding sites (reviewed by Rico-Bautista et al., 2006). Further studies are needed to determine whether SOCS molecules negatively regulate CNTF-evoked STAT3 and ERK activation in nodose neurons. It bears noting that cytokine-evoked induction of SOCS expression occurs within a very short period of time (it can peak after 30 min) and SOCS expression often requires gene transcription and protein synthesis (Bousquet et al., 1999). Second, CNTF and BDNF-evoked signaling occurs at spatially segregated areas of the cell. The GPI-anchored receptor CNTFRα1 accumulates in membrane microdomains called lipid rafts (Port et al., 2007). CNTF binding to membrane-bound CNTFRα1 evokes a rapid translocation of gp130 and LIFR receptor complex into detergent-resistant lipid rafts (Port et al., 2007). This event could potentially segregate CNTF signals to specialized membrane domains. On the other hand, activation of the BDNF receptor trkB does not necessarily require segregation into specialized membrane microdomains in order to promote neuronal survival (Suzuki et al., 2004). Thus, spatial differences in CNTF and BDNF signaling could underlie differences in the nature of ERK phosphorylation in nodose neurons.
The stimulatory effect of cardiac tissue extract on T-type Ca2+ channel expression is also likely mediated by activation of JAK and ERK signaling. Present (see Fig. 1) and previous findings from our laboratory have demonstrated that a tissue extract from older chicken hearts (E20) is equally effective as CNTF in stimulating T-type Ca2+ channel expression in cultured E7 nodose neurons (Pachuau & Martin-Caraballo, 2007b). Now we present evidence showing that similar to CNTF, the heart extract also evokes a considerable activation of the JAK/STAT3 and ERK signaling cascades in both BE(2)-C cells and nodose neurons. Confirming previous findings by Jiao et al. (2003), we have detected a significant increase in STAT3 and ERK phosphorylation following CNTF stimulation of BE(2)-C cells and a similar pattern of activation was also observed following stimulation of BE(2)-C cells with heart extract, providing further support to the idea that the stimulatory effect of heart extract in chicken nodose neurons is mediated by a cytokine-related factor (Pachuau & Martin-Caraballo, 2007b). Although the small number of neurons in the nodose ganglia limits the extent of our biochemical studies, our present studies also reveal that 30 min stimulation of chicken nodose neurons generates a significant increase in STAT3 and ERK activation following exposure of nodose cell cultures to heart extract, similar to the effect generated by CNTF. Our conclusion that activation of the JAK/STAT and ERK signaling molecules mediates the stimulatory effect of heart extract on T-type Ca2+ channel expression is further supported by earlier findings showing that immunodepletion of the heart extract with a chCNTF antibody causes a partial inhibition of T-type Ca2+ channel expression and that pharmacological inhibition of JAK and ERK activation prevents the stimulatory effect of heart extract on channel expression (Pachuau & Martin-Caraballo, 2007b). These results are consistent with the idea that cardiac tissue, through a CNTF or a cytokine-like factor, regulates the functional expression of T-type Ca2+ channels in nodose neurons (Pachuau & Martin-Caraballo, 2007b).
Heart-derived CNTF (or release of a cytokine-like factor from the heart) could underlie the normal expression of T-type Ca2+ channels in nodose neurons. In this regard, it is worth mentioning that the heart is a powerful source of various cytokines including CNTF, cardiotrophin-1 and leukemia inhibitory factor (Yamamori et al., 1989; Ancey et al., 2002; Ohta et al, 1995; Asai et al., 2000). Previous work in chicken hearts has also shown a considerable age-dependent increase in CNTF mRNA expression up to E11 (Wang and Halvorsen, 1998). Changes in the availability of cardiac derived cytokines and/or cytokine-evoked signaling may have important implications in several heart diseases as a result of alterations in cardiac muscle cells or disruptions in the differentiation pattern of neurons innervating the heart including sensory or autonomic neurons (Raju et al., 2007; Kreusser et al., 2008).
Although the role of cardiac tissue and target-derived CNTF in regulating the normal development of T-type Ca2+ channels in nodose neurons has yet to be investigated in vivo, the present results demonstrate that nodose neurons express considerable levels of the CNTF receptor complex molecules CNTFRα1, LIFRβ, and gp130 at early stages of development. Furthermore, stimulation of nodose neurons with CNTF (or heart extract) caused a significant activation of the JAK/STAT and ERK signaling pathways, indicating that by E7, nodose neurons already have the signaling machinery necessary to generate cytokine-evoked responses. It is puzzling then, why channel expression does not occur as early as E7 in vivo (Pachuau & Martin-Caraballo, 2007b). One possibility is that though the receptor and signaling components are expressed at early stages of development, perhaps normal T-type Ca2+ channel expression is limited by accessibility or availability of target-derived CNTF in vivo. In support of this idea, we have found that only heart extract from E20 but not E7 chicken embryos can effectively stimulate T-type Ca2+ channel expression in nodose neurons (Pachuau & Martin-Caraballo, 2007b). This early observation can be explained based on our present results showing that only E20 but not E7 heart extract is effective in causing a significant increase in ERK and JAK/STAT3 activation. We should also point out, however, that changes in receptor expression could also limit the ability of nodose neurons to respond to CNTF in vivo. Thus, our present results clearly demonstrate a significant reduction in CNTFRα1 mRNA expression by E15. Thus, it is possible that a reduction in CNTFRα1 expression also plays a role in limiting T-type Ca2+ channel expression at a specific period of development (around E15). Although it is unclear what mechanism mediates the reduction in CNTFRα1 mRNA expression observed at E15, we speculate that it may be associated with the loss of nodose neurons due to apoptotic cell death. Indeed, previous findings indicate that by E15, there is a ~50% reduction in the number of nodose neurons born at E5 (Harrison et al., 1994).
CNTF-evoked expression of T-type Ca2+ channel expression by activation of the JAK/STAT and ERK signaling pathways could represent an important aspect of neuronal differention of sensory neurons. Although the role of T-type Ca2+ channel expression in nociception has been well established (Todorovic et al., 2002; Dogrul et al., 2003; Bourinet et al., 2005; Choi et al., 2007) little is known about the cellular and molecular mechanisms that regulate T-type Ca2+ channel expression in pain-transmitting neurons. Our present results demonstrate that CNTF or a cardiac tissue-derived extract stimulates T-type Ca2+ channel expression of nodose neurons at early stages of development. This raises the possibility that CNTF or a cytokine-like factor regulates the functional expression of T-type Ca2+ channels during normal development. CNTF-dependent regulation of T-type Ca2+ channel expression could also represent an important regulatory mechanism of neuronal excitability and Ca2+ influx in neurons. T-type Ca2+ channels regulate the action potential waveform and temporal pattern of repetitive firing of nerve cells and therefore play a critical role in defining the electrophysiological phenotype of neurons (Umemiya & Berger, 1994; Huguenard, 1996; Martin-Caraballo & Greer, 2001).
Acknowledgement
We thank Dr. Sheryl White, Thomas Buttolph, and Edward Zelazny from the Cell/Molecular Research Facility at the University of Vermont for technical assistance. This work was supported by NIH grant P20 RR016435 from the Centers of Biomedical Research Excellence Program of the National Center for Research Resources (NCRR) and Vermont Genetics Network through NIH grant P20 RR16462 from the INBRE Program of the NCRR.
Footnotes
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of NCRR or NIH.
REFERENCES
- Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Müller W, Musiani P, Poli V, Davies AM. Role of STAT3 and PI3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci. 2001;18(3):270–82. doi: 10.1006/mcne.2001.1018. [DOI] [PubMed] [Google Scholar]
- Ancey C, Corbi P, Froger J, Delwail A, Wijdenes J, Gascan H, Potreau D, Lecron JC. Secretion of IL-6, IL-11 and LIF by human cardiomyocytes in primary culture. Cytokine. 2002;18(4):199–205. doi: 10.1006/cyto.2002.1033. [DOI] [PubMed] [Google Scholar]
- Asai S, Saito Y, Kuwahara K, Mizuno Y, Yoshimura M, Higashikubo C, Tsuji T, Kishimoto I, Harada M, Hamanaka I, Takahashi N, Yasue H, Nakao K. The heart is a source of circulating cardiotrophin-1 in humans. Biochem Biophys Res Commun. 2000;279(2):320–3. doi: 10.1006/bbrc.2000.3932. [DOI] [PubMed] [Google Scholar]
- Boulton TG, Stahl N, Yancopoulos GD. Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J Biol Chem. 1994;269(15):11648–55. [PubMed] [Google Scholar]
- Bourinet E, Alloui A, Monteil A, Barrere C, Couette B, Poirot O, Pages A, McRory J, Snutch TP, Eschalier A, Nargeot J. Silencing of the Cav3.2 T-type calcium channel gene in sensory neurons demonstrates its major role in nociception. EMBO J. 2005;24(2):315–24. doi: 10.1038/sj.emboj.7600515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet C, Susini C, Melmed S. Inhibitory roles for SHP-1 and SOCS-3 following pituitary proopiomelanocortin induction by leukemia inhibitory factor. J Clin Invest. 1999;104(9):1277–85. doi: 10.1172/JCI7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae KS, Dryer SE. Regulation of neuronal K(Ca) channels by beta-neuregulin-1 does not require activation of Ras-MEK-extracellular signal-regulated kinase signaling cascades. Neuroscience. 2005;135(4):1013–6. doi: 10.1016/j.neuroscience.2005.06.060. [DOI] [PubMed] [Google Scholar]
- Choi S, Na HS, Kim J, Lee J, Lee S, Kim D, Park J, Chen CC, Campbell KP, Shin HS. Attenuated pain responses in mice lacking Ca(V)3.2 T-type channels. Genes Brain Behav. 2007;6(5):425–31. doi: 10.1111/j.1601-183X.2006.00268.x. [DOI] [PubMed] [Google Scholar]
- Dogrul A, Gardell LR, Ossipov MH, Tulunay FC, Lai J, Porreca F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain. 2003;105(1–2):159–68. doi: 10.1016/s0304-3959(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Dziennis S, Habecker BA. Cytokine suppression of dopamine-beta-hydroxylase by extracellular signal-regulated kinase-dependent and -independent pathways. J Biol Chem. 2003;278(18):15897–904. doi: 10.1074/jbc.M212480200. [DOI] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9(5):544–53. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Harrison TA, Stadt HA, Kirby ML. Developmental characteristics of the chick nodose ganglion. Dev Neurosci. 1994;16(1–2):67–73. doi: 10.1159/000112090. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Duhadaway JB, Prendergast GC, Laury-Kleintop LD. RhoB regulates PDGFR-beta trafficking and signaling in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27(12):2597–605. doi: 10.1161/ATVBAHA.107.154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–48. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nakayama C, Noguchi H. Activating mechanism of CNTF and related cytokines. Mol Neurobiol. 1996;12(3):195–209. doi: 10.1007/BF02755588. [DOI] [PubMed] [Google Scholar]
- Jiao J, Kaur N, Lu B, Reeves SA, Halvorsen SW. Initiation and maintenance of CNTF-Jak/STAT signaling in neurons is blocked by protein tyrosine phosphatase inhibitors. Brain Res Mol Brain Res. 2003;116(1–2):135–46. doi: 10.1016/s0169-328x(03)00286-9. [DOI] [PubMed] [Google Scholar]
- Kreusser MM, Buss SJ, Krebs J, Kinscherf R, Metz J, Katus HA, Haass M, Backs J. Differential expression of cardiac neurotrophic factors and sympathetic nerve ending abnormalities within the failing heart. J Mol Cell Cardiol. 2008;44(2):380–7. doi: 10.1016/j.yjmcc.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Lelièvre E, Plun-Favreau H, Chevalier S, Froger J, Guillet C, Elson GC, Gauchat JF, Gascan H. Signaling pathways recruited by the cardiotrophin-like cytokine/cytokine-like factor-1 composite cytokine: specific requirement of the membrane-bound form of ciliary neurotrophic factor receptor alpha component. J Biol Chem. 2001;276(25):22476–84. doi: 10.1074/jbc.M101681200. [DOI] [PubMed] [Google Scholar]
- Lhuillier L, Dryer SE. Developmental regulation of neuronal KCa channels by TGFbeta 1: transcriptional and posttranscriptional effects mediated by Erk MAP kinase. J Neurosci. 2000;20:5616–5622. doi: 10.1523/JNEUROSCI.20-15-05616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lucet IS, Fantino E, Styles M, Bamert R, Patel O, Broughton SE, Walter M, Burns CJ, Treutlein H, Wilks AF, Rossjohn J. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood. 2006;107(1):176–83. doi: 10.1182/blood-2005-06-2413. [DOI] [PubMed] [Google Scholar]
- Martin-Caraballo M, Greer JJ. Voltage-sensitive calcium currents and their role in regulating phrenic motoneuron electrical excitability during the perinatal period. J Neurobiol. 2001;46(4):231–48. doi: 10.1002/1097-4695(200103)46:4<231::aid-neu1005>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ohta K, Hara H, Hayashi K, Itoh N, Ohi T, Ohta M. Tissue expression of rat ciliary neurotrophic factor (CNTF) mRNA and production of the recombinant CNTF. Biochem Mol Biol Int. 1995;35(2):283–90. [PubMed] [Google Scholar]
- Pachuau J, Martin-Caraballo M. Developmental regulation of T-type Ca2+ channels in chick nodose ganglion cells. Dev. Neurobiol. 2007a;67(14):1915–1931. doi: 10.1002/dneu.20560. [DOI] [PubMed] [Google Scholar]
- Pachuau J, Martin-Caraballo M. Extrinsic regulation of T-type Ca2+ channel expression in chick nodose ganglion neurons Dev. Neurobiol. 2007b;67(14):1901–14. doi: 10.1002/dneu.20560. [DOI] [PubMed] [Google Scholar]
- Pedranzini L, Dechow T, Berishaj M, Comenzo R, Zhou P, Azare J, Bornmann W, Bromberg J. Pyridone 6, a pan-Janus-activated kinase inhibitor, induces growth inhibition of multiple myeloma cells. Cancer Res. 2006;66(19):9714–21. doi: 10.1158/0008-5472.CAN-05-4280. [DOI] [PubMed] [Google Scholar]
- Port MD, Gibson RM, Nathanson NM. Differential stimulation-induced receptor localization in lipid rafts for interleukin-6 family cytokines signaling through the gp130/leukemia inhibitory factor receptor complex. J Neurochem. 2007;101(3):782–93. doi: 10.1111/j.1471-4159.2007.04471.x. [DOI] [PubMed] [Google Scholar]
- Raju SV, Zheng M, Schuleri KH, Phan AC, Bedja D, Saraiva RM, Yiginer O, Vandegaer K, Gabrielson KL, O'Donnell CP, Berkowitz DE, Barouch LA, Hare JM. Activation of the cardiac ciliary neurotrophic factor receptor reverses left ventricular hypertrophy in leptin-deficient and leptin-resistant obesity. Proc Natl Acad Sci USA. 2006;6103(11):4222–4227. doi: 10.1073/pnas.0510460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KD, Goureau O, Chen S, Yang XJ. Cytokine-induced activation of signal transducer and activator of transcription in photoreceptor precursors regulates rod differentiation in the developing mouse retina. J Neurosci. 2004;24(44):9779–88. doi: 10.1523/JNEUROSCI.1785-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico-Bautista E, Flores-Morales A, Fernández-Pérez L. Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. 2006;17(Cytokine Growth Factor Rev.)(6):431–9. doi: 10.1016/j.cytogfr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Robertson SE, Setty SR, Sitaram A, Marks MS, Lewis RE, Chou MM. Extracellular signal-regulated kinase regulates clathrin-independent endosomal trafficking. Mol Biol Cell. 2005;17(2):645–57. doi: 10.1091/mbc.E05-07-0662. 2006 Feb. Epub 2005 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Hayashi Y, Harada Y, Okano T, Fukada Y. Role of circadian activation of mitogen-activated protein kinase in chick pineal clock oscillation. J Neurosci. 2000;20(3):986–91. doi: 10.1523/JNEUROSCI.20-03-00986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann WP, Bartoe JL, Nathanson NM. Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor alpha- and gp130-mediated stimulation of mitogen-activated protein kinase. Evidence for participation of multiple signaling pathways which converge at Ras. J Biol Chem. 1997;272(26):16631–6. doi: 10.1074/jbc.272.26.16631. [DOI] [PubMed] [Google Scholar]
- Schonhoff CM, Bulseco DA, Brancho DM, Parada LF, Ross AH. The Ras-ERK pathway is required for the induction of neuronal nitric oxide synthase in differentiating PC12 cells. J Neurochem. 2001;78(3):631–9. doi: 10.1046/j.1471-4159.2001.00432.x. [DOI] [PubMed] [Google Scholar]
- Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13(11):1235–42. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Schweizer U, Gunnersen J, Karch C, Wiese S, Holtmann B, Takeda K, Akira S, Sendtner M. Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult. J Cell Biol. 2002;156(2):287–97. doi: 10.1083/jcb.200107009. Epub 2002 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane M, Nakayama KI. Protrudin induces neurite formation by directional membrane trafficking. Science. 2006;314(5800):818–21. doi: 10.1126/science.1134027. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Numakawa T, Shimazu K, Koshimizu H, Hara T, Hatanaka H, Mei L, Lu B, Kojima M. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J Cell Biol. 2004;167(6):1205–15. doi: 10.1083/jcb.200404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes A, Gearan T, Eby J, Fink JS. Integration of Jak-Stat and AP-1 signaling pathways at the vasoactive intestinal peptide cytokine response element regulates ciliary neurotrophic factor-dependent transcription. J Biol Chem. 1997;272(15):9648–54. doi: 10.1074/jbc.272.15.9648. [DOI] [PubMed] [Google Scholar]
- Takahashi-Tezuka M, Hibi M, Fujitani Y, Fukada T, Yamaguchi T, Hirano T. Tec tyrosine kinase links the cytokine receptors to PI-3 kinase probably through JAK. Oncogene. 1997;14(19):2273–82. doi: 10.1038/sj.onc.1201071. [DOI] [PubMed] [Google Scholar]
- Todorovic SM, Meyenburg A, Jevtovic-Todorovic V. Mechanical and thermal antinociception in rats following systemic administration of mibefradil, a T-type calcium channel blocker. Brain Res. 2002;951(2):336–40. doi: 10.1016/s0006-8993(02)03350-4. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Single-channel properties of four calcium channel types in rat motoneurons. J Neurosci. 1995;15(3 Pt 2):2218–24. doi: 10.1523/JNEUROSCI.15-03-02218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Halvorsen SW. Reciprocal regulation of ciliary neurotrophic factor receptors and acetylcholine receptors during synaptogenesis in embryonic chick atria. J Neurosci. 1998;18(18):7372–80. doi: 10.1523/JNEUROSCI.18-18-07372.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston LA, Hunter T. JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J Biol Chem. 1995;270(52):30837–40. doi: 10.1074/jbc.270.52.30837. [DOI] [PubMed] [Google Scholar]
- Yamamori T, Fukada K, Aebersold R, Korsching S, Fann MJ, Patterson PH. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989;246(4936):1412–6. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]
- York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJ. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392(6676):622–6. doi: 10.1038/33451. 1998 Apr. [DOI] [PubMed] [Google Scholar]
- Xia K, Mukhopadhyay NK, Inhorn RC, Barber DL, Rose PE, Lee RS, Narsimhan RP, D'Andrea AD, Griffin JD, Roberts TM. The cytokine-activated tyrosine kinase JAK2 activates Raf-1 in a p21ras-dependent manner. Proc Natl Acad Sci U S A. 1996;93(21):11681–6. doi: 10.1073/pnas.93.21.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]