Abstract
The cardinal motor symptoms of Parkinson’s disease (PD) are caused by the vulnerability to dysfunction and degeneration of ventral midbrain (VM) dopaminergic (DA) neurons. A major limitation for experimental studies of current ES/iPS cell differentiation protocols is the lack of VM DA neurons with a stable phenotype as defined by an expression marker code of FOXA2/TH/β-tubulin. Here we demonstrate a combination of three modifications that were required to produce VM DA neurons. Firstly, early and specific exposure to 10−8M (low dose) retinoic acid improved the regional identity of neural progenitor cells derived from human ES cells, PD or healthy subject-specific iPS cells. Secondly, a high activity form of human sonic hedgehog established a sizeable FOXA2+ neural progenitor cell population in vitro. Thirdly, early exposure to FGF8a, rather than Fgf8b, and WNT1 was required for robust differentiation of the FOXA2+ floor plate-like human neural progenitor cells into FOXA2+ DA neurons. FOXA2+ DA neurons were also generated when this protocol was adapted to feeder-free conditions. In summary, this new human ES and iPS cell differentiation protocol using FGF8a, WNT1, low dose retinoic acid and a high activity form of SHH can generate human VM DA neurons that are required for relevant new bioassays, drug discovery and cell based therapies for PD.
Introduction
The adult central nervous system (CNS) contains numerous neuronal types with distinct functional characteristics that regulate up to 100 trillion synapses in the most complex vertebrate brains (Pfaff, 2008). Of relevance to Parkinson’s disease (PD) research, the adult brain contains at least 10 types of dopaminergic (DA) neurons (for review see (Ang, 2006)). Different types of DA neurons exhibit region-specific innervation patterns and cell type-specific transcriptional, electrophysiological and neurochemical profiles (Chung et al., 2005; McRitchie et al., 1996; Neuhoff et al., 2002; Wolfart et al., 2001). The cardinal motor symptoms of PD are caused by the relative vulnerability of a specific type of DA neuron in the patient’s ventral midbrain (VM) to degeneration. The substantia nigra A9 type (SN-A9) DA neurons are relatively more vulnerable to significant degeneration than neighboring ventral tegmental area A10 type (VTA-A10) DA neurons (Damier et al., 1999; German et al., 1989; German et al., 1992; Hirsch et al., 1988). With respect to cell therapy for PD, both SN-A9 and VTA-A10 DA neurons exhibit widespread and dense patterns of axonal arborization (Matsuda et al., 2009) but transplanted fetal SN-A9 DA neurons are responsible for appropriate striatal reinnervation and improved drug-induced and spontaneous behavioral measures in models of PD (Grealish et al., 2010; Isacson et al., 2003).
Human embryonic stem (ES) cells are a well-described source of DA neurons (Perrier et al., 2004). With recent progress in cellular reprogramming technology, PD patient specific DA neurons can be generated from induced pluripotent stem (iPS) cells that retain authentic PD risk loads and so can be used for mechanistic studies of PD pathogenesis and drug screening (Soldner et al., 2009). Importantly, iPS cell technology could provide isogeneic cells for cell therapy to eliminate the patient’s immune response to the transplanted neurons. The brain is relatively immunoprivileged but activated microglia can compromise the synaptic function of transplanted neurons (Soderstrom et al., 2008). Nonetheless, the ability of human ES/iPS cells to differentiate in vitro into VM DA neurons that possess the intrinsic properties required for PD modeling or reinnervation after transplantation remains unclear.
Neuronal differentiation of mouse and human ES cells favors telencephalic cell fates (Gaspard et al., 2008; Li et al., 2009), but several strategies for directed differentiation towards mesencephalic cell fates have been described (Cho et al., 2008; Perrier et al., 2004; Roy et al., 2006). However, the ES/iPS cell differentiation protocols do not generate a robust VM DA neuron phenotype as shown by modest behavioral outcomes after transplantation (Chiba et al., 2008; Roy et al., 2006; Sonntag et al., 2007; Yang et al., 2008). The issue of phenotypic robustness of human stem cell-derived DA neurons is compounded by the paucity of DA neuron subtype-specific immunocytochemical markers. For example, SN-A9 but not VTA-A10 DA neurons express the G protein coupled inward rectifying current potassium channel type 2 (GIRK2) in the human ventral midbrain (Mendez et al., 2005). Tyrosine hydroxylase (TH) and GIRK2 coexpression has been adopted as a phenotypic marker of stem cell-derived SN-A9 DA neurons (Roy et al., 2006). Yet forebrain DA neuron subtypes can also coexpress Girk2 and TH (O. Cooper, unpublished observation). Therefore, coexpression of these markers is particularly powerful only when discerning SN-A9 DA neurons from VTA-A10 DA neurons within the ventral midbrain. Recent developmental studies have demonstrated the role of the forkhead transcription factor, FoxA2/HNF3β in specifying and maintaining the VM DA neuron phenotype (Ferri et al., 2007; Kittappa et al., 2007; Lin et al., 2009). Remarkably, immunocytochemical detection of FOXA2 expression by ES/iPS cell-derived DA neurons has not been described, and is conspicuous by it’s absence from existing mouse and human pluripotent stem cell differentiation protocols (Cai et al., 2009).
In the present study, we found that FOXA2 expressing VM DA neurons were not generated by a recently described differentiation protocol (Sonntag et al., 2007). We hypothesized that the regional specification of the differentiation protocol was inadequate. To address this deficiency, we demonstrated that early and specific exposure to retinoic acid (RA) improved the regional identity of neural progenitor cells derived from human ES cells but the differentiated DA neurons did not express the key transcriptional regulator of the VM DA phenotype, FOXA2. To further improve sonic hedgehog (SHH) function in the differentiation protocol, we used a high activity form of human SHH to establish a sizeable FOXA2+ neural progenitor cell population in vitro from human ES or iPS cells. Since Fgf8 splice variants and Wnt1 are also important for the development of the embryonic midbrain (Muhr et al., 1999; Nordstrom et al., 2002; Nordstrom et al., 2006; Olsen et al., 2006), we tested the functions of recombinant FGF8a, Fgf8b and WNT1. We found that early exposure to FGF8a, rather than Fgf8b, and WNT1 were required for robust differentiation of the FOXA2+ floor plate-like neural progenitor cells into FOXA2+ DA neurons. In combination, these factors generated FOXA2+ DA neurons.
Results
Specific midbrain regionalization in response to retinoic acid
To understand the range of human DA neuron subtypes generated by our previously published protocol (Sonntag et al., 2007) and others (Cai et al., 2009; Perrier et al., 2004), we used co-immunoreactivity for FOXA2, tyrosine hydroxylase (TH) and p-tubulin to identify VM DA neurons. While the percentage of TH+/p-tubulin+ cells within the total cell population (Hoechst labeled) was 5.16 ± 2.6%, surprisingly few of the TH+/β-tubulin+ cells coexpressed FOXA2 (approximately 0.00001%). Next, we used co-immunoreactivity for the transcription factors ISLET1 (ISL1) or PAX6, TH and β-tubulin to identify DA neuron subtypes that arise from the dorsal diencephalon (Marin et al., 2005; Mastick and Andrews, 2001). Cell counts revealed that 17.76 ± 4.46% or 33.92 ± 4% of TH+/β-tubulin+ cells coexpressed PAX6 or ISL1, respectively.
In response to these data, we designed experiments to improve the regional specification of the ES cell-derived human DA neurons in culture. We examined a range of retinoic acid (RA) concentrations towards inducing a midbrain-like transcriptional profile. Using human neural progenitor cells, RA concentration-dependent transcriptional profiles were determined after 22 days of differentiation in the absence or presence of 10−6, 10−7, 10−8 or 10−9M RA (Fig. 1A). Higher concentrations of RA (10−6M and 10−7M) reduced OTX2 and PAX2 levels and increased HOXB1 levels relative to differentiation conditions without RA (Fig. 1B). In contrast, 10−8M RA increased the expression levels of the midbrain transcription factor, EN1 by more than 2000-fold. In all conditions tested, expression levels of the ventral neural transcription factor, FOXA2 were consistently low. Upon neuronal differentiation of RA-treated neural progenitor cells, immunocytochemistry rarely detected DA neurons (TH+/β-tubulin+) that coexpressed FOXA2 (data not shown). Therefore, 10−8M retinoic acid (RA) generated a human neural progenitor cell that exhibited a transcriptional profile consistent with midbrain regionalization without the appropriate ventralization signal to generate VM DA neurons.
Fig 1.
Early exposure to mShh-N and 10−8M retinoic acid promotes midbrain regionalization of human ES cell-derived neural progenitor cells. (A) Human ES cells were differentiated by 4 concentrations of RA with mouse Shh for 12 days before exposure to BDNF, ascorbic acid, mouse Shh and Fgf8b for 8 days. (B) Quantitative PCR determined that 10−8M retinoic acid with mouse Shh induced a midbrain-like transcriptional profile by significantly increasing the levels of EN1 without changing the levels of OTX2, HOXB1 and PAX2 (* p < 0.05 ANOVA).
Efficient differentiation of human ES and PD iPS cells into FOXA2 expressing progenitor cells requires a high activity form of recombinant human SHH
To improve the efficiency of ventralization in our RA-treated human neural progenitor cell cultures, we focused our attention to the recombinant Shh protein used in our previous differentiation protocol (Sonntag et al., 2007). As a first step to increasing the yield of FOXA2 expressing neural progenitor cells, we increased the concentration of the recombinant N-terminus truncated form of mouse Shh from 200 ng/ml to 500 ng/ml. At 500 ng/ml, few FOXA2 expressing progenitor cells were observed (data not shown). Next, we exposed the neural progenitor cells to Shh for an additional 7 days without increasing the yield of FOXA2 expressing neural progenitor cells (data not shown).
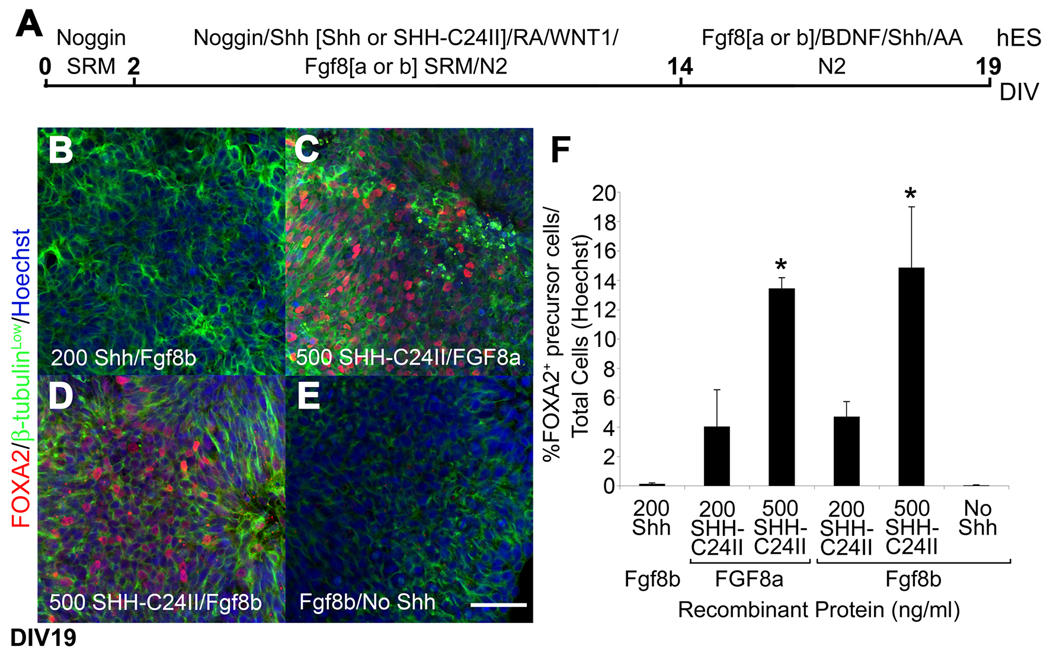
As an alternative to the recombinant N-terminal truncated form of mouse Shh, we decided to examine a recombinant human SHH protein with an N-terminal mutation that significantly increases protein signaling (SHH-C24II) (Taylor et al., 2001). Importantly, the concentration of SHH-C24II markedly changed the yield of FOXA2 expressing progenitor cells that differentiated from RA-treated human ES and PD iPS cells. Furthermore, we explored the ability of another possibly critical reagent in VM DA neurogenesis, FGF8 splice variants (Olsen et al., 2006). At DIV19 (Fig. 2A), 10−8M RA-treated human ES cells efficiently differentiated into FOXA2+/β-tubulinLow progenitor cells in response to 500 ng/ml of high activity human SHH with either FGF8a or Fgf8b (Fig. 2C, D, F). In contrast, relatively few FOXA2+ progenitor cells were observed after exposure to 10−8M RA, Fgf8b with or without mouse Shh (Fig. 2B, E, F).
Fig 2.
High activity form of recombinant SHH promotes the dose-dependent differentiation of FOXA2 expressing neural progenitor cells from 10−8M RA treated human ES cells. (A) Human ES cells were exposed for 12 days to a SHH-C24II and a further 5 days to neural expansion medium. (B–E) Immunocytochemistry at DIV19 revealed few FOXA2+ (red)/β-tubulinLow (green) neural progenitor cells that had differentiated from human ES cells after exposure to mouse Fgf8b with (B) or without mouse Shh (E). In contrast, 500 ng/ml SHH-C24II generated many FOXA2+/β-tubulinLow neural progenitor cells in the presence of either FGF8a (C) or Fgf8b (D). (F) Cell counts revealed a significantly greater percentage of human FOXA2+ neural progenitor cells after exposure to 500 ng/ml SHH-C24II and either FGF8a or Fgf8b (* p < 0.05 ANOVA). Scale bar = 50 µm.
Further phenotypic characterization at DIV19, revealed that the neural progenitor cells exposed to 10−8M RA, Fgf8b with or without mouse Shh organized into rosette-like structures and expressed the forebrain and midbrain marker, OTX2 but not FOXA2 (Fig. 3A, D, E, H, I, L). In contrast, neural progenitor cells exposed to 10−8M RA, SHH-C24II and FGF8a organized into rosette-like structures and coexpressed FOXA2 and OTX2 weakly, indicative of a ventral midbrain-like neural progenitor cell phenotype (Fig. 3B, F, J). Similarly, many of the neural progenitor cells exposed to 10−8M RA, SHH-C24II and Fgf8b coexpressed OTX2 and FOXA2 (Fig. 3C, G, K). However, these FOXA2+ neural progenitor cells did not organize into rosette-like structures nor expressed.
Fig 3.
Phenotypic characterization of FOXA2 expressing neural progenitor cells from RA-treated human ES/iPS cells. (A–L) Immunocytochemistry at DIV19 revealed that human ES cell-derived neural progenitor cells formed rosette-like structures and coexpressed OTX2 (red) but not FOXA2 (green) in response to Fgf8b with (A, E, I) or without mouse Shh (D, H, L). In contrast, isolated neural rosette-like structures were observed in cultures exposed to SHH-C24II and FGF8a (B, F, J). These rosette-like structures coexpressed FOXA2 and OTX2 (B). In cultures exposed to SHH-C24II and Fgf8b, very few rosette-like structures were observed and cells coexpressed FOXA2 and OTX2 (C, G, K). (M) Human ES cells exposed to 500 ng/ml SHH and FGF8a or Fgf8b differentiated into FOXA2+ cells (green) that expressed human-specific nestin (red). (N) In similar culture conditions, FOXA2+ cells (green) coexpressed 3CB2 (red). (O) In parallel cultures, all human iPS cell lines differentiated into FOXA2+ (red)/β-tubulinLow (green) neural progenitor cells. Scale bar A–L, O = 50 µm, M = 20 µm, N = 10 µm.
Furthermore, many of the FOXA2 expressing progenitor cells generated by exposure to 10−8M RA, FGF8a and SHH-C24II expressed nestin (Fig. 3M), the radial glial marker 3CB2 (Fig. 3N) and NCAM (data not shown). Similarly high yields of FOXA2 expressing progenitor cells were obtained from RA-treated PD and healthy control iPS cell lines after exposure to 500 ng/ml SHH-C24II and FGF8a (Fig. 3O, PDC3F-1).
FOXA2+ neural progenitor cell neurogenesis requires exposure to FGF8a rather than Fgf8b during neural induction and expansion
To examine the neurogenic potential of the human neural progenitor cell populations generated by these experimental conditions, we replaced Fgf8 and Shh in the culture medium with cAMP, TGFβ3 and GDNF as described (Sonntag et al., 2007). DA neurogenesis from FOXA2 expressing neural progenitor cells required prior exposure to FGF8a but not Fgf8b (Fig. 4). After 49 days of differentiation (Fig. 4A), few FOXA2 expressing cells were observed after differentiation with 10−8M RA, Fgf8b with or without mouse Shh (Fig. 4B, E, F). After differentiation with 10−8M RA, Fgf8b and SHH-C24II, human ES cell-derived FOXA2 expressing cells were observed but almost none of these cells coexpressed TH and β-tubulin (Fig. 4D, F). In contrast, cultures derived from 10−8M RA-treated human ES cells retained significantly more FOXA2 expressing cells at DIV49 when exposed to SHH-C24II and FGF8a (Fig. 4C, F). Importantly, these cultures contained a significant population of cells that coexpressed FOXA2, TH and β-tubulin (Fig. 4C arrowheads, F). Similar yields of FOXA2+/TH+/β-tubulin+ cells were obtained from PD and healthy control iPS cell lines using similar growth conditions (Fig. 5A, PDC3F-1). In similarly treated human ES cell-derived neuronal cultures, many TH expressing cells coexpressed calbindin or low levels of GIRK2 (Fig. 5B,C).
Fig 4.
Expansion of human ES cell-derived FOXA2+ neural progenitor cells by SHH-C24II and human FGF8a for 28 days leads to the generation of FOXA2 expressing DA neurons. (A) Human ES cell-derived neural progenitor cells were grown in medium supplemented with Shh and FGF8 splice variants before neuronal differentiation. (B–E) Immunocytochemistry at DIV49 revealed many β-tubulin+ neurons (green) and TH+ neurons (blue) but very few FOXA2+ cells (red) differentiated from human ES cells after exposure to Fgf8b with (B) or without mouse Shh (E). In contrast, 500 ng/ml SHH-C24II with FGF8a (C) or Fgf8b (D) generated many FOXA2+ cells. Importantly, FOXA2+/β-tubulinHigh/TH+ neurons were only observed after exposure to FGF8a (C, arrowheads). (F) Cell counts revealed a significantly greater percentage of human FOXA2+ cells (* p < 0.05 ANOVA) and FOXA2+ neurons (# p < 0.05 ANOVA) after exposure to 200 or 500 ng/ml SHH-C24II and FGF8a. A significant increase in the percentage of FOXA2+ DA neurons was observed after exposure to 500 ng/ml SHH-C24II and FGF8a (δ p < 0.05 ANOVA). Scale bar = 50 µm.
Fig 5.

Phenotypic characterization of FOXA2+ dopaminergic neurons generated by SHH-C24II and FGF8a from RA-treated human ES/iPS cells. (A) Human PD-iPS cell lines (PDC3F-1) were competent to generate FOXA2+ (red) dopaminergic neurons (TH, blue; β-tubulin, green; arrowheads). (B,C) In cultures differentiated with 500 ng/ml SHH-C24II and FGF8a, cells coexpressed TH (blue), FOXA2 (red) and calbindin (green, B) or weakly GIRK2 (green, C), indicative of an A10 or A9 DA neuron phenotype, respectively. Scale bar A = 50 µm, B,C = 10 µm.
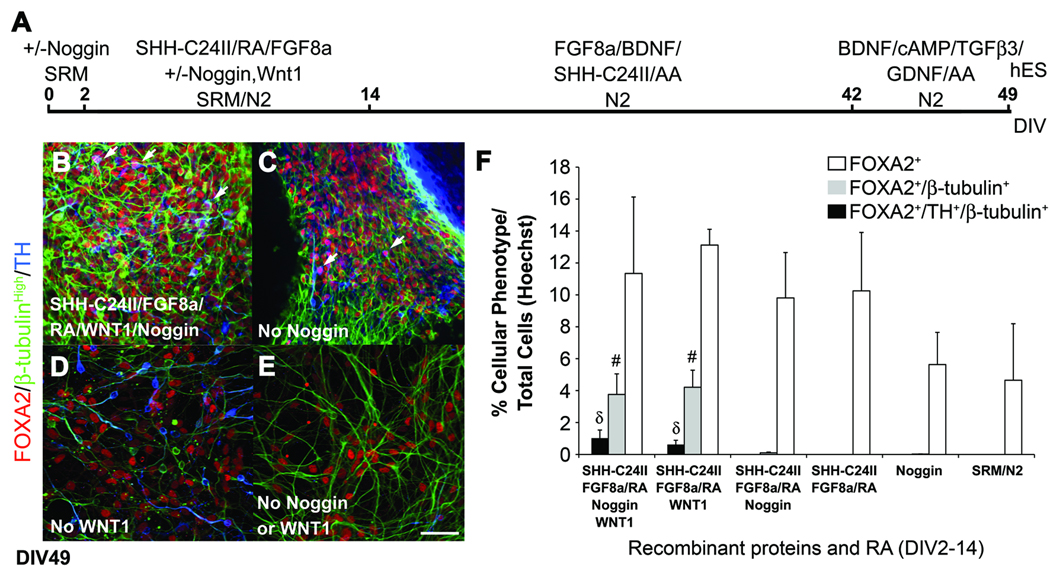
Differentiation of FOXA2+ DA neurons requires exposure to recombinant WNT1 but not Noggin or MS5 feeder cells
With evidence that human ES cells can be directed to differentiate into FOXA2+ DA neurons, we next tested the requirement for recombinant WNT1 and Noggin proteins to generate this type of DA neuron. Human ES cells were cultured with or without WNT1 and/or Noggin, with Noggin alone or without growth factors during the first 14 days of differentiation before exposure to the previously described recombinant protein supplements, including SHH-C24II (Fig. 6A). At DIV16, cultures grown without Noggin contained a few clusters of cells exhibiting neural differentiation (data not shown). By DIV49, human ES cell-derived cultures grown with or without Noggin contained cells that coexpressed FOXA2, TH and β-tubulin (Fig. 6B,C). In contrast, cultures grown without WNT1 and/or Noggin (Fig. 6D,E), with Noggin alone or without growth factors did not contain cells that coexpressed FOXA2, TH and β-tubulin (Fig. 6F).
Fig 6.
Differentiation of human FOXA2 expressing DA neurons from ES cells requires recombinant WNT1 but not Noggin. (A) Human ES cells were differentiated with or without WNT1 and/or Noggin during the first 14 days of the protocol. (B–E) At DIV49, FOXA2+/TH+/β-tubulin+ cells were observed after exposure to SHH-C24II, FGF8A and RA with (B) or without Noggin (C). FOXA2+/TH+/β-tubulin+ cells were not observed at DIV49 without early exposure to WNT1 (D), or WNT1 and Noggin (E). (F) Cell counts revealed a significantly greater percentage of human FOXA2+ neurons (# p < 0.05 ANOVA) and FOXA2+ DA neurons (δ p < 0.05 ANOVA) after exposure to WNT1 with or without Noggin. Scale bar = 50 µm.
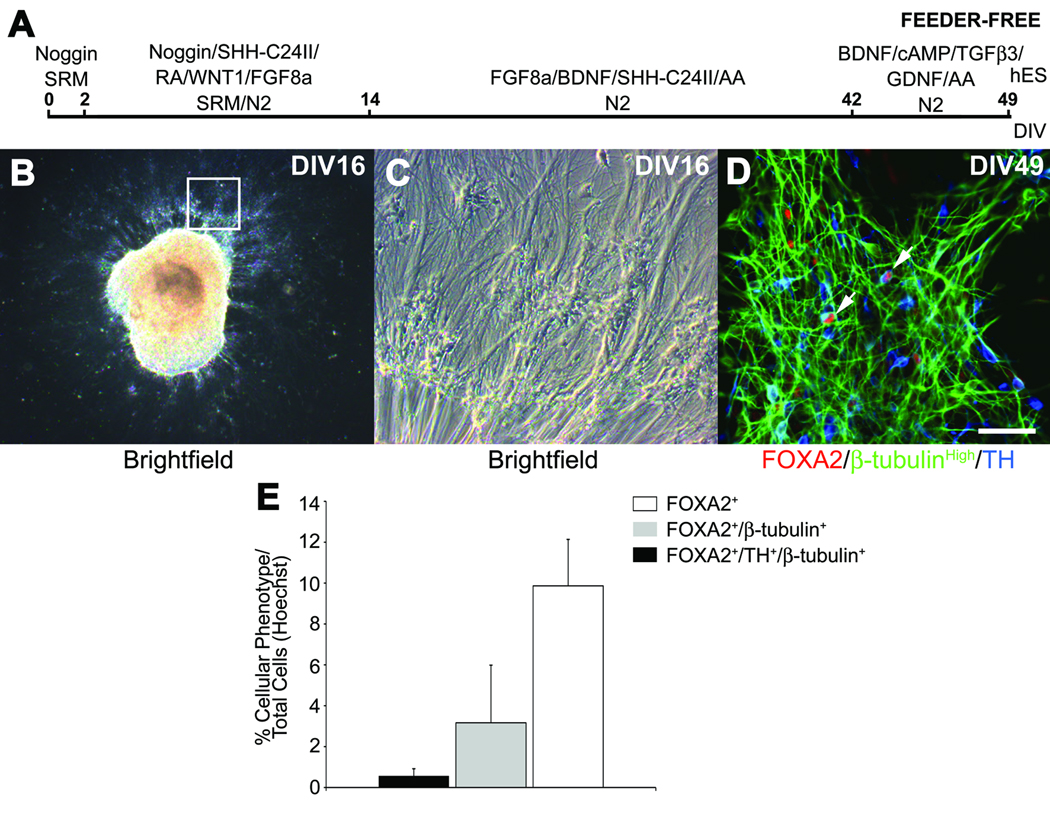
To examine whether MS5-feeder cells were required for the differentiation of human FOXA2+ DA neurons, human pluripotent stem cell lines were differentiated on gelatin-coated dishes using the previously described recombinant protein supplement (Fig. 7A). At DIV16, brightfield microscopy revealed human cultures composed of large cellular masses that exhibited long radial outgrowth consistent with neural differentiation (Fig. 7B,C). Upon neuronal differentiation, the dissociated cultures exhibited many cells that coexpressed TH, β-tubulin and FOXA2 (Fig. 7D,E).
Fig 7.
Feeder cell-free differentiation of stem cell-derived human FOXA2 expressing DA neurons. (A) Human pluripotent stem cells were differentiated without coculture on mouse MS5 feeder cells. (B) At DIV16, large clusters of cells formed that exhibited prominent radial outgrowth. (C) At higher magnification (see inset in B), small clusters of cells were observed adjacent to radial cell processes. (D–E) At DIV49, immunocytochemistry and cell counts revealed many FOXA2 expressing cells (red) that coexpressed TH (blue) and β-tubulin (green). Scale bar = 50 µm.
Discussion
In these experiments, we found that a combination of a specific concentration of RA, high levels of a high activity form of human SHH and exposure to FGF8a and WNT1 was sufficient to direct the fate of human ES/iPS cells towards VM DA neurons.
Early studies of human ES cell directed differentiation generated functional DA neurons (Perrier et al., 2004). While the ability to produce dopamine is an important attribute of neurons required for cell therapy and in vitro assays of PD neurodegeneration, the SN-A9 DA neuron subtype is the optimal dopaminergic neuron subtype for several reasons (Isacson et al., 2003). SN-A9 DA neurons regulate motor function and their degeneration contributes to the cardinal symptoms of PD (Damier et al., 1999; German et al., 1989; German et al., 1992; Hirsch et al., 1988). After transplantation into a PD model, fetal SN-A9 DA neurons reinnervate and increase c-Fos levels in the dorsolateral striatum, leading to improved amphetamine-induced rotations and spontaneous paw reaching activity (Grealish et al., 2010). While these cellular attributes are clearly desirable, few phenotypic markers are available that clearly identify human VM DA neurons. Recent studies have established the transcription factor FoxA2 as a robust cellular marker of VM DA neurons through several stages of development (Ferri et al., 2007; Lin et al., 2009). By using immunocytochemistry to identify FOXA2 expressing cells, we were rarely able to identify VM DA neurons differentiated from human ES cells using an established coculture-based differentiation protocol (Perrier et al., 2004; Sonntag et al., 2007). This finding was surprising but complements a report of an alternative but similarly widely used embryoid body-based human ES/iPS cell differentiation protocol (Cai et al., 2009). Instead, the presence of many ISL1+ DA neurons that likely represented a subthalamic A13 DA neuron cell fate (Mastick and Andrews, 2001) suggested that the specification of the human ES cell-derived neural progenitors required both stronger posteriorizing and ventralizing cues to direct differentiation further from default telencephalic cell fates and generate a meaningful yield of SN-A9 DA neurons (Ang, 2006; Li et al., 2009; Marin et al., 2005; Mastick and Andrews, 2001). RA is one-such example of a potent inducer of specific neural cell fates in a dose-dependent manner in several species including human (Maden, 2007). However, differentiation protocols using RA (10−6–10−7M) were intended to accomplish extensive shifts in cell fate away from the dorsal telencephalon towards motor neuron differentiation (Hu and Zhang, 2009). Using human ES cells, we found that 10−8M RA generated a midbrain-like transcriptional profile consistent with similar studies using mouse ES cells (Okada et al., 2004) but contrasts with a recent study of human ES cell differentiation that did not detect increased levels of EN1 with 10−8M RA (Li et al., 2009). The latter study used an embryoid body-based differentiation protocol, which may explain the low level of EN1 expression observed at a similar RA concentration. Upon differentiation of RA-treated neural progenitor cells, we observed significantly fewer ISL1 expressing DA neurons without FOXA2 expression. Given that diencephalic DA neuron subtypes are poorly characterized, the increased yield of ISL1−/FOXA2−DA neurons suggested a phenotypic shift from subthalamic A13 DA neurons towards a more posterior but still dorsal hypothalamic ISL1− A11 DA spinal projection neuron phenotype (Earley et al., 2009; Qu et al., 2006). While the RA-induced phenotype suggested midbrain regionalization, the lack of FOXA2 expression still suggested inappropriate cell fate specification in the dorsoventral axis.
While Shh is biochemically connected with FoxA2 (Chiang et al., 1996; Roelink et al., 1995; Sasaki et al., 1997), increasing the concentration of recombinant mouse Shh protein did not induce FOXA2 expression by neural progenitor cells. Our alternative strategy was based upon biochemical studies of SHH function that used N-terminal modifications to improve the induction of alkaline phosphatase activity in C3H10T1/2 cells by 8-fold (Taylor et al., 2001). Consistent with these biochemical findings, the recombinant N-terminal high activity human SHH protein induced a dose-dependent increase in the FOXA2+ cellular phenotype. The high dose of an activated form of recombinant protein required to efficiently change cellular fates highlights the functional problems of generating biologically-relevant recombinant proteins and the cost of using them for experimental or cell therapeutic applications.
By DIV49, the different functions of the FGF8 splice variants became apparent. During the early stages of ES/iPS cell differentiation (up to DIV19), both FGF8 proteins supported the generation of FOXA2 expressing progenitor cells. However by DIV49, increased yields of FOXA2 expressing cells required exposure to recombinant FGF8a protein rather than Fgf8b. Due to the heterogeneity and asynchronicity of cell fates during differentiation, human ES cells are not amenable to mechanistic studies of late cell fate specification and our studies do not directly discern the molecular mechanisms for neurogenesis from FOXA2+ progenitor cells. Yet, the different numbers of FOXA2 expressing cells induced by either FGF8a or Fgf8b splice variants at DIV19 and DIV49 may represent an in vitro selection pressure to eliminate non-neural FOXA2+ cell types with a concomitant promotion of ventral midbrain neuronal differentiation. In line with such an interpretation, robust neuronal differentiation of FOXA2 expressing neural progenitor cells was observed only after exposure to FGF8a. In these cultures, the presence of calbindin expressing VM DA neurons demonstrates a commitment to the A10 DA neuron phenotype (Mendez et al., 2005). Yet, the low levels of GIRK2 throughout the cultures at this time point do not represent a lack of an A9 DA neuron phenotype (Mendez et al., 2005). Instead, the cultures are like to be too immature to express this type of functional potassium channel, especially since A9 DA neurons are considered to develop at a slow rate than A10 DA neurons (Joksimovic et al., 2009a). In the embryonic brain, FoxA2 expressing neural progenitor cells generate several neuronal populations, not just VM DA neurons (Joksimovic et al., 2009a). Therefore the FOXA2+/TH−/β-tubulin+ cells observed at DIV49 likely represent either immature VM DA neurons that have not begun expressing TH yet, or terminally differentiated non-DA neurons. This explanation is supported by developmental studies demonstrating that the FGF8a splice variant promotes midbrain development and the Fgf8b splice variant promotes cerebellar development at the expense of midbrain (Lee et al., 1997; Liu et al., 1999; Olsen et al., 2006).
Our studies of the requirement for exogenous WNT1 signaling during human ES cell differentiation into VM DA neurons are interesting for two reasons. Firstly, early exposure of human ES/iPS cells to recombinant WNT1 was required to generate appropriately patterned VM neural progenitor cells, consistent with developmental studies in the embryo (Muhr et al., 1999; Nordstrom et al., 2002; Nordstrom et al., 2006). Secondly, in the context of recent studies of temporal changes in WNT expression during VM DA neurogenesis (Joksimovic et al., 2009b), our findings of neuronal differentiation of FOXA2+ neural progenitor cells without exogenous SHH antagonism are important. This suggests that either SHH antagonists secreted in vitro are sufficient to induce neuronal differentiation or that SHH antagonism is a modest requirement for VM DA neurogenesis in human cells.
Finally, our progress in generating human VM DA neurons without mouse feeder cells provides an opportunity for standardized differentiation conditions free of xenogeneic products that are likely to be required when providing ES/iPS cell-derived neurons for transplantation into PD patients (Mallon et al., 2006). In summary, we have used new phenotypic markers to show that a previously described human ES cell differentiation protocol does not generate VM DA neurons (Sonntag et al., 2007). Several groups have observed the lack of human VM DA neuron differentiation from human ES cells without a significant improvement being proposed (Cai et al., 2009; Erceg et al., 2009). We have now successfully generated human VM DA neurons by targeting both early posteriorizing and ventralizing neural patterning pathways. The markedly improved yield of VM DA neurons from human ES and iPS cells highlights an opportunity to enhance or modify exogenous signaling cues required to specify these cell types. Although isolated non-neural cell types may remain in these cultures, recent progress in flow cytometry can enrich the neuronal component to provide a robust cell source for new bioassays, drug discovery and cell based therapies for PD (Pruszak et al., 2009; Pruszak et al., 2007).
Experimental Methods
Human pluripotent stem cell culture and in vitro differentiation
All experiments were approved by the Partners ESCRO (Embryonic Stem Cell Research Oversight) Committee under protocol number 2006-04-001A. The human ES cell line H9 (WA-09, XX, approximate passage 35) was cultured according to the guidelines established by the National Academy of Sciences. The human iPS cell lines A6 (XX), PDC3F-1 (XY), PDB1lox-17Puro-5 (XY), PDB1lox-21Puro-26 (XY) and PDB1lox21Puro-28 (XY) were generated from parental fibroblast cell lines derived from healthy subjects or patients with sporadic Parkinson’s disease and obtained from Coriell Cell Repositories as described (Soldner et al., 2009). Human ES/iPS cells were propagated as described (Sonntag et al., 2007). The differentiation of human ES/iPS cells was adapted from a published protocol using MS5 feeder cells (Sonntag et al., 2007). Neuroectodermal differentiation was achieved using serum replacement medium (SRM) for 11 days, followed by N2 medium (DMEM/F-12; Invitrogen Corporation, Carlsbad, CA) (N2-A; Stem Cell Technologies, Vancouver, BC, Canada) for 3 days supplemented with 600 ng/ml Noggin (R&D Systems, Inc., Minneapolis, MN), 100 ng/ml human WNT1 (PeproTech EC Ltd., London, UK), 100 ng/ml human FGF8a or mouse Fgf8b (R&D Systems) and all-trans retinoic acid (Sigma-Aldrich, St. Louis, MO) to the culture medium. The amino acid sequences of mouse Fgf8a and human FGF8a are identical but the cDNA sequences are distinct. Media were changed every 2 days. At day 14 of differentiation, total human neuroectodermal colonies were manually picked as described (Karki et al., 2006) and replated on polyornithine and laminin-coated culture dishes in N2 medium supplemented with growth factors. Neural progenitor cells were differentiated toward the DA neuron phenotype with mouse sonic hedgehog (Shh-N), high activity human sonic hedgehog (SHH-C24II), mouse fibroblast growth factor 8b (FGF8b), human FGF8a (all from R&D Systems, Inc.), 20 ng/ml brain derived neurotrophic factor (BDNF) (PeproTech EC Ltd), 1 ng/ml transforming growth factor type β3 (TGF-β3) (Calbiochem, San Diego, CA), 10 ng/ml glial cell line-derived neurotrophic factor (GDNF), 0.5 mM dibutyryl cAMP, and 0.2 mM ascorbic acid (AA) (all from Sigma-Aldrich). After 21 days (Day 35) cells were passaged using Tryple (Invitrogen Corporation) and spun at 1,000 rpm for 5 minutes. Cells were resuspended in N2 medium and plated again at a density of approximately 1,000,000–2,000,000 cells per well (24 well plate) on polyornithine/laminin-coated dishes in the presence of BDNF, AA, SHH, and FGF8. After an additional 7 days of culture (DIV42), cells were differentiated until Day 49 without SHH and FGF8 but in the presence of BDNF, AA, cAMP, GDNF, and TGF-β3. For feeder-free conditions, 3 colonies of each human pluripotent stem cell line were propagated in human ES cell medium (Sonntag et al., 2007) on a 6 well gelatin-coated dish and passaged twice using Accumax (Millipore, Billerica MA) to remove contaminating fibroblasts. For differentiation, human pluripotent stem cells were exposed to recombinant proteins as described. At DIV35, neural cells were split using Tryple (Invitrogen Corporation) and replated for continued growth until DIV49.
Quantitative Real-Time Polymerase Chain Reaction
RNA was purified and real-time polymerase chain reaction (PCR) was performed as described (Chung CY, 2005). For primer sequences, see Supplementary Methods Table 1. Relative gene expression differences were quantified using the 2−ΔΔct method (Livak and Schmittgen, 2001).
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde and analyzed by indirect immunofluorescence (Sonntag et al., 2005). Fluorescent signals were examined using an LSM510 Meta confocal microscope equipped with ultraviolet, argon, and helium-neon lasers (Carl Zeiss, Thornwood, NY). The following primary antibodies were used: rabbit/sheep anti-tyrosine hydroxylase (TH) (1:300, Pel-Freez, Rogers, AK), mouse/goat anti-FoxA2/HNF3β (1:100, Santa Cruz Biotechnology, Santa Cruz, CA); mouse/rabbit anti-β-III-tubulin (TuJ1) (1:500, Covance, Berkeley, CA), mouse anti-Islet1 (1:100 and 1:500, Developmental Studies Hybridoma Bank, Iowa City, IA). The appropriate fluorescent-labeled secondary antibodies (1:500, Alexa Fluor goat or donkey anti-rabbit, -mouse, -rat or -goat 488, 568, 594, 647; Invitrogen Corporation) were applied for visualization, and nuclei were counterstained with Hoechst 33342 (5 µg/ml; Invitrogen Corporation). The specificity of the primary antibodies used in this study has been validated by our laboratory in cells and tissues of several species including human or have been published (Nelander et al., 2009).
Cell Counts
Quantitative analysis of immunocytochemistry was performed on randomly selected confocal fields from at least two independent differentiation experiments. In each field, images of separate channels (Hoechst, 488, 568, 594) were acquired at 40× magnification on an integrated confocal microscope (LSM510/Meta, Carl Zeiss) and stereology workstation (StereoInvestigator, MBF Bioscience, Inc., Williston, VT), where images of cells in independent channels and merged images were counted. For β-tubulin expression, we identified high expression when signal was detectable using the following parameters on the confocal microscope: pinhole 322 µm, Detector gain 393, Amplifier Offset 0 V. We considered low expression when signal was detectable using the following parameters on the confocal microscope: pinhole 163 µm, Detector gain 588, Amplifier Offset 0 V. On average, 20–25 visual fields were acquired per 16-mm coverslip, and a total of 4,000–8,000 Hoechst cells were counted per experiment in a blinded manner by at least two investigators.
Statistical Analysis
Data were analyzed by Tukey’s ANOVA using software (JMP, SAS Institute Inc, Cary, NC). Statistical significance was achieved at p < 0.05.
Supplementary Material
Acknowledgements
During the review of this paper, another paper providing mechanistic data for human FOXA2 expressing neural precursor cell differentiation from ES cells was published (Fasano et al., 2010). Our study was supported by the Udall Parkinson`s Disease Center of Excellence Grant P50 NS39793, Michael Stern Foundation (WX81XWH-05-1-0555), Orchard Foundation, the Consolidated Anti-Aging Foundation and the Harold and Ronna Cooper Family. G.H. received a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft (HA5589/1–1). T.O. received a Molecular Biology of Neurodegeneration postdoctoral fellowship from Harvard Medical School/National Institute on Aging (5T32AG00222-17).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ang SL. Transcriptional control of midbrain dopaminergic neuron development. Development. 2006;133:3499–3506. doi: 10.1242/dev.02501. [DOI] [PubMed] [Google Scholar]
- Cai J, Donaldson A, Yang M, German MS, Enikolopov G, Iacovitti L. The role of Lmx1a in the differentiation of human embryonic stem cells into midbrain dopamine neurons in culture and after transplantation into a Parkinson's disease model. Stem Cells. 2009;27:220–229. doi: 10.1634/stemcells.2008-0734. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Chiba S, Lee YM, Zhou W, Freed CR. Noggin enhances dopamine neuron production from human embryonic stem cells and improves behavioral outcome after transplantation into Parkinsonian rats. Stem Cells. 2008;26:2810–2820. doi: 10.1634/stemcells.2008-0085. [DOI] [PubMed] [Google Scholar]
- Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY SH, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Human Molecular Genetics, Epub ahead of printing. 2005 doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Earley CJ, Allen RP, Connor JR, Ferrucci L, Troncoso J. The dopaminergic neurons of the A11 system in RLS autopsy brains appear normal. Sleep Med. 2009;10:1155–1157. doi: 10.1016/j.sleep.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erceg S, Ronaghi M, Stojkovic M. Human embryonic stem cell differentiation toward regional specific neural precursors. Stem Cells. 2009;27:78–87. doi: 10.1634/stemcells.2008-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ferri AL, Lin W, Mavromatakis YE, Wang JC, Sasaki H, Whitsett JA, Ang SL. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development. 2007;134:2761–2769. doi: 10.1242/dev.000141. [DOI] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, Gaillard A, Vanderhaeghen P. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson's disease: computer visualization. Ann Neurol. 1989;26:507–514. doi: 10.1002/ana.410260403. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: sparing of calbindin-D28k–containing cells. Ann. N. Y. Acad. Sci. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- Grealish S, Jonsson ME, Li M, Kirik D, Bjorklund A, Thompson LH. The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson's disease. Brain. 2010 doi: 10.1093/brain/awp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Hu BY, Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacson O, Bjorklund LM, Schumacher JM. Towards full restoration of synaptic and terminal function of the dopaminergic system in Parkinson's disease from regeneration and neuronal replacement by stem cells. Annals of neurology. 2003;53:135–148. doi: 10.1002/ana.10482. [DOI] [PubMed] [Google Scholar]
- Joksimovic M, Anderegg A, Roy A, Campochiaro L, Yun B, Kittappa R, McKay R, Awatramani R. Spatiotemporally separable Shh domains in the midbrain define distinct dopaminergic progenitor pools. Proc Natl Acad Sci U S A. 2009a;106:19185–19190. doi: 10.1073/pnas.0904285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic M, Yun BA, Kittappa R, Anderegg AM, Chang WW, Taketo MM, McKay RD, Awatramani RB. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nature neuroscience. 2009b;12:125–131. doi: 10.1038/nn.2243. [DOI] [PubMed] [Google Scholar]
- Karki S, Pruszak J, Isacson O, Sonntag KC. ES cell-derived neuroepithelial cell cultures. J Vis Exp. 2006:118. doi: 10.3791/118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittappa R, Chang WW, Awatramani RB, McKay RD. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 2007;5:e325. doi: 10.1371/journal.pbio.0050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Danielian PS, Fritzsch B, McMahon AP. Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development. 1997;124:959–969. doi: 10.1242/dev.124.5.959. [DOI] [PubMed] [Google Scholar]
- Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Metzakopian E, Mavromatakis YE, Gao N, Balaskas N, Sasaki H, Briscoe J, Whitsett JA, Goulding M, Kaestner KH, Ang SL. Foxa1 and Foxa2 function both upstream of and cooperatively with Lmx1a and Lmx1b in a feedforward loop promoting mesodiencephalic dopaminergic neuron development. Dev Biol. 2009;333:386–396. doi: 10.1016/j.ydbio.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Liu A, Losos K, Joyner AL. FGF8 can activate Gbx2 and transform regions of the rostral mouse brain into a hindbrain fate. Development. 1999;126:4827–4838. doi: 10.1242/dev.126.21.4827. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Park KY, Chen KG, Hamilton RS, McKay RD. Toward xeno-free culture of human embryonic stem cells. Int J Biochem Cell Biol. 2006;38:1063–1075. doi: 10.1016/j.biocel.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin F, Herrero MT, Vyas S, Puelles L. Ontogeny of tyrosine hydroxylase mRNA expression in mid- and forebrain: neuromeric pattern and novel positive regions. Dev Dyn. 2005;234:709–717. doi: 10.1002/dvdy.20467. [DOI] [PubMed] [Google Scholar]
- Mastick GS, Andrews GL. Pax6 regulates the identity of embryonic diencephalic neurons. Mol Cell Neurosci. 2001;17:190–207. doi: 10.1006/mcne.2000.0924. [DOI] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRitchie DA, Hardman CD, Halliday GM. Cytoarchitectural distribution of calcium binding proteins in midbrain dopaminergic regions of rats and humans. J Comp Neurol. 1996;364:121–150. doi: 10.1002/(SICI)1096-9861(19960101)364:1<121::AID-CNE11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, Dagher A, Isacson O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhr J, Graziano E, Wilson S, Jessell TM, Edlund T. Convergent inductive signals specify midbrain, hindbrain, and spinal cord identity in gastrula stage chick embryos. Neuron. 1999;23:689–702. doi: 10.1016/s0896-6273(01)80028-3. [DOI] [PubMed] [Google Scholar]
- Nelander J, Hebsgaard JB, Parmar M. Organization of the human embryonic ventral mesencephalon. Gene Expr Patterns. 2009;9:555–561. doi: 10.1016/j.gep.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nature neuroscience. 2002;5:525–532. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- Nordstrom U, Maier E, Jessell TM, Edlund T. An early role for WNT signaling in specifying neural patterns of Cdx and Hox gene expression and motor neuron subtype identity. PLoS Biol. 2006;4:e252. doi: 10.1371/journal.pbio.0040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Shimazaki T, Sobue G, Okano H. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev Biol. 2004;275:124–142. doi: 10.1016/j.ydbio.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Olsen SK, Li JY, Bromleigh C, Eliseenkova AV, Ibrahimi OA, Lao Z, Zhang F, Linhardt RJ, Joyner AL, Mohammadi M. Structural basis by which alternative splicing modulates the organizer activity of FGF8 in the brain. Genes Dev. 2006;20:185–198. doi: 10.1101/gad.1365406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff SL. Developmental neuroscience: Hox and Fox. Nature. 2008;455:295–297. doi: 10.1038/455295a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszak J, Ludwig W, Blak A, Alavian K, Isacson O. CD15, CD24 and CD29 Define a Surface Biomarker Code for Neural Lineage Differentiation of Stem Cells. Stem Cells. 2009 doi: 10.1002/stem.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszak J, Sonntag KC, Aung MH, Sanchez-Pernaute R, Isacson O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells. 2007;25:2257–2268. doi: 10.1634/stemcells.2006-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S, Ondo WG, Zhang X, Xie WJ, Pan TH, Le WD. Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res. 2006;168:152–156. doi: 10.1007/s00221-005-0075-1. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nature medicine. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- Soderstrom KE, Meredith G, Freeman TB, McGuire SO, Collier TJ, Sortwell CE, Wu Q, Steece-Collier K. The synaptic impact of the host immune response in a parkinsonian allograft rat model: Influence on graft-derived aberrant behaviors. Neurobiol Dis. 2008;32:229–242. doi: 10.1016/j.nbd.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R, Isacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag KC, Simantov R, Bjorklund L, Cooper O, Pruszak J, Kowalke F, Gilmartin J, Ding J, Hu YP, Shen MM, Isacson O. Context-dependent neuronal differentiation and germ layer induction of Smad4−/− and Cripto−/− embryonic stem cells. Mol Cell Neurosci. 2005;28:417–429. doi: 10.1016/j.mcn.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Taylor FR, Wen D, Garber EA, Carmillo AN, Baker DP, Arduini RM, Williams KP, Weinreb PH, Rayhorn P, Hronowski X, Whitty A, Day ES, Boriack-Sjodin A, Shapiro RI, Galdes A, Pepinsky RB. Enhanced potency of human Sonic hedgehog by hydrophobic modification. Biochemistry. 2001;40:4359–4371. doi: 10.1021/bi002487u. [DOI] [PubMed] [Google Scholar]
- Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.