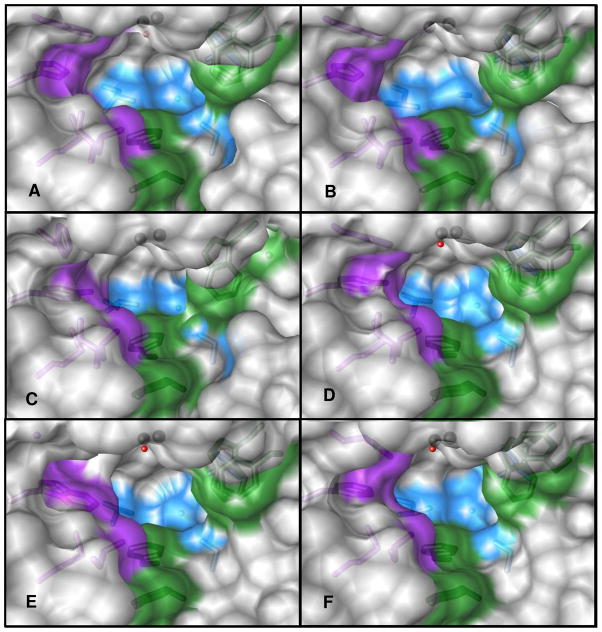
Figure 4.
The active site structure of wild-type PTE and five mutant enzymes (A) Wild-type; (B) G60A; (C) I106G/F132G/H257Y; (D) H257Y/L303T; (E) H254G/H257W/L303T; and (F) H254Q/H257F. The images were drawn with Chimera (45, 46). The surfaces are colored grey; the surface for Cα and side chains for residues 254, 257, 317, and 271were colored purple. The surfaces for residues 60, 303, 308 and 106 were colored blue and the surface for residues 131, 132, 306, and 309 were colored green. The stick figures of the residues were colored a shade darker than their corresponding surface. The metals are represented as black spheres and the bridging water, when present, was colored as a red sphere. A transparency of 15% was placed on the surface with a “one layer” transparency.