Abstract
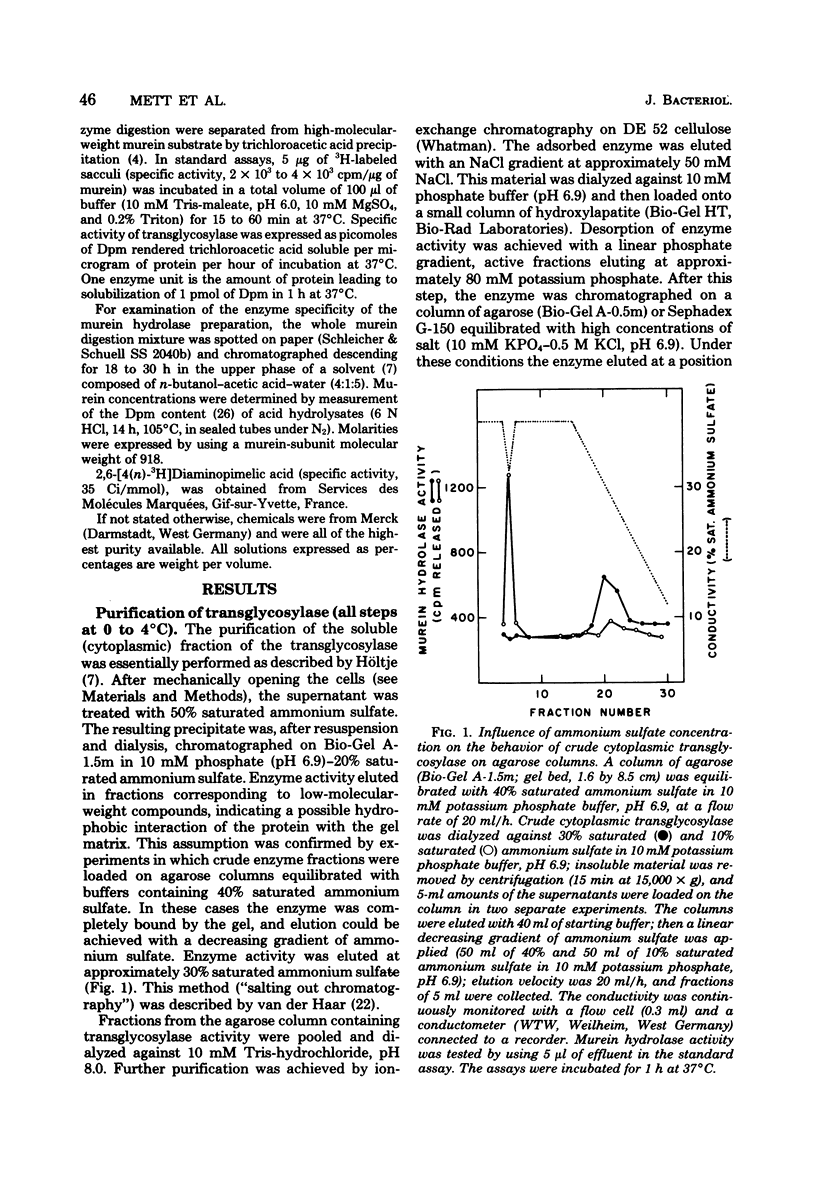
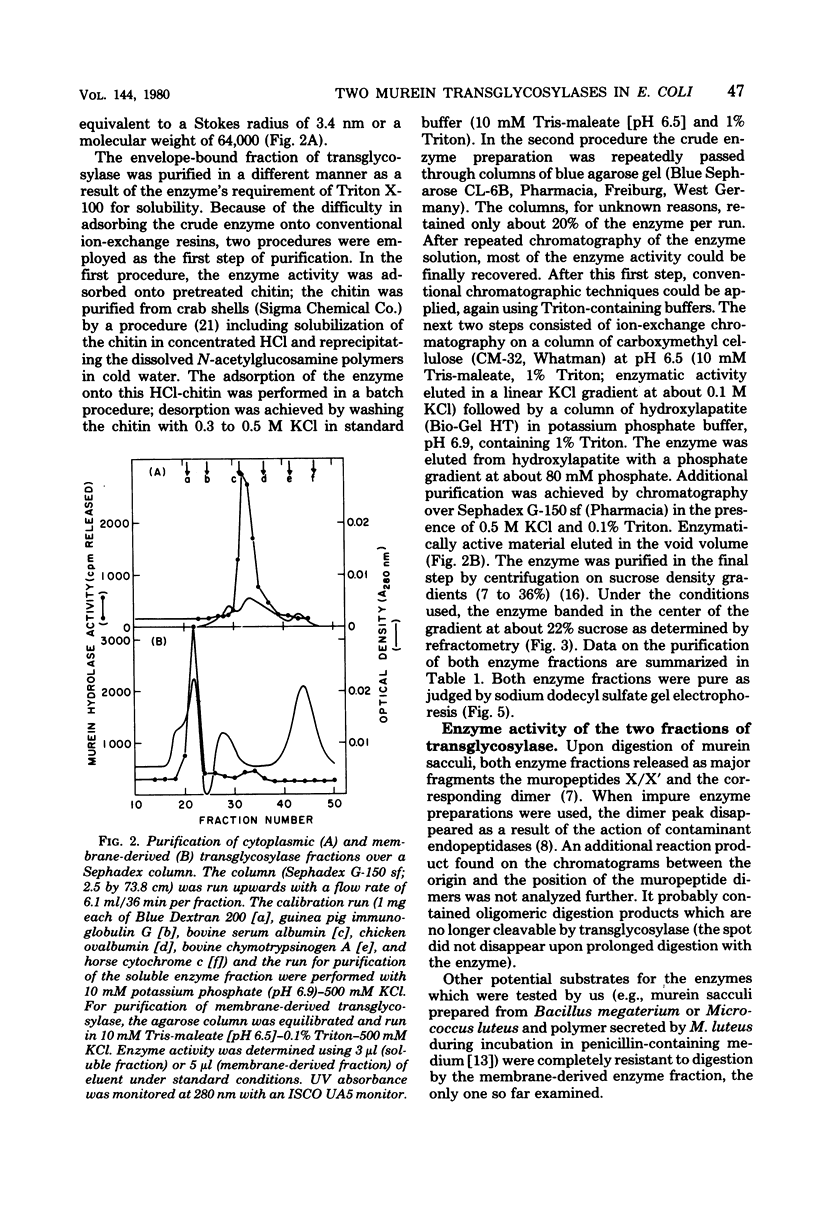
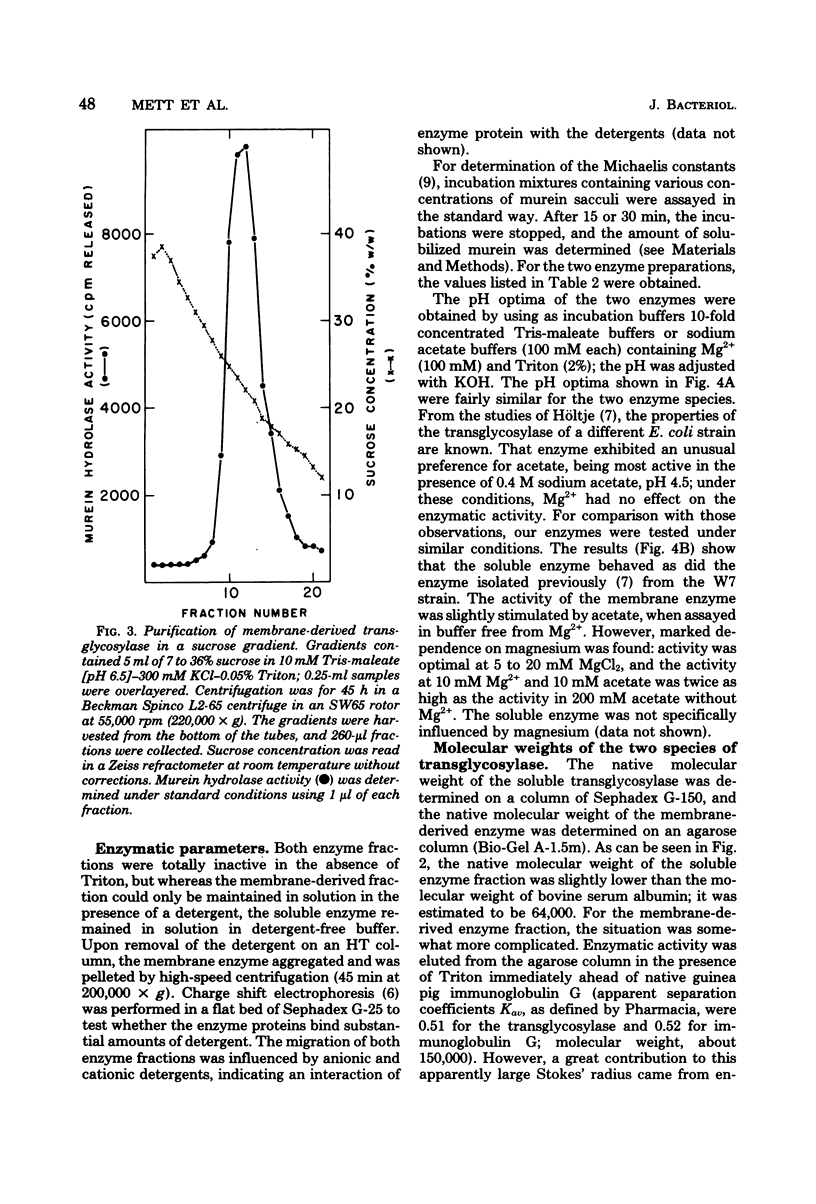
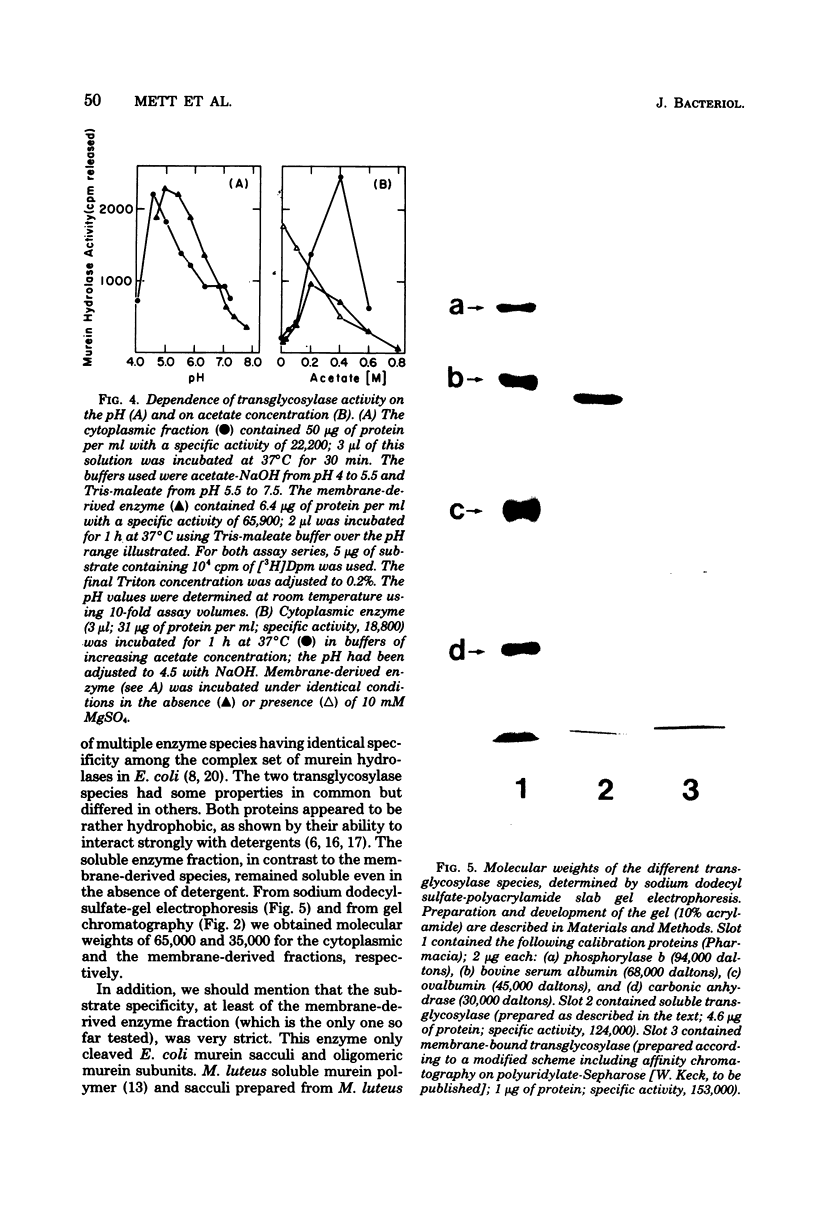
We demonstrated that Escherichia coli murein transglycosylase exists in two forms. After mechanical disruption of the cells, one form was found in the soluble fraction and the other, in the cell envelope. The two enzymes differed with respect to molecular weight, isoelectric point, solubility in aqueous buffers, and to some extent in their requirements for maximal catalytic activity. The molecular weight of the membrane-bound transglycosylase (35,000) was half that of the soluble enzyme. Whether the high-molecular-weight soluble protein is a precursor of the membrane-bound enzyme species remains to be elucidated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Activity of murein hydrolases in synchronized cultures of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1239–1244. doi: 10.1128/jb.129.3.1239-1244.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Messer W. Oscillations in the synthesis of cell wall components in synchronized cultures of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1234–1238. doi: 10.1128/jb.129.3.1234-1238.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Bock-Hennig S. B., Schwarz U. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur J Biochem. 1974 Jan 3;41(1):203–208. doi: 10.1111/j.1432-1033.1974.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Charge shift electrophoresis: simple method for distinguishing between amphiphilic and hydrophilic proteins in detergent solution. Proc Natl Acad Sci U S A. 1977 Feb;74(2):529–532. doi: 10.1073/pnas.74.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. The binding of detergents to lipophilic and hydrophilic proteins. J Biol Chem. 1972 Jun 10;247(11):3656–3661. [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck W., Schwarz U. Escherichia coli murein-DD-endopeptidase insensitive to beta-lactam antibiotics. J Bacteriol. 1979 Sep;139(3):770–774. doi: 10.1128/jb.139.3.770-774.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Penicillin-induced secretion of soluble, uncross-linked peptidoglycan by Micrococcus luteus cells. Biochemistry. 1974 Nov 19;13(24):5045–5053. doi: 10.1021/bi00721a028. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Peptidoglycan biosynthesis in a thermosensitive division mutant of Escherichia coli. Biochemistry. 1976 May 4;15(9):1781–1790. doi: 10.1021/bi00654a001. [DOI] [PubMed] [Google Scholar]
- Osborne H. B., Sardet C., Helenius A. Bovine rhodopsin: characterization of the complex formed with Triton X-100. Eur J Biochem. 1974 May 15;44(2):383–390. doi: 10.1111/j.1432-1033.1974.tb03495.x. [DOI] [PubMed] [Google Scholar]
- Reyenolds J. A., Stoeckenius W. Molecular weight of bacteriorhodopsin solubilized in Triton X-100. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2803–2804. doi: 10.1073/pnas.74.7.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Effet des sels et autres composés sur le phénotype de mutants thermosensibles de Escherichia coli. Ann Microbiol (Paris) 1973 Jan;124(1):29–43. [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- TRACEY M. V. Chitinase in some Basidiomycetes. Biochem J. 1955 Dec;61(4):579–586. doi: 10.1042/bj0610579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- WORK E. Reaction of ninhydrin in acid solution with straight-chain amino acids containing two amino groups and its application to the estimation of alpha epsilon-diaminopimelic acid. Biochem J. 1957 Nov;67(3):416–423. doi: 10.1042/bj0670416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfels B. Developmental and mutational changes of glycoproteins in the mouse neuronal retina: studies with bovine galactosyltransferase. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3223–3227. doi: 10.1073/pnas.76.7.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- von der Harr F. The ligand-induced solubility shift in salting out chromatography: a new affinity technique, demonstrated with phenylalanyl- and isoleucyl-tRNA synthetase from baker's yeast. FEBS Lett. 1978 Oct 15;94(2):371–374. doi: 10.1016/0014-5793(78)80980-6. [DOI] [PubMed] [Google Scholar]




