Abstract
Nonsurgical methods represent the majority of cosmetic procedures performed in the United States and include the use of toxins and injectable medical devices for soft-tissue augmentation. In some cases, both nonsurgical and surgical modalities can be used synergistically for optimal facial rejuvenation. Aesthetic surgical procedures remove and reposition lax or sagging skin and tighten and/or resuspend facial musculature. Injectable medical devices can enhance the aesthetic effect of cosmetic surgery by replacing lost volume and restoring the three-dimensional appearance of the face while maintaining natural facial contours. Soft-tissue augmentation can also provide additional support for the skin, correcting natural variations in facial symmetry. This article provides a descriptive review of the age-related facial changes and suggests a method for the use of three-dimensional volumetric augmentation for soft-tissue facial rejuvenation. Age-related changes in skin elasticity, fat distribution, and facial contours require a three-dimensional treatment approach that addresses the pathophysiology of chronological aging. Volume replacement restores the youthful appearance of the face in patients opting for nonsurgical rejuvenation and complements surgical approaches as well. Optimal three-dimensional soft-tissue augmentation can be achieved using a combination of aesthetic surgery and injectable medical devices, such as collagen, hyaluronic acid, calcium hydroxylapatite, and injectable poly-L-lactic acid to improve facial volume changes and contour irregularities. Injectable medical devices replace lost volume and restore facial convexity, reestablishing the bloom of youth.
Objective perception of a youthful face is largely based on the face’s three-dimensional (3-D) appearance.1 This includes the convexity, or roundness, of the midface, which is often described as the bloom of youth and is frequently lost or changed with advancing age.2 Consequently, an accurate assessment for facial volumetric restoration to enhance the 3-D features of the face is essential. Aesthetic surgery can remove and reposition lax or sagging skin and tighten and/or resuspend facial musculature, but does not replace lost volume. Injectable medical devices restore the 3-D appearance of the face by replacing lost volume while maintaining natural contours. Volume restoration also provides additional support for the skin, correcting natural variations in facial symmetry.1,3,4 Injectable fillers offer reduced downtime,5,6 decrease cost,7 and eliminate visible scars. Moreover, less invasive, nonsurgical procedures eliminate the risks of general anesthesia.8 The purpose of this article is to provide a discussion of how to best assess the patient’s face both pretreatment and post-treatment and to suggest a method for optimal 3-D volumetric soft-tissue augmentation.
Clinical Assessment Prior to Soft-Tissue Augmentation
Differences in appearance between the young and old face may be the result of extrinsic factors, including sun exposure, diet, exercise, alcohol use, and smoking, and intrinsic physiological factors, such as the redistribution of facial fat, weakening and laxity of facial muscles, and skeletal changes.2 The redistribution of superficial and deep fat is a primary determinant of volume loss.9–11 The loss of underlying support from facial skeleton remodeling may also contribute to the deflated appearance of the face.12–15
Age-related changes in the structural features of the face result in an undulating or wavy quality rather than a presentation of smooth, full, symmetrical features.11 Facial convexity represents the characteristic bloom of youth; therefore, the concavity and flattening that occur with aging are corrected to restore a more youthful appearance.11 Consequently, facial enhancement is best done using a 3-D approach for volumetric augmentation.1,4
Volumetric restoration provides added dimensionality to the surgical face-lift. Most surgical approaches to facial rejuvenation reposition facial tissues in one or two planes, which is why a face-lift without volume replacement may not achieve optimal results.4 With age, soft tissues sag inferiorly and inferomedially and the subcutaneous fat layer thins, resulting in wrinkles and folds.4 To correct this, concave and adjacent convex areas are blended to restore a full yet contoured face.1 Surgically moving soft tissue superiorly and posteriorly, however, may accentuate the flattened and deflated appearance in patients with volume loss. Injectable medical devices help reestablish the convexity of the face by replacing lost volume and addressing age-related changes in facial muscles and bone.
Selecting an appropriate injectable begins with an understanding of the benefits and limitations of each device. There are three types of injectable fillers: short-acting, long-acting, and permanent fillers. Collagens (bovine and human) and the hyaluronic acids generally have a short duration of effect, lasting 3 to 12 months.16,17 Bovine-derived collagen necessitates skin testing prior to use.17 Long-acting deep dermal fillers include calcium hydroxylapatite and injectable poly-L-lactic acid (PLLA).18 The effects of injectable PLLA have been shown to last up to two years, while the duration of effect of calcium hydroxylapatite is approximately 12 months.18,19 Permanent fillers, such as polymerized methyl methacrylate (PMMA) and medical grade silicone, offer the longest duration of effect (about 4–5 years).7,17,20,21 However, as the face continues to age and as facial volume continues to change, permanent implants may become visible.17 The permanence of PMMA and silicone can be problematic if the patient becomes dissatisfied with the effect, as these materials are not easily removed from tissue.
All fillers are subject to immediate and delayed adverse events. Immediate adverse events include pain, swelling, redness, and bruising, which may be treated with massage and ice packs and typically resolve over a few days.1 Delayed events, reported with all dermal fillers, include papules, nodules, and granulomas.22 These may resolve spontaneously or may require intralesional steroid injections and/or other treatment options.1
Three-Dimensional Approach to Soft-Tissue Augmentation
The target area or cosmetic unit requiring treatment and the nature of the defect (e.g., fine line, moderate or deep wrinkle, or area of volume loss) dictate the filler choice. The collagens and hyaluronic acids are suitable for shallow-to-deep wrinkles; however, these products are limited by their short duration. For wrinkles and folds associated with underlying volume loss, correction may be best achieved with injectable PLLA or calcium hydroxylapatite. These products are implanted at or below the level of the dermal-subcutaneous junction. In the author’s clinical experience, injectable PLLA has a longer duration and superior cosmetic effect compared with other available options. Injectable PLLA was recently approved for use in immune-competent people for the correction of shallow-to-deep nasolabial fold contour deficiencies and other facial wrinkles in which deep dermal grid pattern injection technique is appropriate.23 Injectable PLLA is also approved in the United States for the restoration and/or correction of the signs of facial fat loss (lipoatrophy) in people with human immunodeficiency virus (HIV).18
Injectable PLLA can restore lost volume by repositioning soft tissue in medial to lateral, superior, and anterior planes for 3-D volume restoration.1 The effects of injectable PLLA are gradual, and several treatments are usually required initially for optimal results that have been shown to last for a period of 25 months. The gradual correction is hypothesized to be due to a cellular response involving macrophages, lymphocytes, and mast cells, which has been observed after the implantation of solid PLLA particles in animal studies.24,25 Similar cellular events may occur after the injection of reconstituted PLLA in humans.26 In each study, implantation resulted in an increase in collagen formation over time.24–26 Based on this hypothesized mode of operation, it is thought that administration of injectable PLLA begins the process of restoration to the lost collagenous network. However, the gradual effect of injectable PLLA requires sufficient time between injections to avoid overcorrection and to assess the need for additional treatments.1,27,28 The principle of “treat to repair, wait to restore, and assess to refine” will guide the physician through the overall injection series.1,29 As with other injectable devices, patient response to injectable PLLA may vary.
The proper injection depth for injectable PLLA must be at or below the dermal-subcutaneous junction to effectively add volume and minimize safety concerns. The dermal-subcutaneous junction or superficial subcutaneous fat is the most common plane for injectable PLLA deposition.1 Other potential planes that can be expanded with additional volume include the mid-deep subcutaneous fat and supramuscular, submuscular, and supraperiosteal spaces. As with other volumizing agents, injectable PLLA may be layered to yield the optimal effect—appropriate volume with contour. In the author’s practice, injectable PLLA for facial injection is reconstituted using 6 to 8mL of United States Pharmacopeia sterile bacteriostatic water per vial, which differs from the 5mL sterile water for injection recommended by the manufacturer. The reconstituted product is then allowed to stand overnight prior to injection. Another difference in the author’s practice from the manufacturer’s recommendation is the gradual addition of 1mL of lidocaine immediately prior to injection. Although 26-gauge needles are recommended in the product insert, injectable PLLA may be administered with a 25-gauge, 1-inch needle, using a linear retrograde injection technique, with volumes ranging from 0.025 to 0.1mL per injection.1 These small amounts spaced approximately 2 to 4mm apart will blanket the lower two-thirds of the face from the temples down, using gridlike injection patterns (Figure 1) and supporting vectors for uniform distribution to volumize and blend concavity with convexity for a 3-D effect. Titration of lower total injection volume as the injector moves from superior to inferior areas helps maintain facial contour. Postinjection massage by both the injector and the patient helps further ensure a uniform distribution of injectable PLLA.1,28,30 Massage by the physician during the treatment session aids in the even distribution of the product and may reduce the incidence of papules and nodules.18,23,28,30-32 The physician should also educate the patient on the proper massage technique31,33 and instruct the patient to massage the treated area a minimum of five minutes twice daily for at least seven days.
Figure 1.

A schematic representation of the gridlike injection pattern recommended for uniformly distributed injectable PLLA23
As one progresses through the series of injection sessions, facial assessment may change due to an improvement in volume. Consequently, adjustments in depth, volume, and distribution of injectable PLLA may be required.1 Volume changes can be detected not only through visual examination and palpation of facial skin, but also during the injection process. In the author’s experience, tissue reaction to injectable PLLA can be detected at various levels of implantation as increased resistance in previously treated areas. Areas of reduced resistance may be noted as the needle is advanced. Such areas may be reinjected using small, titrated aliquots of injectable PLLA to further enhance facial volume and maintain contour.
Guidelines for injectable PLLA treatment of HIV–associated facial lipoatrophy. The goal of restoring facial volume in patients with HIV-associated facial lipoatrophy is to achieve a more normal facial appearance to alleviate the psychosocial stress and debilitation associated with this stigmatizing antiretroviral treatment–related physical appearance.1,34 The grading of facial lipoatrophy severity is based on the extent of facial fat loss and protuberance of the underlying facial musculature and skeleton, ranging from mild and localized deficits limited to the cheeks (i.e., grade 1; Figure 2) to clearly visible panfacial lipoatrophy with associated changes in muscle and bone (i.e., grades 4 and 5).34
Figure 2.
Global facial assessment. Grade 1 (upper row, left): Early grade of lipoatrophy characterized by a mild flattening of several facial areas, including the cheek, temple, preauricular, and periorbital areas; grade 2 (upper row, middle): An intermediate stage between grades 1 and 3; grade 3 (upper row, right): Typified by moderate concavity and increasing prominence of bony landmarks; grade 4 (lower left): Intermediate between grades 3 and 5; grade 5 (lower right): Severe indentation of one or more facial regions and severe prominence of bony landmarks.34 Adapted with permission from Ascher B, et al. Dermatol Surg. 2006;32:1058–1069.
In the author’s clinical experience, injectable PLLA treatment is best performed during early-onset facial lipoatrophy. When implemented during grade 1 lipoatrophy (Figure 2), treatment for submalar fat loss may be more localized.1,34 In grade 2, the lipoatrophy includes a wider and longer area of submalar loss and perceptible involvement of adjacent facial zones, including the nasolabial folds, medial and midcheek, and anterior jawline (Figure 2).34 Restoring volume to the primary area of submalar loss and blending with adjacent facial zones that are only beginning to show volume change will result in a more natural appearance and promote longevity of effect.1 Patients with early facial lipoatrophy (grades 1 and 2) usually require 2 to 3 sessions using 1 to 1.5 vials per session (reconstituted to a total volume of 7 to 9mL/vial, including 1 to 2mL lidocaine) injected at 4- to 6-week intervals. Fat loss in grade 3 lipoatrophy is accompanied by a more pronounced protuberance of facial muscles and bone (Figure 2).34 The treatment area broadens across the medial, mid, and lateral cheeks; the submalar region; nasolabial folds; and anterior jawline. Patients with grade 3 facial lipoatrophy may require 1 to 1.5 vials per session (reconstituted to a total volume of 7–9mL/vial) injected at 4- to 6-week intervals over 3 to 5 sessions. In grade 4 (panfacial lipoatrophy, clear muscle and bony protuberance, and inferior temporal fossa concavity) and grade 5 lipoatrophy (panfacial lipoatrophy, muscle and bony changes, and both inferior and superior temporal fossa concavity), the full lower two-thirds of the face including the temples is treated.34 Treatment of grades 4 and 5 HIV-associated facial lipoatrophy typically requires two vials per session (reconstituted to a total volume of 7–9mL/vial) injected at 4- to 6-week intervals over 5 to 6 treatment sessions. As improvement occurs, the volume injected in later sessions may decrease, and the number of sessions may vary depending on patient response. Hence, treatment plans should be individualized, especially in early stages of lipoatrophy.
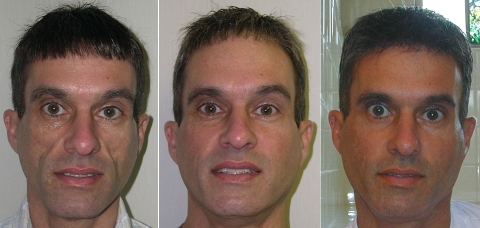
An example of treatment of HIV-associated facial lipoatrophy is shown in Figure 3. This patient presented with grade 4 lipoatrophy involving the medial, mid, and lateral cheeks; zygomatic arches; temples; nasolabial folds; and submalar regions. The scleral show in the photo before treatment (Figure 3, far left) was due to fat loss and diminished support from the superior aspect of the medial, mid, and lateral cheeks. Six months after treatment initiation (Figure 3, middle), the patient’s facial contours were normalized and the skin looked and felt natural. The convexity in the preauricular areas, resulting from parotid gland enlargement, and shown in the pretreatment photo, is commonly seen as a consequence of antiretroviral treatment for HIV35 and appears to have been minimized by the addition of injectable PLLA up to this convexity. A more normal appearance was achieved, and the patient remained content with the results 17 months after the last treatment (shown in Figure 3, far right). No adverse events were reported.
Figure 3.
Patient with grade 4 HIV-associated facial lipoatrophy before (left), 6 months after the start of treatment with injectable PLLA (middle), and 17 months after the last treatment with injectable PLLA (right). Treatment involved two vials of injectable PLLA per session (reconstitution 6mL/vial) at 4- to 6-week intervals for six sessions with a total injection volume of 72mL over approximately six months.
Guidelines for 3-D soft-tissue augmentation for immune-competent patients. The aesthetic goal of 3-D volumization with the use of injectable PLLA is restoration of a more youthful appearance. For immune-competent patients, the trend is for higher reconstitution volumes and prolonged injection intervals. A total reconstitution volume of 8 to 9mL/vial (including 1–2mL lidocaine added immediately prior to injection) has been suggested to minimize the frequency of nodule formation.22,29,31,36,37 In the author’s clinical experience, this increased total reconstitution volume has not diminished patient response to injectable PLLA. It has also been the author’s experience that older patients may have their first and second sessions scheduled at 4- to 6-week intervals and their third and fourth sessions (if required) at 2- to 3-month intervals. In younger patients, the author has recommended up to 6 to 8 weeks between the first and second sessions and as long as 2 to 4 months between a second and third session. These intervals between treatments help minimize adverse events, reduce the likelihood of overcorrection, and allow for the hypothesized formation of collagen.24–26,28 Any deposition of collagen can affect the volume and distribution of future injectable PLLA treatments.
Examples of patients who may be interested in volumetric augmentation are patients with lean, athletic physiques (Figure 4); patients in search of a nonsurgical option to restore facial volume; facelift patients 4 to 6 months after surgery, when the pseudovolumizing effect of facial edema has disappeared (Figure 5); and patients several years post-facelift who simply wish to restore volume. The use of injectable PLLA for aesthetic purposes (which was considered off-label at the time of treatment of patients discussed here) varies by patient and by the desired cosmetic result. Treatment plans are therefore individualized. For example, a naturally lean or athletic patient may wish only to restore a youthful appearance by alleviating deeper, more severe volume deficiencies while maintaining a lean and contoured face to match body type.
Figure 4.
The first three treatment sessions administered to the lower third of this 44-year-old woman’s face were 4 to 6 weeks apart and utilized 3 to 6mL of injectable PLLA (reconstituted to 6mL/vial). Three sessions were required in the mid-third of the face and utilized 4 to 6mL of injectable PLLA per session (reconstituted to 6mL/vial for the first and second sessions; 7mL/vial for the third). The first and second sessions for the mid-third of the face were 6 to 8 weeks apart, and the second and third sessions were three months apart. The photographs show the patient’s face at baseline (left) and 12 months later (right), approximately eight months after her last treatment.
Figure 5.
For this 78-year-old woman, the first three sessions were performed at 4- to 6-week intervals. The fourth session was performed approximately two months following the third treatment. Injection volume per session was 5 to 6mL (reconstituted to 6mL/vial). The images show her face at baseline (left) and approximately 18 months (right) after her last treatment with injectable PLLA.
Benefits for the patient with a lean body type include volume augmentation and skin tightening to minimize skin laxity. Figure 4 shows a 44-year-old lean woman who complained of cheek hollowness, although her face showed global volume changes and skin laxity. She was unfamiliar with injectable PLLA as a treatment option and elected to begin treating only the submalar region as a “test” area. Her first three sessions concentrated on treatment of the lower third of the face, including the submalar regions, the lateral chin, prejowl sulcus, and areas overlying the masseter muscles (Figure 4). Pleased with the effects on the lower third of the face, she elected to continue with volumization of the mid-third of the face, including the medial, mid, and lateral cheeks; inferior temples; zygomatic arches; and preauricular areas (Figure 4). Her last treatment involved touch ups in the temple and nasolabial folds. She remains pleased with her results and has not experienced any adverse events. She also received a total of 45 units of botulinum toxin to smooth the forehead and crow’s feet.
In post-facelift patients, injectable PLLA may also provide volume restoration. As the edema fades, patients may be treated with injectable PLLA approximately 4 to 6 months (or longer) post-facelift to restore volume. This 3-D volumization of the face will tighten lax skin, which may reappear as post-surgical swelling resolves. In the elderly patient (generally >65 years) who may have had one or more facelifts, additional surgery and general anesthesia may pose a risk and/or a cosmetically unacceptable appearance. Some patients may also be averse to additional surgery. Figure 5 shows a 78-year-old woman who had her first facelift at age 69 and a second facelift at age 71 to correct the unsatisfactory results of the initial procedure. After both facelifts, she remained concerned about her age-related redundant folds, laxity, and pleated appearance of the perioral skin. She desired a nonsurgical option with longevity. Injectable PLLA was layered from the level of the dermal-subcutaneous junction to the supraperiosteal layer. The regions injected included the nasolabial folds, the submalar areas, prejowl sulcus, lateral chin, and marionette lines (Figure 5). No adverse events have been reported to date.
These patient cases demonstrate that in individual instances there may be clinical reasons for not adhering to the guidelines outlined above or to the FDA-approved instructions for use. However, the approach used in these patients depends on individualized therapy administered by an experienced and well-trained physician.
Discussion
Injectable medical devices for soft-tissue augmentation have become safe and effective alternatives to surgical procedures while minimizing the downtime, costs, and risks associated with general anesthesia. Injectable devices, however, are not meant to replace aesthetic surgery but to enhance a surgically performed facelift. Where surgical procedures reposition facial tissues in 1 or 2 planes, injectable devices replace lost facial volume for 3-D augmentation of the aging face.
The duration of aesthetic effect of an injectable medical device depends mostly on the specific product. Injectable PLLA provides a longer duration of effect when compared with hyaluronic acid derivatives, calcium hydroxylapatite, and bovine- or human-derived collagen. In a randomized study of injectable PLLA for the treatment of nasolabial fold wrinkles in immune-competent subjects, significant improvements from baseline in wrinkle assessment score were maintained from Week 3 through Month 25 (p<0.001 at all time points).38 Because the effect of injectable PLLA is gradual, it is essential to allow sufficient time between treatment sessions to avoid overcorrection, to ensure that the effect of the previous treatment has occurred, and to assess the necessity for additional treatments. This process of treat, wait, and assess helps to facilitate optimal results while minimizing potential adverse events.1,29 Understanding this approach and obtaining proper training for 3-D volumization with injectable PLLA will enable physicians to maximize volume restoration for their aesthetic surgery patients.
References
- 1.Sherman RN. Sculptra: the new three-dimensional filler. Clin Plast Surg. 2006;33(4):539–550. doi: 10.1016/j.cps.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Friedman O. Changes associated with the aging face. Facial Plast Surg Clin North Am. 2005;13(3):371–380. doi: 10.1016/j.fsc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Glogau RG. Aesthetic and anatomic analysis of the aging skin. Semin Cutan Med Surg. 1996;15(3):134–138. doi: 10.1016/s1085-5629(96)80003-4. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez OM. Full face rejuvenation in three dimensions: a “face-lifting” for the new millennium. Aesthetic Plast Surg. 2001;25(3):152–164. doi: 10.1007/s002660010114. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers JD, Carruthers A. Facial sculpting and tissue augmentation. Dermatol Surg. 2005;31(11 pt 2):1604–1612. doi: 10.2310/6350.2005.31248. [DOI] [PubMed] [Google Scholar]
- 6.de Maio M. The minimal approach: an innovation in facial cosmetic procedures. Aesthetic Plast Surg. 2004;28(5):295–300. doi: 10.1007/s00266-004-0037-1. [DOI] [PubMed] [Google Scholar]
- 7.Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg. 2004;20(1):21–29. doi: 10.1055/s-2004-822955. [DOI] [PubMed] [Google Scholar]
- 8.Gordon NA, Koch ME. Duration of anesthesia as an indicator of morbidity and mortality in office-based facial plastic surgery: a review of 1200 consecutive cases. Arch Facial Plast Surg. 2006;8(1):47–53. doi: 10.1001/archfaci.8.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Le Louarn CL, Buthiau D, Buis J. Structural aging: the facial recurve concept. Aesthetic Plast Surg. 2007;31(3):213–218. doi: 10.1007/s00266-006-0024-9. [DOI] [PubMed] [Google Scholar]
- 10.Rohrich RJ, Pessa JE. The fat compartments of the face: anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119(7):2219–2227. doi: 10.1097/01.prs.0000265403.66886.54. [DOI] [PubMed] [Google Scholar]
- 11.Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg. 2000;26(12):1107–1112. [PubMed] [Google Scholar]
- 12.Mendelson BC, Hartley W, Scott M, et al. Age-related changes of the orbit and midcheek and the implications for facial rejuvenation. Aesthetic Plast Surg. 2007;31(5):419–423. doi: 10.1007/s00266-006-0120-x. [DOI] [PubMed] [Google Scholar]
- 13.Pessa JE, Chen Y. Curve analysis of the aging orbital aperture. Plast Reconstr Surg. 2002;109(2):751–755. doi: 10.1097/00006534-200202000-00051. [DOI] [PubMed] [Google Scholar]
- 14.Shaw RB, Jr, Kahn DM. Aging of the midface bony elements: a three-dimensional computed tomographic study. Plast Reconstr Surg. 2007;119(2):675–681. doi: 10.1097/01.prs.0000246596.79795.a8. [DOI] [PubMed] [Google Scholar]
- 15.Vleggaar D, Fitzgerald R. Dermatological implications of skeletal aging: a focus on supraperiosteal volumization for perioral rejuvenation. J Drugs Dermatol. 2008;7(3):209–220. [PubMed] [Google Scholar]
- 16.Juvederm Ultra Plus [package insert] Santa Barbara, Calif: Allergan, Inc; 2008. [Google Scholar]
- 17.Narins RS, Bowman PH. Injectable skin fillers. Clin Plast Surg. 2005;32(2):151–162. doi: 10.1016/j.cps.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Sculptra [package insert] Bridgewater, NJ: Dermik Laboratories, a business of Sanofi-Aventis U.S. LLC; 2006. [Google Scholar]
- 19.Radiesse Injectable Implant [package insert] Franksville, WI: BioForm Medical Inc.; 2006. [Google Scholar]
- 20.Cohen SR, Berner CF, Busso M, et al. ArteFill: a long-lasting injectable wrinkle filler material—summary of the U.S. Food and Drug Administration trials and a progress report on 4- to 5-year outcomes. Plast Reconstr Surg. 2006;118(3 suppl):64S–76S. doi: 10.1097/01.prs.0000234873.00905.a4. [DOI] [PubMed] [Google Scholar]
- 21.Narins RS, Beer K. Liquid injectable silicone: a review of its history, immunology, technical considerations, complications, and potential. Plast Reconstr Surg. 2006;118(3 Suppl):77S–84S. doi: 10.1097/01.prs.0000234919.25096.67. [DOI] [PubMed] [Google Scholar]
- 22.Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005;31(11 pt 2):1616–1625. [PubMed] [Google Scholar]
- 23.Sculptra Aesthetic [package insert] Bridgewater, NJ: Dermik Laboratories, a business of Sanofi-Aventis U.S. LLC; 2009. [Google Scholar]
- 24.Gogolewski S, Jovanovic M, Perren SM, et al. Tissue response and in-vivo degradation of selected polyhydroxyacids: polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA) J Biomed Mater Res. 1993;27(9):1135–1148. doi: 10.1002/jbm.820270904. [DOI] [PubMed] [Google Scholar]
- 25.Lemperle G, Morhenn VB, Pestonjamasp V, Gallo RL. Migration studies and histology of injectable microspheres of different sizes in mice. Plast Reconstr Surg. 2004;113(5):1380–1390. doi: 10.1097/01.prs.0000112764.22839.7a. [DOI] [PubMed] [Google Scholar]
- 26.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27(5):354–366. doi: 10.1007/s00266-003-3022-1. [DOI] [PubMed] [Google Scholar]
- 27.Keni SP, Sidle DM. Sculptra (injectable poly-L-lactic acid) Facial Plast Surg Clin North Am. 2007;15(1):91–7, vii. doi: 10.1016/j.fsc.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Vleggaar D. Facial volumetric correction with injectable poly-L-lactic acid. Dermatol Surg. 2005;31(11 pt 2):1511–1517. doi: 10.2310/6350.2005.31236. [DOI] [PubMed] [Google Scholar]
- 29.Lowe NJ. Optimizing poly-l-lactic acid use. J Cosmet Laser Ther. 2008;10(1):43–46. doi: 10.1080/14764170701840074. [DOI] [PubMed] [Google Scholar]
- 30.Lam SM, Azizzadeh B, Graivier M. Injectable poly-L-lactic acid (Sculptra): technical considerations in soft-tissue contouring. Plast Reconstr Surg. 2006;118(3 suppl):55S–63S. doi: 10.1097/01.prs.0000234612.20611.5a. [DOI] [PubMed] [Google Scholar]
- 31.Narins RS. Minimizing adverse events associated with poly-L-lactic acid injection. Dermatol Surg. 2008;34(suppl 1):S100–S104. doi: 10.1111/j.1524-4725.2008.34250.x. [DOI] [PubMed] [Google Scholar]
- 32.Rotunda AM, Narins RS. Poly-L-lactic acid: a new dimension in soft tissue augmentation. Dermatol Ther. 2006;19(3):151–158. doi: 10.1111/j.1529-8019.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 33.Beer K. Optimizing patient outcomes with collagenic stimulators. J Drugs Dermatol. 2007;6(suppl 1):S9–S12. [Google Scholar]
- 34.Ascher B, Coleman S, Alster T, et al. Full scope of effect of facial lipoatrophy: a framework of disease understanding. Dermatol Surg. 2006;32(8):1058–1069. doi: 10.1111/j.1524-4725.2006.32230.x. [DOI] [PubMed] [Google Scholar]
- 35.James J, Carruthers A, Carruthers J. HIV-associated facial lipoatrophy. Dermatol Surg. 2002;28(11):979–986. doi: 10.1046/j.1524-4725.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 36.Woerle B, Hanke CW, Sattler G. Poly-L-lactic acid: a temporary filler for soft tissue augmentation. J Drugs Dermatol. 2004;3(4):385–389. [PubMed] [Google Scholar]
- 37.Lowe NJ. Dispelling the myth: appropriate use of poly-L-lactic acid and clinical considerations. J Eur Acad Dermatol Venereol. 2006;20(suppl 1):2–6. doi: 10.1111/j.1468-3083.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- 38.Narins RS, Baumann L, Brandt FS, et al. A randomized study of the efficacy and safety of injectable poly-L-lactic acid versus human-based collagen implant in the treatment of nasolabial fold wrinkles. J Am Acad Dermatol. 2010;62(3):448–462. doi: 10.1016/j.jaad.2009.07.040. [DOI] [PubMed] [Google Scholar]