Abstract
The number of products available to dermatologists for soft tissue augmentation has grown significantly over the past several years in the United States. This manuscript reviews the various hyaluronic acid fillers and other United States Food and Drug Administration-approved products utilized for patients in the rejuvenation process. It is the hope that through this paper, clinicians will feel more comfortable using these products in their everyday practice of dermatology.
Introduction
The world of fillers has changed significantly over the past several years and clinicians now have at their disposal a number of very reliable and safe products available to use for soft tissue augmentation. The purpose of this manuscript is to review the fillers currently available in the United States and to comment on some of the newer fillers that are available in other parts of the world and may become available in the United States at some point in the near future.
The popularity of fillers dates back to the early 1980s when bovine collagen was introduced to treat lines and wrinkles as well as volume defects. Since then, numerous fillers have been introduced to the market and now the most popular injected fillers are hyaluronic acids (HAs). Several other filler products that have also been shown to be effective and are available in the United States will also be reviewed in this manuscript.
The popularity of these new fillers parallels the use of botulinum toxin A in the US market. Botulinum toxin A injections for lines and wrinkles has become the most popular cosmetic procedure performed in the United States and worldwide. According to the American Society for Aesthetic Plastic Surgery (ASAPS), there were 2,557,068 botulinum toxin A procedures performed and 1,313,038 HA injections performed in the United States in 2009. This makes the injection of HAs the second most common cosmetic procedure performed in the United States.1 What is interesting and of note in regard to these statistics is that for many years, ASAPS data had listed laser hair removal as the number two cosmetic procedure being performed in the United States; now it is number three. And with other fillers besides HA available in the United States, the actual number of filler injections is higher than the ASAPS numbers. As noted, filler injections are popular in the United States, and with newer fillers becoming available and an increase in consumer awareness of these products, the number of filler procedures will likely rise in the next several years.
What do clinicians want in their filler? What makes the “ideal” filler? These are questions that we have been asking for almost 30 years. Are we closer to that ideal filler than ever? Or do we still have more work to do in the development process for fillers? The ideal filler, according to most, would be easy to inject, consistently provide reproducible results, and have longevity, which makes the filler procedure worthwhile to the patient receiving the therapy. How long each filler injection should last is also a question that has been around for years. Many have argued various positions over the years, but most would now agree that fillers should last for at least one year and perhaps as long as two years. Any filler that lasts less than one year, at least at this point, seems to have fallen out of favor among most clinicians. Discussions regarding pain upon injection have been around for many years, and most of the commonly used fillers in 2010 have lidocaine for patient comfort, which has been a major accomplishment in 2010. Physicians also want fillers to be nonallergenic, nonteratogenic, and to have no potential allergies from the injection itself. This means that skin testing, which was commonplace when the bovine collagens were popular, is something that physicians want to avoid as do the patients who often want their injections on the day of their consultations. Additionally, physicians want fillers to have long shelf lives, be free from any transmissible diseases, have minimal potential for untoward effects (which can be easily treated if they do arise), and be cost-appropriate, which means affordable to both patients and clinicians.2
Several different classifications for fillers have been used over the years. For simplicity and for the purpose of this manuscript, fillers can be divided into non-permanent, semi-permanent, and permanent categories.3 Most clinicians are more comfortable injecting the non-permanent and semi-permanent fillers; however, there is a place for the permanent variety, especially in the hands of skilled physician injectors.
In the United States in 2010, non-permanent fillers comprise HA fillers, which include Restylane® and Perlane® (Medicis Aesthetics Inc., Scottsdale, Arizona) along with their new lidocaine fillers, Juvederm Ultra and Juvederm Ultra Plus (Allergan, Inc., Irvine, California) with their new lidocaine fillers, Hydrelle (Anika Therapeutics, Bedford, Massachusetts), and Prevelle Silk (Mentor Corporation, Santa Barbara, California). The semi-permanent fillers comprise two fillers—poly L-lactic acid (Sculptra, Sanofi-Aventis US LLC, Bridgewater, New Jersey) and calcium hydroxylapetite (Radiesse, BioForm Medical, Inc., San Mateo, California). The permanent filler category has one United States Food and Drug Administration (FDA)-approved product known as ArteFill (Suneva Medical, Inc., San Diego, California). This article reviews these fillers as well as some of the newer products being evaluated for FDA submission, which may be available in the future.
HA Fillers
HA fillers comprise the largest group of fillers currently available in the United States. HA, or hyaluronan, is a glycosaminoglycan that consists of repeating nonsulfated disaccharide units of glucuronic acid and N-acetylglucosamine.4 HA is a naturally occurring substance, is a biopolymer, and exhibits no species or tissue specificity. HA is an essential component of the extracellular matrix in all animal tissues and is an abundant part of this matrix in all animal species. It is highly hydrophilic and so therefore attracts water to help it form large concentrations, which can occupy large volumes relative to its mass. It forms gels even at low concentrations. When water is drawn into the HA matrix, it creates a swelling pressure or turgor that enables the HA complex to withstand compressive forces. It is these characteristics that have helped make HA fillers the most popular among clinicians injecting patients to improve fine lines and wrinkles, and for volume enhancement. HAs do not exhibit tissue or species specificity, playing a crucial role in minimizing any potential immunological reactions or other allergic potentials, and skin testing is not required with these products. This has been one of the major reasons these products have gained so much popularity in the past several years.
The first HA was developed as a dermal filler in 1989 when Balazs et al described their first injectable HA filler.5 It was not a long-lasting dermal filler, but paved the way for the advent of this new class of fillers. Others soon began looking for new HA fillers and several differentiations have become important in their development. These have included the source of the HA, the concentration of the HA being utilized, the particulate size of the HA, the cross-linking of the HA and type of cross-linking agent being used, whether the HA is monophasic or biphasic, and whether an anesthetic is added to the syringe or not. Some of the original HA fillers used avian rooster combs as the source for their HAs, but more commonly the source is bacteria-based, mainly from the fermentation of the Streptococcus equine bacterium. Most of the newer HA fillers have higher concentrations of HA compared to the original one described. Those HA fillers with higher concentrations of HA may be longer lasting, so those with concentrations of greater than 20mg/mL are considered ideal for HA fillers at this time. Cross-linking is important and most HA fillers utilize ether cross-link bonds to help stabilize the HA. The newer nonparticulate HA fillers contain double cross-linking, multiple cross-linking, or are in monophasic gels, in an attempt to stabilize the molecule even more. Cross-linking makes the HAs less resistant to degradation, making for longer lasting fillers. As a result of the cross-linking process, and because the newer HAs are nonparticulate in formation, they require the higher HA concentrations to prevent biodegradation from free radicals and other enzymatic activity to enhance HA filler duration. 1,4-butanediol diglycidyl ether (BDDA) has been used as the cross-linking agent for many of the HA fillers, as well as newer agents including 1,2,7,8-diepoxyoctane. Also, larger HA particles tend to last longer as fillers and are usually designed for deeper filler injections. Monophasic HA fillers are predominantly cohesive gels rather than just HA particles. Biphasic HA fillers are made into particle form. Each one of these, monophasic and biphasic, has its benefits. Monophasic HA fillers may last longer and may not migrate as much following injection; biphasic HA fillers are more easily customized to particle size per indication and anatomic area being treated.2
The first HA filler in the United States was Restylane. Restylane received its FDA approval in the United States in December 2003, although it received its EU clearance much earlier, in 1996. It has been injected in well over 10 million treatment sessions worldwide and is considered the standard for which all HAs are measured. It is manufactured by Q-Med AB (Uppsala, Sweden) and is marketed in the United States and Canada by Medicis Pharmaceutical Corporation. Restylane is a non-animal stabilized HA, known commonly as NASHA, produced from the fermentation of equine streptococci. It is cross-linked with BDDA, with a one-percent degree of cross-linking. Restylane has an HA concentration of 20mg/mL and its gel particulate size is 400µm. It has a particulate size of 100,000 gel particles per milliliter and was the first of the Restylane family of products available from Q-Med and Medicis. Restylane’s FDA approval is for mid-to-deep dermal implantation for the correction of moderate-to-severe facial wrinkles and folds, such as nasolabial folds. It received an initial FDA approval for six months duration of correction. Restylane has also been successfully used in the treatment of tear trough deformities. The second product released in the United States, which was in the Restylane family, is known as Perlane. Perlane contains 8000 gel particles per milliliter and is indicated for deeper injections and deeper clinical defects. In other parts of the world, this product is known as Restylane Perlane.6
Two pivotal European clinical trials led to the approval of Restylane in Europe. These trials by Duranti et al7 and Olenius8 showed the safety and efficacy of Restylane in correction of the nasolabial folds. In the first trial by Duranti et al, 78 percent of the patients enrolled found they were able to maintain moderate-to-marked clinical improvement at eight months following the injection.7 In the second study, by Olenius, there was correction noted at 12 weeks of 82 percent and at 26 weeks of 69 percent. Adverse events (AEs) noted in these first two clinical trials were predominantly injection-related AEs, including treatment-site erythema, hyperpigmentation at the treatment site, and pain from the injection itself, reported in 13 percent of the patients in these trials. As experience grew with the product and injection techniques were refined, Friedman et al9 re-examined the AE rate in a large series of patients and found that these injection-related AEs were, in fact, only occurring in 0.15 percent of patients receiving Restylane injections.
Shortly after these reports, several cases of what was described as delayed implant hypersensitivity were reported in the European literature10–12 and through these evaluations it was determined that there was a 0.4- to 3.7-percent risk of this occurring following Restylane injection. As a result of this delayed implant hypersensitivity occurring in more than what was felt to be acceptable, Q-Med manufactured a more purified Restylane product, which is the current NASHA product available today. Clinical evaluations of the new purified Restylane used by clinicians who mastered their injection technique found that AEs were reduced to 0.06 percent and hypersensitivity reactions were reduced to 0.02 percent, which is considered acceptable for continued use of this new NASHA.
The United States pivotal clinical trials for Restylane compared Restylane in one nasolabial fold with Zyplast (Allergan) collagen, the then standard collagen injectable material, being injected into the other nasolabial fold. Narins et al13 evaluated 138 individuals. The majority of the patients enrolled were female (93%) and Caucasian (89%). The study showed that optimal correction was achieved in 1.4 sessions for both of the products being injected. The volume needed for volume correction with Restylane showed a mean of 1.0mL (range 0.3–2.8mL) while the amount of Zyplast used showed a mean of 1.6mL (range of 0.1–5.0mL). The Wrinkle Severity Rating Scale (WSRS) score for Restylane was superior at all time points evaluated as compared to the Zyplast side. This was true at two months, four months, and six months following the optimal correction of the nasolabial folds. At the six-month evaluation, Restylane was rated superior in 56.9 percent of patients compared to Zyplast in 9.5 percent of patients. The Global Aesthetic Improvement Scale (GAIS) was also superior for Restylane at all time points, with 62 percent rating Restylane superior at six months, as compared to eight percent rating Zyplast superior.
Adverse events were evaluated at each follow-up visit during the course of the study. Mild-to-moderate injection-site reactions occurred in a similar and nonstatistical fashion with both of the products (93.5% Restylane, 90.6% Zyplast). These were short-lasting in all cases, usually resolving within seven days. For all treatment-related AEs during the evaluation, 26.4 percent were reported for Restylane and 39.1 percent for Zyplast. Delayed-onset reactions were noted in 8.7 percent; all resolved within 2 to 3 months without intervention. There were no reports of hypersensitivity reactions reported during the trial.
Further evaluations have been performed with Restylane over the past several years in the United States.14–16 They have continued to show the safety and efficacy of this product in each and every study. Two further US clinical evaluations are very important and warrant consideration in this manuscript. The first, by Odunze et al,17 evaluated 60 patients who received Restylane injections, one-third of which were injected into darker skin types (Fitzpatrick skin types IV–VI). In their evaluation, they noted no untoward AEs in the darker skin color group, providing evidence that Restylane can be safely injected into patients of all skin types. The second study, by Narins et al,18 also studied Restylane, but looked at repeat injections and longevity associated with those repeat injections. Seventy-five patients were enrolled into this multicenter evaluation of two different retreatment schedules looking at the longevity of those schedules, with data presented at 18 months. The patients were randomized to receive retreatment of one of their nasolabial folds at 4.5 months and the contralateral fold at nine months after correction of both folds at the initial visit. The WSRS improved significantly (p<0.001) from baseline, with mean improvement noted from 1.1 to 1.7 grades. Ninety-seven percent of all the patients responded to this retreatment program, and the efficacy of the retreatment schedules did not differ significantly. AEs reported, all local and consisting of swelling and bruising at the treatment site, occurred in 33 percent and were not rated as serious in this study. Thus, Restylane was shown to maintain correction at 18 months following repeat injection at 4.5 months. This study led to a second submission to the FDA, known as a supplemental Premarket Approval Application for its label, giving a new indication for Restylane—longevity up to 18 months with repeat injection at 4.5 months.
Following the approval of Restylane, Perlane received its FDA approval for deeper dermal defects, especially in those who have deep nasolabial folds and for other lines and wrinkles that require a larger particle size HA filler.
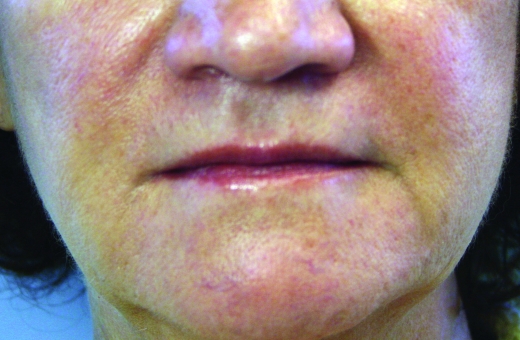
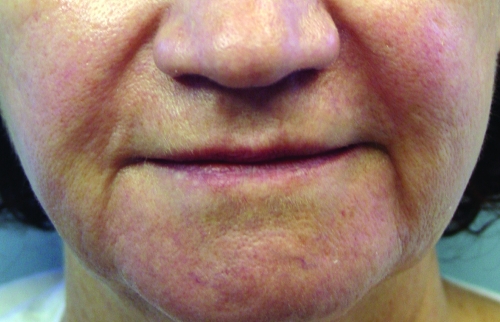
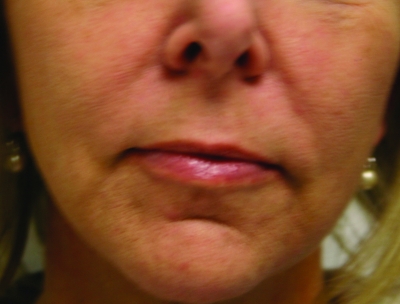
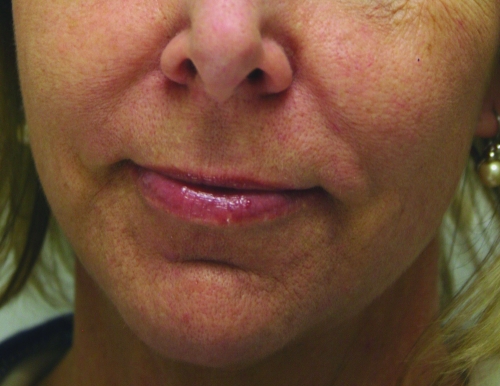
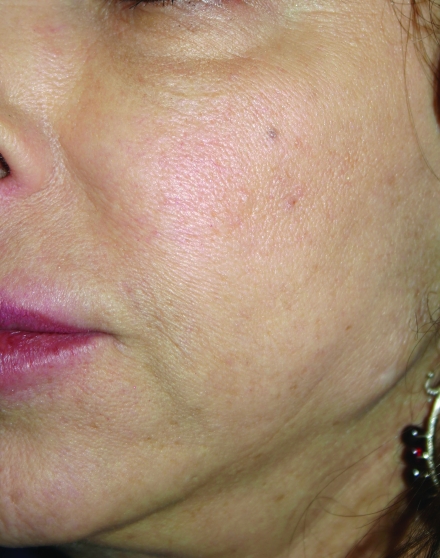
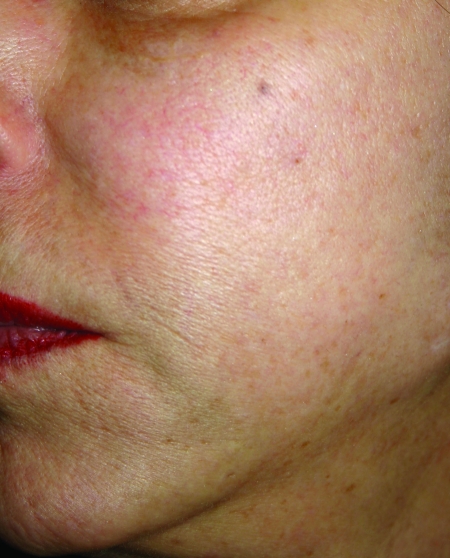
In February 2010, Restylane-L and Perlane-L were approved by the FDA. These are lidocaine-containing products of Restylane and Perlane and the clinical trials performed for these products confirmed their safety and efficacy in reducing the pain associated with injection of these fillers. In the clinical trial performed, 71.7 percent of patients in the Restylane-L study and 95 percent of patients in the Perlane-L study had a within-patient difference in the validated visual analog scale (VAS) of at least 10mm favoring Restylane-L and Perlane-L.19 A clinical example of Restylane is shown in Figure 1.
Figure 1.
Before treatment (A) and post-treatment with Restylane-L (3mL) to the nasolabial folds (B)
The second group of HAs available in the US is known as Juvederm. Juvederm is manufactured by Lea Derm, a subsidiary of Corneal Group (Paris, France). It was brought to the United States by Inamed, which was acquired by Allergan, Inc. in 2006. Allergan, the makers of Botox, are the worldwide distributors for Juvederm. There are four current formulations of Juvederm available in the United States—Juvederm Ultra and Juvederm Ultra Plus, along with their lidocaine equivalents, known as Juvederm Ultra XC and Juvederm Ultra Plus XC. The available United States products contain 24mg/mL of HA respectively with Juvederm Ultra Plus containing 24mg/mL of HA in high viscosity. The original US Juvederm formulations were FDA approved in June 2006—Juvederm Ultra for deep wrinkles and defects; Juvederm Ultra Plus for deeper furrows, such as the nasolabial folds. The Juvederm family is produced from the bacterial fermentation of equine streptococci. The HA is cross-linked with a patented, single-phase, BDDE-phosphate buffered to 6.5 to 7.3 pH. With a higher concentration of HA and more cross-linking than other HA fillers, it is felt that perhaps the Juvederm family of products may persist longer than other HA fillers and have a more smooth injection flow.20
The pivotal US clinical trial by Baumann et al21 was a comparison of three Juvederm products compared to Zyplast collagen in the treatment of nasolabial folds. Four hundred twenty-three patients completed the clinical trial of a 24-week evaluation. More than 300 patients received an additional treatment of the HA filler at the conclusion of the clinical trial to further examine the long-term effects. Results showed that the Juvederm used and the Zyplast collagen showed significant improvements at all points in the 24-week clinical trial. The Juvederm family of three products studied showed significantly greater efficacy than the bovine collagen product; the efficacy increased with time and was greatest at 24 weeks after the last treatment. Utilizing a 4-point scale, an improvement of at least 1 point was seen in more than 80 percent of Juvederm-treated patients compared to a 0.5 improvement, on average, in the Zyplast-treated side. For those having an end-of-24-week injection, long-term results showed that 57 percent had duration of effect at eight months, 37 percent at 10 months, and 18 percent at 12 months.
AEs were similar for both the Juvederm and Zyplast sides that were treated and were similar for all of the Juvederm products studied. Mild-to-moderate treatment-site reactions were seen in the majority of patients, all of which were resolved within seven days. No long-term adverse reactions were noted. Patient preference data suggested a 78-percent preference with Juvederm 30, 88 percent with Juvederm 24HV, and 84 percent with Juvederm 30HV. From this clinical study, Juvederm 24HV and Juvederm 30HV were chosen for the US market, both of which contain 24mg/mL of HA, with Juvederm Ultra having nine-percent cross-linking and Juvederm Ultra Plus having 11-percent cross-linking.
Patients were included in a longer term follow up during this multi-center clinical trial and showed longevity at one year following optimal correction; henceforth, Juvederm received FDA clearance for up to one year.22
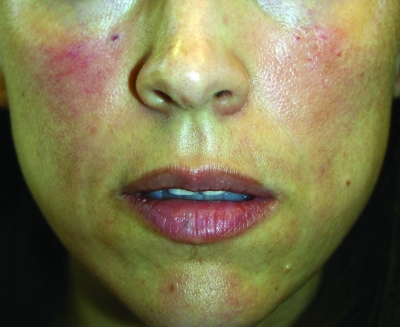
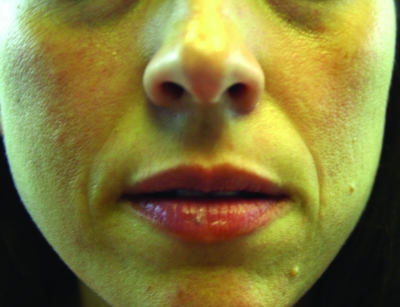
A clinical example of Juvederm is shown in Figure 2. A lidocaine-containing Juvederm clinical trial was recently published and clinical data support its effectiveness in reducing the pain associated with injections of Juvederm.23 The mean difference in procedural pain during the clinical trial was 3.4 (p<0.00001) and 93 percent of the patients felt there was less pain associated with the incorporation of lidocaine in this side-by-side clinical evaluation and less pain noted after the injection as well. Improvement in nasolabial fold correction was equal for the products studied. Juvederm Ultra XC and Juvederm Ultra Plus XC with lidocaine have been approved since early 2010.
Figure 2.
Before treatment (A) and immediately post-treatment with Juvederm (1cc) to the nasolabial folds and tear trough (B)
The next HA filler to be described is known as Hydrelle, originally called Elevess. Hydrelle is marketed through Coapt Systems (Palo Alto, California) and is manufactured by Anika Therapeutics. Hydrelle contains the highest concentration of HA on the market, 28mg/mL and it also contains 0.3% lidocaine, the first of the US products receiving FDA clearance for an HA with lidocaine. It is cross-linked with p-phenylene bisethyl carbodiimide or biscarbodiimide, or BCDI, which is a novel HA cross-linker. Its source of HA is from equine streptococci. The US clinical pivotal study for Hydrelle (Elevess) studied 191 individuals who received Elevess in one nasolabial fold and CosmoPlast (Allergan) in the other nasolabial fold. Patients had a significant improvement in the Elevess side at both four and six months following optimal correction. AEs were similar in both groups and not significant. They consisted mainly of treatment-site reactions and were resolved in the majority of cases within seven days (personal communication, Anika Therapeutics, 2008). Patients who still were improved at the six-month time frame were eligible to enter a nine (n=90) and 12 (n=84) month extension follow-up clinical trial. Patients maintained their improvement at these time frames as well; with the Elevess side improved more so than the CosmoPlast side (personal communication, Anika Therapeutics, 2008). The adoption of this filler has not been widespread at the time of this writing.
The next HA filler that received FDA approval is known as Prevelle Silk. This product is the second generation of an earlier HA filler known as Captique, which is no longer available. Captique was manufactured by Genzyme Corporation (Cambridge, Massachusetts) and was originally marketed by Inamed, then Allergan, and was sold to Mentor Corporation. Recently, Mentor was purchased by Johnson & Johnson (Skillman, New Jersey). The product contains 4.5 to 6.0mg/mL of HA, is 20-percent cross-linked with divinyl sulfone, and has a gel particle size of 500µm. Because of the low concentration of HA in the product, clinical results were of short duration in the 3- to 6-month time period.
Prevelle Silk combines Captique with 0.3% lidocaine. The pivotal trial for this product, conducted by Monheit et al,24 showed that Prevelle Silk had a significant difference in pain associated with the injection process and pain postprocedure. Patient preference was also significantly in favor of the Prevelle Silk over Captique. Mentor also has another HA product pending FDA approval. It is known as Dermal Gel Extra (DGE), or Prevelle Lift. This product, with lidocaine, may be available in the United States later this year. It is also a more robust HA filler, and clinical trials have demonstrated its safety and efficacy.
Semi-Permanent Fillers
The semi-permanent fillers include Sculptra and Radiesse. Radiesse, also known as calcium hydroxylapetite (CaHA), are synthetic CaHA microspheres (30%) suspended in a carboxy-methylcellulose resorbable aqueous gel carrier (70%). This process allows for the body’s stimulation of collagen. Skin testing is not required for Radiesse injections. Radiesse was approved by the FDA in December 2006 and has indications for the treatment of facial wrinkles and folds as well as the correction of facial wasting as a result of HIV-associated lipoatrophy. Radiesse was the first filler to receive these two FDA indications. Pivotal US clinical trials for both of these indications showed significant improvements,25,26 and many studies have demonstrated longevity with Radiesse for over one year and up to two years.27–29
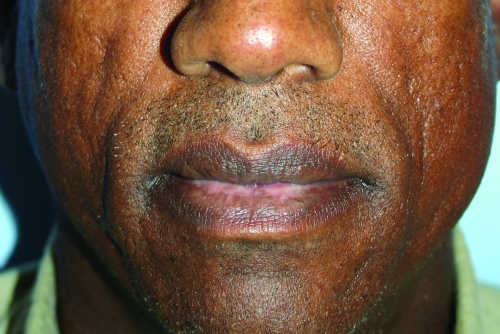
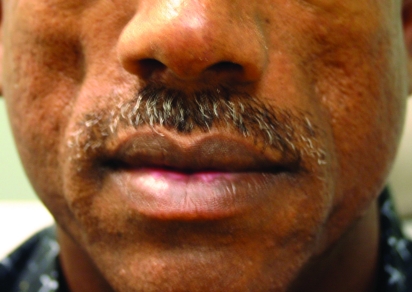
Radiesse has found a home with many clinicians who are looking for a more “robust” filler and long-lasting results. It also has become one of the favorite fillers for hand rejuvenation. Physicians have been injecting Radiesse into the dorsal of the hands and massaging the area to mold the Radiesse into the skin; however, this indication for Radiesse is not FDA approved. Many clinicians also have incorporated lidocaine into the Radiesse syringe through an adaptor process, which has recently received FDA approval.30,31 Clinical studies post-FDA approvals have shown Radiesse is safe in patients of skin of color and studies comparing Radiesse to HA fillers have shown that Radiesse lasts longer than its HA equivalents. A clinical example of Radiesse is shown in Figure 3.
Figure 3.
Before treatment (A) and immediately post-treatment with Radiesse (1.3cc) to the nasolabial folds (B)
Sculptra, or poly-L-lactic acid, has been in the US market for the past several years with FDA approval (2004) to treat HIV-associated lipoatrophy. In July 2009, Sculptra received FDA clearance to treat lines and wrinkles for aesthetic considerations. It is best used as a volume-enhancement treatment and it requires several treatment sessions to achieve the desired effect.
In the United States, two pivotal FDA clinical trials were performed in HIV-associated lipoatrophy patients. They are known as the APEX002 (n=95) and the Blue Pacific (n=68) studies. Both of these studies showed the effectiveness of Sculptra in HIV-associated lipoatrophy.32,33 More recent studies confirm these original trial results and the effectiveness of Sculptra for several years duration.34,35
As noted, patients receive a series of injections in order for Sculptra to achieve its full correction. Clinicians usually space the injections 4 to 6 weeks apart and inform their patients with HIV-lipoatrophy that from 2 to 4 injection sessions may be required for the poly-L-lactic acid to stimulate new collagen and reverse the signs of lipoatrophy. For cosmetic enhancement, 1 to 3 sessions are usually sufficient. There are also various techniques to prepare the product for injection and each clinician will develop their “favorite” technique. In the author’s practice, they usually mix 5cc of sterile water with 1cc of 0.3% lidocaine and let the medicine set for 24 hours prior to the Sculptra injection. An example of a patient treated with Sculptra is shown in Figure 4.
Figure 4.
Before treatment (A) and post-treatment with Sculptra (6cc) (B)
Permanent Fillers
The last of the fillers that will be reviewed is a more permanent filler known as ArteFill. This filler has had several precursor fillers and is currently being promoted by Suneva Medical, Inc.. This filler comprises polymethyl methacrylate (PMMA) microspheres suspended in a more rapidly dissolving bovine collagen carrier with 0.3% lidocaine added to the syringe. It was designed in this fashion to induce “reactive” long-term collagen deposition. The PMMA microspheres are from 30 to 50µm in size, too big to be phagocytized within the body but small enough to easily be injected through a 26-gauge needle. This product, first known as ArtePlast, then ArteColl, and now ArteFill has had a rocky history. The previous generation products differ from today’s products in many ways, but the current product is safe and effective. ArteFill received FDA approval in October 2006. In the US pivotal clinical trial, ArteFill was compared to Zyplast or Zyderm collagen in the nasolabial folds. Two hundred fifty-one patients were enrolled in this trial and at six months the collagen sides were crossed-over to also receive ArteFill. At six months, there was a significant change in the nasolabial folds that received ArteFill, while the collagen sides had returned to their baselines. AEs were similar between the groups.36 Safety studies for ArteFill continued successfully for 12 months. Five-year ArteFill data is also available.37 Currently, there is an ongoing five-year safety trial for ArteFill, now in year three and showing very good safety data to this point.38
ArteFill is a very nice filler for patients with deep dermal defects who understand that the filler being placed will last anywhere from 1 to 5 years, depending on numerous factors, including the skill level of the injector and the proper placement of the product. A patient treated with ArteFill is shown in Figure 5.
Figure 5.
Before treatment (A) and post-treatment with Artefill (2.4cc) to the nasolabial folds (B)
Conclusion
Many new dermal fillers have been introduced over the past several years, some of which are available in the United States. Many other filler products exist in Europe and elsewhere around the world and are likely to be available in the United States over the next several years. These products include Prevelle Lift, Merz’s Belotero, and three HA fillers already available in Europe and poised to enter the United States in 2010. Other companies exploring HA fillers include Pierre Fabre, the makers of Glytone, with two HA fillers currently available in Europe, and Teosyal, a European group that markets the popular European HA filler known as Teoxane. These fillers are more likely to enter the United States in 2011 or later, and others are sure to join them.
References
- 1.American Society for Aesthetic Plastic Surgery. [July 22, 2010]. Cosmetic Surgery National Data bank: 2009 Statistics. www.surgery.org/sites/ default/files/2009stats.pdf.
- 2.Gold MH. Use of hyaluronic acid fillers for the treatment of the aging face. Clin Interventions in Aging. 2007;2(3):369–376. doi: 10.2147/cia.s1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith KC. Reversible vs. nonreversible fillers in facial aesthetics: concerns and considerations. [July 22, 2010];Derm Online Journal. 2008 14(8) http//dermatology.cdlib.org/148/commentary/facial_aesthetics/smith.html. [PubMed] [Google Scholar]
- 4.Alberts B, Johnson A, Lewis J, et al., editors. The Molecular Biology of the Cell. Cell Junctions, Cell Adhesion, and the Extracellular Matrix. New York: Garland Sciences; 2002. pp. 1065–1126. [Google Scholar]
- 5.Balazs EA, Demlinger JL. Clinical uses of hyaluronan. Ciba Found Symp. 1989;142:265–275. doi: 10.1002/9780470513774.ch16. [DOI] [PubMed] [Google Scholar]
- 6.Matarasso SL, Carruthers JD, Jewell ML, et al. Consensus recommendations for soft tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane) Plast Reconstr Surg. 2006;117(s):3s–34s. doi: 10.1097/01.prs.0000204759.76865.39. [DOI] [PubMed] [Google Scholar]
- 7.Duranti F, Salti G, Bovani B, et al. Injectable hyaluronic acid gel for soft tissue augmentation: a clinical and histologic study. Dermatol Surg. 1998;24:1317–1325. doi: 10.1111/j.1524-4725.1998.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 8.Olenius M. The first clinical study using a new biodegradable implant for the treatment of the lips, wrinkles, and folds. Aesthet Plast Surg. 1998;22:97–101. doi: 10.1007/s002669900172. [DOI] [PubMed] [Google Scholar]
- 9.Friedman PM, Mafong EA, Kauvar ANB, et al. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002;28:491–494. doi: 10.1046/j.1524-4725.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- 10.Lowe NJ, Maxwell CA, Lowe P, et al. Hyaluronic acid skin fillers: adverse reactions and skin testing. J Am Acad Dermatol. 2001;45:930–933. doi: 10.1067/mjd.2001.117381. [DOI] [PubMed] [Google Scholar]
- 11.Lupton JR, Alster TS. Cutaneous hypersensitivity reaction to injectable hyaluronic acid. Dermatol Surg. 2000;26:135–137. doi: 10.1046/j.1524-4725.2000.99202.x. [DOI] [PubMed] [Google Scholar]
- 12.Micheels P. Human anti-hyaluronic acid antibodies: is it possible? Dermatol Surg. 2001;27:185. doi: 10.1046/j.1524-4725.2001.00248.x. [DOI] [PubMed] [Google Scholar]
- 13.Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–595. doi: 10.1046/j.1524-4725.2003.29150.x. [DOI] [PubMed] [Google Scholar]
- 14.Baumann LS, Shamban AT, Lupo MP, et al. Comparison of hyaluronic acid-based fillers with cross-linked bovine collagen: a double-masked, multicenter, randomized, within-subject study. Poster presented at: American Academy of Dermatology 64th Annual Meeting; March 3-7, 2006; San Francisco, CA. [Google Scholar]
- 15.DeLorenzi C, Weinberg M, Solish N, Swift A. Multicenter study of the efficacy and safety of subcutaneous non-animal-stabilized hyaluronic acid in aesthetic facial contouring: interim report. Dermatol Surg. 2006;32:2105–2111. doi: 10.1111/j.1524-4725.2006.32035.x. [DOI] [PubMed] [Google Scholar]
- 16.Rao J, Chi GC, Goldman MP. Clinical comparison between two hyaluronic acid-derived fillers in the treatment of the nasolabial folds: Hylaform versus Restylane. Dermatol Surg. 2005;31:1591–1598. doi: 10.2310/6350.2005.31245. [DOI] [PubMed] [Google Scholar]
- 17.Odunze M, Cohn A, Few JW. Restylane and people of color. Plast Reconstr Surg. 2007;120:2011–2016. doi: 10.1097/01.prs.0000287330.94038.63. [DOI] [PubMed] [Google Scholar]
- 18.Narins RS, Dayan SH, Brandt FS, Baldwin EK. Persistence and improvement of nasolabial fold correction with nonanimal-stabilized hyaluronic acid 100,000 gel particles/mL filler on two retreatment schedules: results up to 18 months on two treatment schedule. Dermatol Surg. 2008;34:S2–S8. doi: 10.1111/j.1524-4725.2008.34236.x. [DOI] [PubMed] [Google Scholar]
- 19. [July 26, 2010]; Restylane-L and Perlane-L dermal fillers approved. Available at www.empr.com/restylane-l-and-perlane-l-dermal-fillers-approved/article/163061/ [Google Scholar]
- 20. [May 2010]; Juvederm Fact Sheet. http://www.allergan.com/assets/pdf/juvederm_ultra_and_juvederm_ultra_plus_fact_sheet.pdf. [Google Scholar]
- 21.Baumann LS, Shamban AT, Lupo MP, Monheit GD, et al. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: a multicenter, double-masked, randomized, within-subject study. Dermatol Surg. 2007;33(Suppl 2):S128–S135. doi: 10.1111/j.1524-4725.2007.33352.x. [DOI] [PubMed] [Google Scholar]
- 22.Allemann I, Baumann L. Hyaluronic acid gel (Juvederm™) preparations in the treatment of facial wrinkles and folds. Clin Interventions in Aging. 2008;3(4):629–634. doi: 10.2147/cia.s3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinkle SH, Bank DE, Boyd CM, Gold MH, et al. A multi-center, double-blind, randomized controlled study of the safety and effectiveness of Juvéderm injectable gel with and without lidocaine. J Cosm Derm. 2009;8:205–210. doi: 10.1111/j.1473-2165.2009.00451.x. [DOI] [PubMed] [Google Scholar]
- 24.Monheit, Holmdahl L, Garcia E. Reduced pain with the use of Prevelle Silk, a divinyl sulfone cross-linked non-animal derived hyaluronic acid dermal gel formulated with lidocaine, for correction of nasolabial folds. Epresentation; ASAPS; May 2009.
- 25.Moers-Carpi MM, Tufet JO. Calcium hydroxylapatite versus nonanimal stabilized hyaluronic acid for the correction of nasolabial folds: a 12-month, multicenter, prospective, randomized, controlled, split-face trial. Dermatol Surg. 2008;34:210–215. doi: 10.1111/j.1524-4725.2007.34039.x. [DOI] [PubMed] [Google Scholar]
- 26.Carruthers A, Carruthers J. Evaluation of injectable calcium hydroxylapatite for the treatment of facial lipoatrophy associated with human immunodeficiency virus. Dermatol Surg. 2008;34:1486–1499. doi: 10.1111/j.1524-4725.2008.34323.x. [DOI] [PubMed] [Google Scholar]
- 27.Sadick NS, Katz BE, Roy D. A multicenter, 47-month study of safety and efficacy of calcium hydroxylapatite for soft tissue augmentation of nasolabial folds and other areas of the face. Dermatol Surg. 2007;33:122–126. doi: 10.1111/j.1524-4725.2007.33351.x. [DOI] [PubMed] [Google Scholar]
- 28.Jacovella PF, Peiretti CB, Cunille D, Salzamendi M, Schechtel SA. Long-lasting results with hydroxylapatite (Radiesse) facial filler. Plast Reconstr Surg. 2006;118:15–21. doi: 10.1097/01.prs.0000234902.61284.c9. [DOI] [PubMed] [Google Scholar]
- 29.Beer K, Yohn M, Cohen JL. Evaluation of injectable CaHA for the treatment of mid-face volume loss. J Drugs Dermatol. 2008;7:359–366. [PubMed] [Google Scholar]
- 30.Busso M, Voigts R. An investigation of changes in physician properties of injectable calcium hydroxylapetite in a carrier gel when mixed with lidocaine and with lidocaine-epinephrine. Dermatol Surg. 2008;34:S16–S24. doi: 10.1111/j.1524-4725.2008.34238.x. [DOI] [PubMed] [Google Scholar]
- 31.Marmur E, Green L, Busso M. Controlled, randomized study of pain levels in subjects treated with calcium hydroxylapatite premixed with lidocaine for correction of nasolabial folds. Dermatol Surg. 2010;36(3):309–315. doi: 10.1111/j.1524-4725.2009.01435.x. [DOI] [PubMed] [Google Scholar]
- 32.Valantin MA, Aubron-Olivier C, Ghosn J, Laglenne E, Pauchard M, Schoen H, et al. Polylactic acid implants (New-Fill) to correct facial lipoatrophy in HIV-infected patients: results of the open-label study VEGA. AIDS. 2003;17:2471–2477. doi: 10.1097/00002030-200311210-00009. [DOI] [PubMed] [Google Scholar]
- 33.Moyle GJ, Lysakova L, Brown SE. Randomized open-label study of immediate versus delayed polylactic acid injections for the cosmetic management of facial lipoatrophy in persons with HIV infection. HIV Med. 2004;5:82–87. doi: 10.1111/j.1468-1293.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 34.Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201–205. doi: 10.1080/14764170500451404. [DOI] [PubMed] [Google Scholar]
- 35.Mest DR, Humble GM. Duration of correction for human immunodeficiency virus-associated lipoatrophy after retreatment with injectable poly-L-lactic acid. Aesthetic Plast Surg. 2009;33:654–656. doi: 10.1007/s00266-008-9226-7. [DOI] [PubMed] [Google Scholar]
- 36.Lowe NJ, Maxwell CA, Lowe P, Shah A, Patnaik R. Injectable poly-L-lactic acid: 3 years of aesthetic experience. Dermatol Surg. 2009;35:344–349. doi: 10.1111/j.1524-4725.2008.01061.x. [DOI] [PubMed] [Google Scholar]
- 37.Mest DR, Humble GM. Duration of correction for human immunodeficiency virus-associated lipoatrophy after retreatment with injectable poly-L-lactic acid. Aesthetic Plast Surg. 2009;33:654–656. doi: 10.1007/s00266-008-9226-7. [DOI] [PubMed] [Google Scholar]
- 38.Cohen SR, Holmes RE. Artecoll: a long-lasting injectable wrinkle filler material: report of a controlled, randomized, multicenter clinical trial of 251 subjects. Plast Reconstr Surg. 2004;114:964–976. doi: 10.1097/01.prs.0000133169.16467.5f. [DOI] [PubMed] [Google Scholar]