Abstract
Current methods for measuring collagen content in engineered tissues are incompatible with monitoring of collagen production because they require destruction of the tissue. We have implemented a luciferase-based strategy to monitor collagen production noninvasively. Fibrin-based tissue constructs made using vascular smooth muscle cells stably transfected with a collagen I promoter/luciferase transgene developed with collagen content comparable to control cells, but could be imaged noninvasively to follow collagen transcription during tissue growth in vitro. We showed that these cells reported collagen I production at the transcriptional level in response to the growth factor transforming growth factor-β1 and fibrinolytic inhibition by ɛ-aminocaproic acid and that these changes were consistent with changes at the mRNA and protein levels. As these cells report collagen changes instantly and without tissue destruction, they will facilitate construct optimization using multiple stimuli to produce functional engineered tissues.
Introduction
Members of the collagen family are vital components of virtually every tissue in the human body. Their critical role in tissue mechanical function is highlighted by the linkage of human disease to collagen gene mutations.1 Type I collagen is a heterotrimeric molecule composed of two α1(I) chains and one α2(I) chain. The gene for the α1(I) chain of type I collagen, COL1A1, is located on the human chromosome 17 and is conserved across a broad number of species. COL1A1 transcription has been shown to be positively regulated by interleukin-4 and transforming growth factor (TGF-β) and negatively regulated by vitamin D and tumor necrosis factor-α via response elements in the promoter.2–5 A region of the mouse promoter from −2.3 kb to the start site has been useful for targeting the expression of reporter genes to collagen-expressing cells and contains the aforementioned response elements.6,7 Once collagen has been transcribed, it is translated and then modified posttranslationally by proline hydroxylation before secretion, trimerization, telopeptide removal, and crosslinking.
There is a key need for mature collagen fibers in engineered tissue. Collagen provides both the strength needed to obviate mechanical tissue failure and the stiffness to confer appropriate deformations in response to physiological loads. Alignment of collagen fibers in native tissues is well known to be essential for normal mechanical functions. In our studies, we achieved this alignment through the use of fibrin-based tissue constructs. Entrapped cells compact the initial fibrin gel, leading to aligned fibrin fibrils that cause co-alignment of deposited collagen fibrils.8
As yet, determining collagen production in engineered tissue has required destruction of the growing tissue construct (1) to extract RNA for analysis of collagen transcript level, (2) to extract collagen protein for determination of 4-hydroxyproline levels via biochemical assay, and thereby infer collagen content, or (3) to embed the tissue for histological analysis using collagen staining dyes, such as trichrome or picrosirius red. All of these methods eliminate the possibility of implanting the cultured tissue and do not allow for collagen production to be monitored so that culture conditions can be optimized during incubation.
We report here a luciferase-based method for monitoring collagen production noninvasively. Similar methods have been previously used for monitoring the activation of TGF-β and Wnt-induced signaling.9,10 In our system, the COL1A1 promoter, which is responsive to multiple stimuli as aforementioned, is used to drive the production of firefly (Photinus pyralis) luciferase; therefore, luciferase will be made whenever collagen is transcribed. Activity of this enzyme can be measured by incubating tissue with the substrate for luciferase, luciferin; as, in fireflies, the luciferase reaction produces light, which can be easily monitored. The reporter enzyme has a moderate turnover rate, with a half-life of ∼2 h in mammalian cells,11 fast enough to report on daily changes in collagen transcription, but slow enough that luciferase activity will be maintained during imaging. We entrapped vascular smooth muscle cells (vSMCs) stably transfected with the reporter transgene in fibrin gel to create adherent disc-shaped tissue constructs. As in our previous studies, they were subjected to stimuli to promote collagen production while remodeling the fibrin into a more tissue-like extracellular matrix, including TGF-β1 and altered fibrinolysis via the inhibitor ɛ-aminocaproic acid (ACA). They were periodically incubated with luciferase substrate and imaged to measure collagen I transcription via luminescence. Selected samples were also harvested to measure COL1A1 RNA by quantitative reverse transcriptase (qRT)-polymerase chain reaction (PCR), collagen content by hydroxyproline assay, and cellularity by DNA assay for interpretation of the luminescence signal.
Materials and Methods
Cell culture
ACA, bovine fibrinogen (F4753, diluted to total protein 30 mg/mL for working solution), thrombin, Pluronics F127, ascorbate, insulin (all from Sigma, St. Louis, MO), and active recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) were used for constructs and cell culture. vSMCs were isolated from newborn Fisher rat pups and grown as previously described.12 vSMCs were cultured in standard Dulbecco's modified Eagle's medium/F12 medium [supplemented with 100 U/mL penicillin, 100 U/mL streptomycin (antibiotics) (Invitrogen, Carlsbad, CA), and 15% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA)] and passaged upon reaching confluence.
Stable transfection of vSMCs
pColLuc, comprised of the human COL1A1 promoter (−2351 to +55) cloned into the multiple cloning site of pGL4.14 (Promega, Madison, WI), was a generous gift from Dr. Albert Banes, FlexCell International, and was generated via standard recombinant DNA techniques. Briefly, the COL1A1 promoter region was amplified by PCR from genomic DNA extracted from MG63 human osteoblast cells, using forward and reverse primers containing NheI and BglII sites, respectively, at their 5' ends. The PCR amplification product and pGL4.14 vector were digested with NheI and BglII restriction enzymes and ligated with T4 DNA ligase to generate pColLuc. vSMCs were cultured near confluence, changed to antibiotic-free medium overnight, then transfected with pColLuc using Fugene HD (Roche, Indianapolis, IN) according to the manufacturer's protocol. Thirty microliters of Fugene HD and 10 μg plasmid were used for each 10 cm dish. Transfection efficiency was estimated at ∼20% by counting the percentage of fluorescent cells transfected in parallel with a plasmid for yellow fluorescent protein (pYFP). Twenty-four hours posttransfection, the cells were passed 1:1.5 and cultured in a medium supplemented with 50 μg/mL hygromycin B (Invitrogen) for selection. Pooled selection was chosen instead of clonal selection because of the importance of obtaining large amounts of transfected cells with as few cell doublings as possible. The surviving cells were then propagated as above in a selection medium and were observed to have 1.7 ± 0.2 doublings/week, when compared with 2.7 ± 0.2 doublings/week for control cells in a standard medium.
Fabrication of fibrin-based adherent disc tissue constructs
The fabrication of these constructs has been previously described.12 For these studies, the initial concentrations were 5 × 105 vSMCs/mL and 3.3 mg (ACA study) or 6.6 mg (TGF-β1 study) fibrin per mL; 200 μL of fibrin gel was used for each 1 cm diameter tissue construct having an approximate initial thickness of 3 mm. After fibrin gelation, an incubation medium (Dulbecco's modified Eagle's medium/F12, antibiotics, 10% fetal bovine serum, 50 μg/mL ascorbate, 1 ng/mL TGF-β1, 2 μg/mL insulin, and 1 mg/mL ACA, unless otherwise specified) was added at a volume of 1 mL/105 initial cells and changed every 2–3 days.
Fluorescence measurements
Fluorescent microspheres (Dragon Green, 15 μm; Bangs Laboratories, Fishers, IN) were introduced into constructs for studying the effect of fibrin compaction on optical properties. All microspheres were sterilized by thoroughly rinsing 10 μL microspheres per 1 mL final gel volume with 70% ethanol before adding them to the fibrinogen mixture. Varying amounts of control vSMCs were used upon fabrication to generate constructs, after 2 days of culture, with varying thickness but identical microsphere content.
Fluorescence was measured after rinsing constructs in phosphate-buffered saline by reading in a plate reader (Bio-Tek, Winooski, VT) using emission/excitation wavelengths of 485 ± 20/528 ± 20 nm and the top probe with sensitivity 50. Values were scaled using a value of 100 for one of the cell-free constructs before reporting the value as relative light units (RLU).
By fixing constructs in formalin after fluorescent imaging to stiffen them for testing and then rinsing thoroughly with phosphate-buffered saline, the thickness was measured (n = 5 readings per construct) using a force probe attached to a displacement transducer as previously described.13
Luminescence measurements
For luminescence measurements, the medium was removed from the constructs and replaced with 1 mM Luciferin-EF (Promega) diluted in Hank's buffered saline solution. Luminescence values were then recorded using a plate reader (Bio-Tek) or an in vivo imaging system (IVIS®; Caliper, Hopkinton, MA) as indicated. The plates were read for luminescence using a sensitivity setting of 200; the average of six readings per sample was adjusted by subtracting the luminescence of a well containing only substrate before reporting the value as RLU. All samples imaged using IVIS used an exposure time of 30 s, bin setting of 1 or 4, and f-stop of 1. Downstream image analysis was performed using Image J (National Institutes of Health, Bethesda, MD). Briefly, the constructs were outlined to define an area of interest and then measured for mean gray level. Normalized luminescence (RLU) was calculated by subtracting the intensity of a nonluminescent “blank” area of equal size. Different vSMC transfections exhibited changes in luminescence magnitude but identical trends upon TGF-β1 or ACA treatment, and so for figures combining multiple transfections, two samples with equivalent treatment and days in culture were scaled to a value of 50 RLU. All other values for a transfection in that experimental group were also scaled accordingly. For estimation of cumulative collagen transcription at each time of luminescence measurement, the average of the rate at day i + 4 (TGF-β1) or week i + 0.5 (ACA) and the rate at day/week i was added to the cumulative value at day/week i; in Figure 1B this added value was multiplied by 4/3 to account for the additional day in culture.
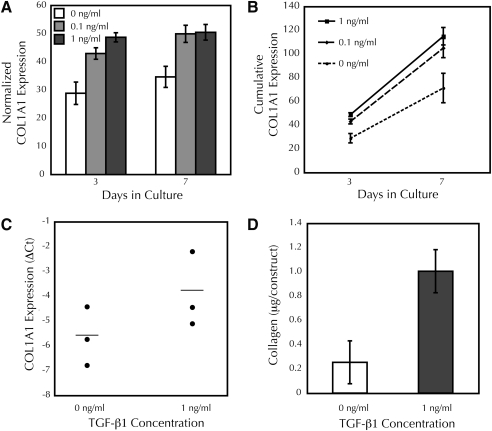
FIG. 1.
Fibrin-based adherent tissue constructs fabricated with vascular smooth muscle cells transfected with the reporter construct pColLuc demonstrate increased collagen promoter activity, transcription, and deposition in response to TGF-β1 treatment. (A) TGF-β1-stimulated COL1A1 promoter activity in constructs. Constructs treated with 0, 0.1, or 1 ng/mL TGF-β1 were imaged using IVIS®; n = 11 per group, except for 1 ng/mL TGF-β1 at day 7, n = 19. (B) Integration of COL1A1 promoter activity predicted higher collagen accumulation in TGF-β1-treated constructs. Propagated error is shown but significance was not assessed. (C) TGF-β1 promoted collagen transcription in constructs. ΔCt values are shown along with the mean, p < 0.02. (D) TGF-β1 increased collagen deposition in constructs; total collagen content in constructs after 7 days is shown (n = 5 and 8 for 0 and 1 ng/mL TGF-β1, respectively). TGF-β1, transforming growth factor-β1.
Real-time qRT-PCR
The constructs were harvested immediately after luminescence imaging and were stored in RNAlater (Qiagen, Valencia, CA) at −20°C until extraction. RNA was extracted from constructs using 350 μL lysis buffer + 7 μL 2 M dithiothreitol per construct, according to the manufacturer's protocol (RNeasy; Qiagen). After a 30 s sonication step in lysis buffer, debris was pelleted by centrifugation and removed before purifying the lysate with the RNeasy kit (Qiagen). An on-column DNase digest was performed to reduce genomic DNA contamination. Total RNA, eluted in 50 μL RNase-free water, was quantified by spectrophotometry (NanoDrop, Thermo Scientific). cDNA was generated from 30 ng total RNA using Superscript III (Invitrogen) and random hexamer primers (50 ng/reaction) according to the manufacturer's protocol. Three nanograms of cDNA was used as the template for real-time PCR using a Mx3000P instrument (Agilent, Santa Clara, CA), master mix (Brilliant SyBr Green; Agilent), ROX reference dye, and 10 pmol each of forward (f) and reverse (r) primers (fCol1A1: ATCAGCCCAAACCCCAAGGAGA; rCol1A1: CGCAGGAAGGTCAGCTGGATAG; frS9: GGGCCTGGCCAAATCTATTC; rrS9: GCCGACCCTGATGTGACGTTG). A common threshold (Ct) was determined using software (MxPro, Agilent) and then the Ct values for each sample were calculated. Each reaction was run in triplicate with each primer set along with a negative control where the cDNA reaction was performed without reverse transcriptase. ΔCt was calculated in triplicate for each construct as (Ct(rS9) − Ct(Col1A1)) and compared between TGF-β1-treated and control groups (n = 3 constructs per group). Fold increase upon TGF-β1 treatment was calculated as follows: 2(ΔCt(Treated) − ΔCt(Control)).
Biochemical analysis of constructs
Analysis of cellular content by DNA determination and quantification of collagen content by hydroxyproline determination has been previously described.12 Briefly, for cellular content, the constructs were digested with protease K at 56°C for 16 h and quantified (using a standard curve of calf thymus DNA) with a modified Hoechst assay, assuming 7.6 pg of DNA per cell. For collagen content, the constructs were extracted for 1 h at 98°C with 0.1 M NaOH, the supernatants obtained by centrifugation were dried and hydrolyzed overnight at 110°C in 6 N HCl, and hydroxyproline content was determined by Chloramine-T assay, using a standard curve of known 4-hydroxyproline concentration. The collagen content was calculated assuming 7.7 mg collagen per mg hydroxyproline.
Statistical analysis
Unless otherwise noted, all data points show the mean and standard error with n = 3 or greater in each experiment. Significance was assigned by Student's t-test between sample groups, and p < 0.05 was reported as significant. The samples with the same cell type and day of culture with nonoverlapping error bars showed significant differences.
Results
Reporter cells entrapped in fibrin-based tissue constructs could be monitored nondestructively for collagen transcription
We transfected a population of vSMCs with either a plasmid containing a transgene that reported transcription from the human COL1A1 promoter, pColLuc, or the empty parent vector, pGL4.14. Nontransfected (control) cells were also maintained for comparison. Cells containing the empty parent vector should make insignificant amounts of the reporter protein luciferase because the vector was engineered to contain few, if any, enhancer elements upstream of the luc2 gene. In contrast, cells containing the reporter vector (reporter cells) should produce luciferase only when the COL1A1 promoter is active, that is, when the vSMC are transcribing message for type I collagen α1 chain.
After pooling and selection of the transgene-positive clones, monolayers of reporter cells were assayed for luciferase activity. In comparison with control cells, which showed negligible luciferase activity, confluent monolayers of reporter cells showed luminescence when exposed to the luciferase substrate luciferin [639 ± 41 (n = 1, pColLuc) vs. −11 ± 8 (n = 1, control), uncertainty of measurement over 11 readings is indicated]. After exposure to luciferin and measurement of luminescence, the cells could be returned to the incubator with no obvious cell loss or contamination.
Having confirmed that our reporter cells could be assayed for luciferase activity without complication, we entrapped them in fibrin-based tissue constructs. Over 4 weeks of incubation, vSMCs remodeled the constructs by compacting and degrading the fibrin as well as depositing new extracellular matrix (ECM) similar to previous reports.12,14 The constructs containing reporter cells, but not those containing control vSMC, showed luminescence upon addition of luciferin [1792 ± 208 (n = 5, pColLuc) vs. 12 ± 7.5 RLU (n = 6, control) on plate reader, p < 0.001]. As with the cell monolayers, this assay maintained sterility of the samples. Notably, the luminescence of fibrin-based reporter constructs increased each week during culture (28 ± 9, 104 ± 24, 200 ± 74, 381 ± 70 RLU for weeks 1–4, respectively, for n = 3 constructs) reflecting an increase in luminescence per cell and/or cellularity over the 4 weeks. These possibilities are discussed below. In contrast, cells transfected with pGL4.14 alone showed only basal luminescence levels (4 ± 2, 11 ± 8, 38 ± 3, 32 ± 10 RLU for weeks 1–4, respectively, for n = 4 constructs). Two hours after addition of the substrate at 4 weeks, the luminescence was reread, and the signal was equivalent (381 ± 70 vs. 319 ± 60 RLU before and after a 2 h incubation at 37°C, n = 3 constructs).
TGF-β1 promoted collagen transcription in fibrin-based tissue constructs
TGF-β1 has been shown to lead to increased collagen transcription by fibroblasts15–17 and secretion by vSMCs.18 We have also shown increased collagen deposition in fibrin-based constructs with entrapped vSMCs that are treated with TGF-β1 during static incubation and an associated improvement in mechanical properties.19 Therefore, to verify the responsiveness of our reporter cells to this growth factor, constructs were cultured in medium with or without TGF-β1 supplementation and assayed for luminescence over 7 days.
As early as day 3 of culture, the constructs treated with 1 ng/mL TGF-β1 exhibited increased luminescence compared with untreated constructs (Fig. 1B). During the culture period, this increase in transcription varied between 1.4- and 1.6-fold. Intermediate TGF-β1 treatment (0.1 ng/mL) led to intermediate luminescence at day 3, but this group was not different from the 1 ng/mL group by 7 days and, therefore, was not analyzed further. To rule out the possibility that the increased luminescence with TGF-β1 treatment was solely due to increased cellularity, cell proliferation in the reporter cell constructs was assessed. In contrast to the increased luminescence, TGF-β1 treatment trended toward a decrease in cellularity [1.3- and 1.4-fold at day 3 (n = 1) and day 7 (n = 4 or 5, p = 0.068), respectively].
Assuming a linear change in transcription rate between time points, and that the observed promoter activity led to protein that accumulated in the construct, we integrated the rate curves of Figure 1A at each TGF-β1 concentration for comparison with actual values for deposited collagen from samples harvested after 7 days of culture (Fig. 1B). The integrated transcription rates predicted that the collagen contents differ through 7 days of culture between TGF-β1-treated (0.1 or 1 ng/mL) and untreated groups.
A subset of these constructs was harvested after 3 days of culture to confirm the effect of TGF-β1 on collagen expression at the RNA level. TGF-β1 treatment led to a 1.73 ± 0.65 difference in ΔCt, equivalent to a 3.3-fold increase in normalized Col1A1 expression upon TGF-β1 treatment (Fig. 1C). As this assay uses a housekeeping gene (rS9) for normalization, these data reflect the expression of collagen on a per-cell basis. For this subset, the treated group had 1.3-fold more luminescence, 1.3-fold less cells, and therefore 1.7-fold more luminescence per cell than the untreated group at day 3.
After 7 days of incubation, the constructs were analyzed for hydroxyproline content to quantify collagen protein levels. In agreement with the luminescence signal, constructs treated with TGF-β1 showed an increase in collagen content (Fig. 1D), as expected from our earlier studies using vSMCs in fibrin-based constructs.19
Inhibition of fibrinolysis led to a parallel decrease in both collagen transcription and deposition by vSMCs in fibrin-based tissue constructs
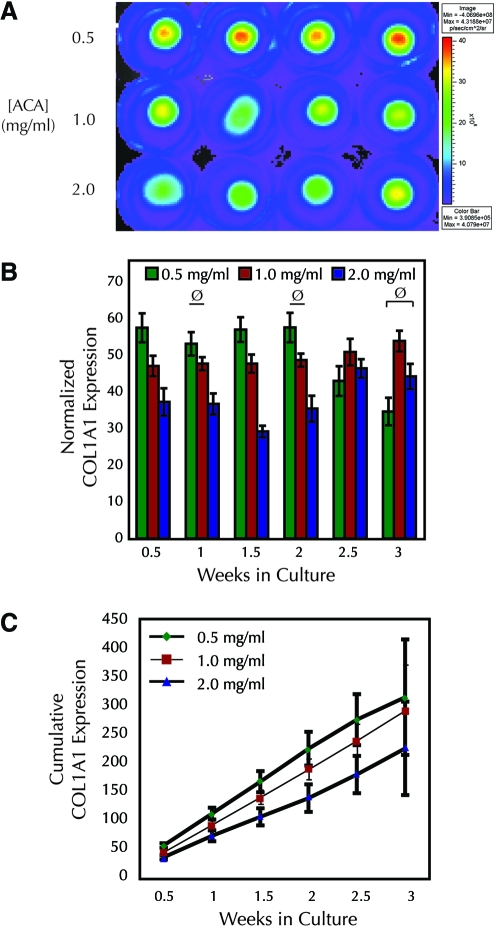
As further validation that luminescence of our constructs relates to collagen production, we took advantage of our finding that the plasmin inhibitor ACA leads to reduced fibrinolysis and decreased collagen deposition in fibrin-based tissue constructs (unpublished data). We hypothesized that this decrease in collagen deposition resulted from decreased collagen transcription rather than a defect in posttranslational processing or assembly. To test this hypothesis, constructs were cultured in varying amounts of ACA [0.5 mg/mL (low), 1.0 mg/mL (intermediate), or 2.0 mg/mL (high)] and imaged during culture using the live luminescence assay (Fig. 2A). As early as the first luminescence reading midway through the first week, ACA had a dose-dependent inhibitory effect on overall collagen transcription rate (Fig. 2B). This difference persisted for 2 weeks. At week 3, luminescence in the low ACA group had decreased below that of the intermediate group.
FIG. 2.
Inhibition of fibrinolysis with ACA leads to a transient decrease in collagen transcription rate in fibrin-based adherent tissue constructs, predicting lower collagen deposition. (A) Representative pseudocolor image of luminescence from IVIS® after 9 days in culture; each row represents a different ACA treatment group. (B) ACA decreased COL1A1 promoter activity of reporter cell constructs during the first 2 weeks of culture (n = 4 for 2.5 week groups, n ≥ 11 for all others). With the exception of cases marked with “ø” all samples with the same cell type and day of culture with nonoverlapping error bars showed significant differences. (C) Integration of COL1A1 promoter activity from (B) predicted lower collagen accumulation in constructs treated with high versus low ACA. Propagated error is shown, but significance was not assessed. Intermediate ACA data were illustrated with a thin line for clarity. ACA, ɛ-aminocaproic acid. Color images available online at www.liebertonline.com/ten.
As in the TGF-β1 study, cumulative COL1A1 expression was determined from the luminescence values obtained biweekly over 3 weeks of culture. No differences were seen after the first time point between the low and intermediate ACA groups throughout the 3 week culture period (Fig. 2C). In contrast, the high ACA group had lower accumulated collagen than the low ACA group through the first 2.5 weeks according to this predictive model. This difference ended by 3 weeks.
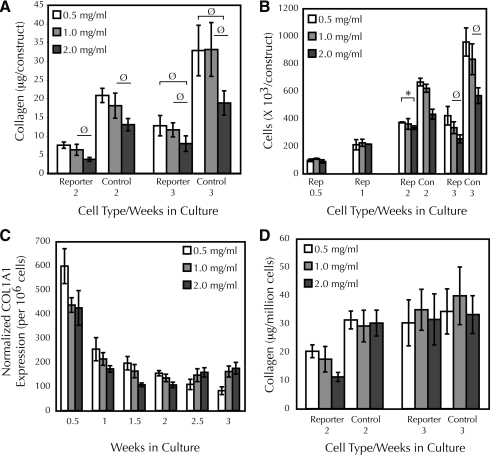
Consistent with the predicted accumulated collagen from the transcription rates, constructs cultured in high ACA deposited less collagen when compared with the group treated with low ACA at week 2 (Fig. 3A). By 3 weeks, there was no difference among treatment groups. The net collagen deposited in the reporter cell constructs was about one-third of that found in constructs fabricated in the same experiment with control vSMCs, which were of lower passage number (Fig. 3A). As decreased collagen deposition could result entirely from a decreased number of cells, cell proliferation in the reporter cell constructs was assessed. During the first week of culture, the cell number in reporter constructs doubled, independently of ACA concentration (Fig. 3B). By 2 weeks, there was less cellularity in the high, compared with the low, ACA treatment group. However, this also occurred for constructs fabricated with control cells. Relative to control cell constructs, the reporter cell constructs possessed approximately one-half the cellularity.
FIG. 3.
ACA decreased collagen deposition and cellularity in fibrin-based adherent tissue constructs. (A) Collagen content of reporter and control cell constructs after 2 and 3 weeks of culture; n ≥ 5 for each group. (B) Cellularity of reporter (Rep) and control (Con) cell constructs during culture (n = 2 for 0.5 and 1 weeks; n ≥ 3 for other groups). For clarity, * indicates p < 0.05 between 0.5 and 2.0 mg/mL. With the exception of cases marked with “Ø,” all samples with the same cell type and day of culture with nonoverlapping error bars showed significant differences. (C) COL1A1 promoter activity of reporter cell constructs normalized to cellularity. Cellularity at 1.5 and 2.5 weeks was assumed to be the average of the adjacent weekly values. Error bars represent propagated error from Figures 2B and 3B; significance was not assessed. (D) Collagen content of reporter and control cell constructs normalized to cellularity. Error bars represent propagated error from (A) and (B); significance was not assessed.
To determine if the decreased cellularity accounted for the decreased collagen content in reporter cell constructs in the high ACA group, the luminescence (Fig. 2B) and collagen values (Fig. 3A) were re-expressed on a per-cell basis using linear interpolations of the cellularity data (Fig. 3B). Because of the equal cellularity over the first 2 weeks of culture, the normalized luminescence values of the reporter cell constructs still differed between treatment groups (Fig. 3C). After 2 weeks, the per-cell luminescence trend appeared to reverse, with higher normalized transcription rates in the high ACA group. At week 3, there was no difference in normalized collagen content between control and reporter cell constructs; however, values for reporter cell constructs were lower at week 2 (Fig. 3D). All differences in the collagen content of ACA-treated control cell constructs disappeared after normalization. The improvement in collagen content of the reporter cell constructs can thus be attributed to an increase in collagen deposition per cell over the third week. This implied that the reporter transgene was stable over 3 weeks in the constructs without selection.
Optical properties of fibrin-based constructs are maintained after compaction, and reporter cells are both viable and synthetic after imaging
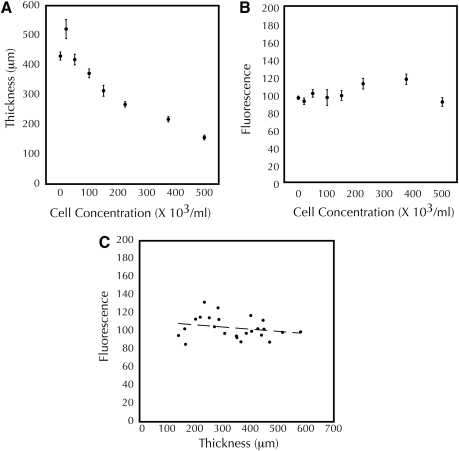
Significant differences in thickness were seen during culture for both TGF-β1 (261 ± 16 μm for untreated at day 7 vs. 339 ± 6 μm for 1 ng/mL TGF-β1, p < 0.03) and ACA (the low ACA group being ∼25% the thickness of the high ACA group) constructs. To determine whether these differences in thickness altered the optical properties of the constructs for luminescence measurements, we fabricated tissue constructs containing equal numbers of microspheres conjugated to a fluorescent dye as surrogates for luminescent cells. These microspheres have been used successfully previously to assess strain in collagen-based tissue constructs.20 By varying the initial cell content, different thickness constructs were obtained after incubation, ranging from 140 to 580 μm (Fig. 4A). Over this range of cell concentration, fluorescence varied from 86 to 132 RLU (Fig. 4B). There was no correlation between thickness and fluorescence (R2 < 0.06) when paired values were plotted (Fig. 4C).
FIG. 4.
Fluorescence of fibrin-based adherent tissue constructs is independent of thickness. (A, B) Thickness after 2 days of compaction varies with initial cell concentration, whereas fluorescence does not. (C) No correlation exists between construct thickness and fluorescence. Dashed line shows linear fit of the data, with R2 < 0.06.
To rule out a negative effect of the imaging process, which involved a duration of ∼20 min away from the 37°C humidified environment of the incubator and replacement of the culture medium with Hank's buffered saline solution containing luciferin reagent, cellularity and collagen content of assayed constructs were compared with those of constructs incubated at 37°C until harvest after 3 weeks in a standard construct medium. There was no difference in either cellularity (192 ± 11 × 103 vs. 210 ± 4 × 103 cells per construct, n = 2, p = 0.35) or collagen content (7.2 ± 0.3 vs. 8.3 ± 0.2 μg per construct, n = 2, p = 0.13) between the assayed and nonassayed groups.
Discussion
The technology described in this study provides great utility for the fabrication of engineered tissues with maximum collagen content. Reporter cell constructs showed increased luminescence, indicative of type I collagen transcription rate, in response to TGF-β1 stimulation and decreased luminescence in response to lower fibrinolysis via the plasmin inhibitor ACA. The changes in type I collagen transcription rates among treatment groups measured by the luciferase assay successfully predicted similar changes in collagen mRNA expression and deposition, although lower in magnitude.
In this study, we found that the final cellularity and collagen content of reporter cell constructs was lower compared with constructs prepared with control cells. Cell proliferation data on tissue culture plastic argue that this discrepancy is more likely a consequence of using reporter cells that were passaged in culture longer than the control cells and therefore have decreased proliferative capacity than an artifact of the luminescence assay. To address this issue, we have begun transfecting vSMCs sooner after primary isolation, with a hope that this discrepancy can be eliminated. Ideally, there would be no difference in proliferation or COL1A1 transcription rates between reporter and control cells, and so reporter cells could be used to “spike” nontransfected cells used in fabrication and the luminescence signal would simply reflect the total transcription rate of the entire cell population. If differences cannot be ruled out, using a pure population of reporter cells would be required to ensure that the interpretation is accurate.
The use of luciferase as a reporter enzyme in live mammalian cells has been well studied. The Michaelis–Menten constants for the production of light from HEK-293T cells incubated with luciferin in a culture medium have been experimentally determined, including a Km for luciferin of ∼1 mM and a half-life for firefly luciferase of 2 h,11 consistent with the luciferin concentration and assay time used in this study. The same investigators modeled the production of luminescence in live cells, assuming diffusive transport of luciferin across the cell membrane.11 Their model can be analyzed to suit our study by assuming steady-state luciferin and luciferase concentrations over the course of one imaging session. The former assumption is justified by the excess luciferin reagent in our assay, and the latter by the finding that the luminescence signal persisted for 2 h, far greater than the 30 s image acquisition period. We further assume that the luciferase synthesis rate (ks in their model) is proportional to the COL1A1 transcription rate. Effectively, these assumptions simplify the model equations to a case where the rate of light production (v in their model) is proportional to the product of the rate of collagen transcription with the number of cells in the construct. The luminescence measurement is effectively this product multiplied by the 30 s time interval of image acquisition. Because the luminescence values used for normalization at each time point varied over the time course of each experiment, the normalized per-cell luminescence values cannot be used to compare transcription rates chronologically but only at discrete sampling times. However, the data at each time point were informative as snapshots of collagen transcription rates and their relative dependence on treatment condition, and they were predictive of differences in accumulated collagen when integrated over the course of the experiment.
Differences in construct thickness were seen in our study with both medium supplements, which could have affected our measurements. However, as no effect of construct thickness was found on the optical properties of fibrin-based constructs in the fluorescence system, a “worst-case scenario” for light scatter, absorption, and so forth, it is unlikely that thickness is affecting the luminescence measurements.
In this study, we have chosen to validate our findings with both an independent assay for collagen message at the level of mRNA and an assay for collagen deposition. In response to both TGF-β1 stimulation and decreased fibrinolysis, our results are consistent with collagen transcription and deposition being highly correlated. However, it is conceivable that some stimuli may decouple these two phenomena by having effects on mRNA stability, posttranslational processing, or degradation of the final protein product. Discrepancies between luminescence and collagen content could still be informative for biological mechanism, for example, increased collagen content in response to a drug without an increase in collagen transcription would imply that the drug's action is posttranscriptional rather than upstream of the collagen gene. Therefore, reporter cell findings should be periodically analyzed biochemically in parallel in future studies, but constant analysis should be unnecessary. Alternately, collagen content could increase without an increase in type I collagen transcription because of enhanced type III collagen transcription, as multiple collagens are detected with our biochemical assay; however, studies have shown that transcription of these two genes is typically coupled in vSMCs.14
Collagen only represents a starting point for the use of this technology in tissue engineering. In the field of cardiovascular tissue engineering, another critical ECM component is elastin. To this point the portion of the elastin promoter that defines its temporal and spatial expression patterns remains unknown, leading to the use of a 98 kb bacterial artificial chromosome to direct the expression of human elastin in mice.21 However, validation methods similar to those used in this study could be used to find a smaller promoter acceptable for use in monitoring the elastin content of tissue-engineered constructs.
The utility of promoter-driven luciferase is not limited to cardiovascular tissue engineering. Other engineered tissues could benefit from reporters as well, such as cartilage and pancreas using the hyaluronic acid synthase and insulin promoters, respectively. Ultimately, this technology will enable monitoring and thereby screening of multiple mechanical and chemical stimuli en route to the production of functional engineered tissue possessing maximum (or optimum) contents of critical ECM components.
Acknowledgments
The authors thank Stephen Stephens and Cary Valley for performing biochemical assays, and Lee Meier and Naomi Ferguson for assistance in maintaining cells and tissue constructs. This study was funded by the NIH Grant R01 HL083880 (to R.T.T.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Myllyharju J. Kivirikko K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Buttner C. Skupin A. Rieber E.P. Transcriptional activation of the type I collagen genes COL1A1 and COL1A2 in fibroblasts by interleukin-4: analysis of the functional collagen promoter sequences. J Cell Physiol. 2004;198:248. doi: 10.1002/jcp.10395. [DOI] [PubMed] [Google Scholar]
- 3.Iraburu M.J. Dominguez-Rosales J.A. Fontana L. Auster A. Garcia-Trevijano E.R. Covarrubias-Pinedo A. Rivas-Estilla A.M. Greenwel P. Rojkind M. Tumor necrosis factor alpha down-regulates expression of the alpha1(I) collagen gene in rat hepatic stellate cells through a p20C/EBPbeta- and C/EBPdelta-dependent mechanism. Hepatology (Baltimore, MD) 2000;31:1086. doi: 10.1053/he.2000.5981. [DOI] [PubMed] [Google Scholar]
- 4.Pavlin D. Bedalov A. Kronenberg M.S. Kream B.E. Rowe D.W. Smith C.L. Pike J.W. Lichtler A.C. Analysis of regulatory regions in the COL1A1 gene responsible for 1,25-dihydroxyvitamin D3-mediated transcriptional repression in osteoblastic cells. J Cell Biochem. 1994;56:490. doi: 10.1002/jcb.240560409. [DOI] [PubMed] [Google Scholar]
- 5.Ritzenthaler J.D. Goldstein R.H. Fine A. Lichtler A. Rowe D.W. Smith B.D. Transforming-growth-factor-beta activation elements in the distal promoter regions of the rat alpha 1 type I collagen gene. Biochem J. 1991;280(Pt 1):157. doi: 10.1042/bj2800157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J.E. Nakashima K. de Crombrugghe B. Transgenic mice expressing a ligand-inducible cre recombinase in osteoblasts and odontoblasts: a new tool to examine physiology and disease of postnatal bone and tooth. Am J Pathol. 2004;165:1875. doi: 10.1016/S0002-9440(10)63240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossert J. Eberspaecher H. de Crombrugghe B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol. 1995;129:1421. doi: 10.1083/jcb.129.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barocas V.H. Girton T.S. Tranquillo R.T. Engineered alignment in media equivalents: magnetic prealignment and mandrel compaction. Journal of biomechanical engineering. 1998;120:660. doi: 10.1115/1.2834759. [DOI] [PubMed] [Google Scholar]
- 9.Abe M. Harpel J.G. Metz C.N. Nunes I. Loskutoff D.J. Rifkin D.B. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 10.Sussman D.J. LEF/TCF-dependent, fluorescence-based reporter gene assay for Wnt signaling. BioTechniques. 2002;32:1000. doi: 10.2144/02325bm04. [DOI] [PubMed] [Google Scholar]
- 11.Ignowski J.M. Schaffer D.V. Kinetic analysis and modeling of firefly luciferase as a quantitative reporter gene in live mammalian cells. Biotechnol Bioeng. 2004;86:827. doi: 10.1002/bit.20059. [DOI] [PubMed] [Google Scholar]
- 12.Grassl E.D. Oegema T.R. Tranquillo R.T. A fibrin-based arterial media equivalent. J Biomed Mater Res. 2003;66:550. doi: 10.1002/jbm.a.10589. [DOI] [PubMed] [Google Scholar]
- 13.Syedain Z.H. Weinberg J.S. Tranquillo R.T. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci USA. 2008;105:6537. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross J.J. Tranquillo R.T. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 2003;22:477. doi: 10.1016/s0945-053x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 15.Ignotz R.A. Endo T. Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987;262:6443. [PubMed] [Google Scholar]
- 16.Raghow R. Postlethwaite A.E. Keski-Oja J. Moses H.L. Kang A.H. Transforming growth factor-beta increases steady state levels of type I procollagen and fibronectin messenger RNAs posttranscriptionally in cultured human dermal fibroblasts. J Clin Invest. 1987;79:1285. doi: 10.1172/JCI112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varga J. Rosenbloom J. Jimenez S.A. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987;247:597. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson J.M. Zoia O. Liu J.M. Modulation of transforming growth factor-beta 1 stimulated elastin and collagen production and proliferation in porcine vascular smooth muscle cells and skin fibroblasts by basic fibroblast growth factor, transforming growth factor-alpha, and insulin-like growth factor-I. J Cell Physiol. 1993;155:149. doi: 10.1002/jcp.1041550119. [DOI] [PubMed] [Google Scholar]
- 19.Long J.L. Tranquillo R.T. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 2003;22:339. doi: 10.1016/s0945-053x(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 20.Girton T.S. Barocas V.H. Tranquillo R.T. Confined compression of a tissue-equivalent: collagen fibril and cell alignment in response to anisotropic strain. J Biomech Eng. 2002;124:568. doi: 10.1115/1.1504099. [DOI] [PubMed] [Google Scholar]
- 21.Hirano E. Knutsen R.H. Sugitani H. Ciliberto C.H. Mecham R.P. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ Res. 2007;101:523. doi: 10.1161/CIRCRESAHA.107.153510. [DOI] [PubMed] [Google Scholar]