Abstract
Extracts prepared from turmeric (Curcuma longa L., [Zingiberaceae]) containing bioactive phenolic curcuminoids were evaluated for bone-protective effects in a hypogonadal rat model of postmenopausal osteoporosis. Three-month female Sprague Dawley rats were ovariectomized (OVX) and treated with a chemically complex turmeric fraction (41% curcuminoids by weight) or a curcuminoid-enriched turmeric fraction (94% curcuminoids by weight), both dosed at 60mg/kg 3x per week, or vehicle alone. Effects of two months of treatment on OVX-induced bone loss were followed prospectively by serial assessment of bone mineral density (BMD) of the distal femur using dual-energy x-ray absorptiometry (DXA), while treatment effects on trabecular bone microarchitecture were assessed at two months by micro-computerized tomography (μCT). Chemically complex turmeric did not prevent bone loss, however, the curcuminoid-enriched turmeric prevented up to 50% of OVX-induced loss of trabecular bone and also preserved the number and connectedness of the strut-like trabeculae. These results suggest that turmeric may have bone-protective effects but that extract composition is a critical factor.
Keywords: Turmeric, Curcuma longa L, Curcuminoids, Ovariectomy, Bone mineral density, Bone μCT
Introduction
Given the significant health and economic impact of postmenopausal osteoporosis on individuals and society, effective, safe, and readily available preventative measures are needed for early intervention to stop the progressive loss of bone structure that begins during perimenopause. The use of dietary supplements for this purpose, in addition to the current recommended use of calcium and vitamin D supplementation, could be a valuable approach if safe and effective agents can be identified. Public use of natural products and botanical dietary supplements has increased steadily in this country within the last decade, particularly among women between the ages of 40 – 69 (1). Despite the popularity of natural product supplements, rigorous scientific evidence supporting their use is lacking for most botanicals (2). Only three percent of traditionally used medicinal plants have been studied under western scientific methods for therapeutic potential (3); therefore, botanical products may represent an untapped resource in the arsenal for osteoporosis prevention. Botanical phytoestrogens have already been targeted for scientific evaluation as an alternative to hormone replacement therapy for preservation of bone during menopause (4–6). However, any agent that acts via estrogen receptors (ERs) may also have ER-mediated side effects. The identification of botanical dietary supplements that preserve bone via non-ER pathways may provide a safer alternative.
Botanical extracts isolated from the rhizome of turmeric (Curcuma longa L., Zingiberaceae), a perennial plant native to Asia, have been utilized as anti-inflammatory agents in Ayurvedic and Traditional Chinese Medicine (TCM) for centuries. During the course of in vivo studies assessing turmeric’s anti-arthritic effects, our laboratory discovered that curcuminoid-containing turmeric extracts also prevent osteoclastogenesis and peri-articular bone destruction in a model of rheumatoid arthritis, with a chemically complex turmeric fraction (only 41% curcuminoids by weight), offering more bone-protective effects than a curcuminoid-enriched turmeric fraction (94% curcuminoids by weight; 7–9). In these arthritis studies, turmeric extracts prevented the activation of the transcription factor NF-κB and suppressed the subsequent expression of genes mediating the destruction of cartilage and bone, including RANK-L, the ligand that binds to and activates the receptor activator for NF-κB (RANK) on osteooclast precursors, which is a necessary and sufficient stimulus for the differentiation of bone-destroying osteoclasts (10). These in vivo findings are consistent with prior in vitro investigations of the anti-inflammatory effects of curcumin, one of turmeric’s three major phenolic curcuminoids that constitute 3–5% of the powdered rhizome (Figure 1), as numerous studies have identified blockade of NF-κB activation as a major mediator of curcumin’s biological effects, including its in vitro ability to prevent osteoclast differentiation (11–14). Novel pharmaceutical therapeutics targeting the RANK pathway are currently under development as an alternative to selective ER modulators (SERMs) and bisphosphonates for the treatment of osteoporosis (15). Thus, we posited that curcuminoid-containing turmeric extracts may be a botanical product of benefit in the prevention of RANK-mediated osteoporotic bone loss (11–14).
FIGURE 1.
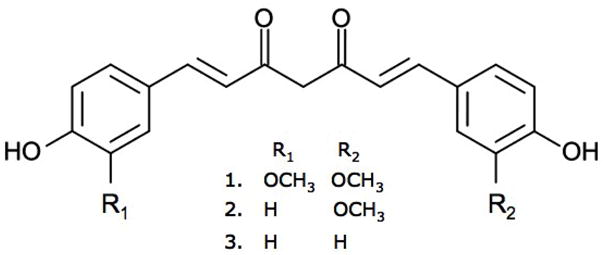
Chemical structures of curcumin (1), demethoxycurcumin (2), and bis-demethoxycurcumin (3).
Previous analyses by our laboratories of a random selection of marketed turmeric dietary supplements revealed that the botanical content of these products is variable and often not well reflected by product labeling (9). While most turmeric supplements assayed were labeled as containing an extract enriched (>95%) for curcumin, all of the products contained a mixture of turmeric’s three major curcuminoids in varying proportions, with recoverable total curcuminoid levels, with one exception, being only ~38% of that indicated on the label (9). The majority of turmeric dietary supplements tested did not contain turmeric’s essential oils. Guided by these data on turmeric dietary supplement composition, we chose to evaluate and compare two turmeric extracts analogous to those marketed today, both essential oil-free and containing a mixture of the 3 major curcuminoids, for efficacy in the prevention of hypogonadal bone loss in the ovariectomized rat model of postmenopausal osteoporosis. The extracts to be tested, which are the same as those previously demonstrated to prevent bone loss in the rat rheumatoid arthritis model (7–9), differ in their chemical complexity as one is a complex turmeric fraction containing only 41% curcuminoids, and the second is a curcuminoid-enriched turmeric fraction containing 94% curcuminoids. In the OVX rat model, we postulated that, analogous to the findings in arthritic rats, both curcuminoid-containing turmeric extracts would prevent bone loss, with the more complex turmeric fraction being the most efficacious.
Materials and Methods
Botanical extract isolation
As previously described, a complex turmeric fraction (Table 1) devoid of the essential oils containing 41% curcuminoids was prepared by methanol extraction of the marc obtained from an initial hexane extraction of ground turmeric rhizome (San Francisco Herb Co.; 8). A curcuminoid-enriched turmeric product (Table 1), sold as >98% curcumin, but composed of an essential oil-free 94% mixture of the three main curcuminoids was purchased from Fisher Scientific (#218580100, Lot A017528901; 8). Chemical content of the complex turmeric fraction and purified curcuminoids were assessed as previously described using an Agilent 1100 series high performance liquid chromatography (HPLC) system (Agilent, Palo Alto, CA) and stock solutions of pure curcumin, demethoxycurcumin, and bis-demethoxycurcumin (8). The turmeric fraction and the curcuminoid-enriched turmeric extract each contained the three major curcuminoids (curcumin > demethoxycurcumin > bis-demethoxycurcumin; Table 1, Figure 1) and were essential oil-depleted (8).
TABLE 1.
Composition of botanical extracts tested
| Turmeric extract | Total curcuminoids (% by weight) | Curcuminoid content (% of total) | ||
|---|---|---|---|---|
| curcumin | demethoxy-curcumin | bis-demethoxy-curcumin | ||
| Essential oil-free turmeric fraction (“complex turmeric”) | 40.6 | 63.3 | 21.4 | 15.3 |
| Curcuminoid-enriched turmeric fraction (“curcuminoid-enriched turmeric”) | 93.6 | 79.3 | 15.9 | 4.8 |
Animal procedures
Female three-month old Sprague Dawley rats were purchased from Harlan Laboratories, housed in plastic cages, and maintained on 12L/12D cycles at 22 ± 2°C, with access to water ad libidum. Animals were allowed to acclimate to the animal facility for one week prior to initiation of treatment, after which they were randomly assigned to the following treatment groups (n=10–13/group): 1) sham surgery + vehicle (DMSO) treatment, 2) surgical ovariectomy (OVX) + vehicle, 3) OVX + complex turmeric fraction, and 4) OVX + curcuminoid-enriched turmeric. Dosing began on the day of surgery (day 0) and continued for two months (56 days). All treatments were administered via intraperitoneal (ip) injection at 0.5 μl/g 3x per week. Botanical dosing was normalized to curcuminoid content (60mg curcuminoids/kg 3x per week, equivalent to a mean of 25mg curcuminoids/kg/d) so that any differential effect of treatment between the two extracts could be attributed to additional components present in the complex turmeric fraction. Because bioefficacy was a primary outcome, ip administration of the extracts, normalized to curcuminoid content, was utilized to eliminate variable absorption of curcuminoids due to matrix effects of the more complex product. The botanical dose tested was chosen based on its efficacy in preventing bone loss in previous in vivo arthritis experiments (9). All animals were pair-fed in order to minimize excessive weight gain in OVX animals (2016 Teklad Global 16% protein rodent diet, Harlan Laboratories; 16 g/d; 16% protein, 12.6 kJ/g, 4% fat, 3% crude fiber). At the termination of the experiment, liver, spleen, kidney, and uterus were weighed, and circulating white blood cell counts were determined using a Hemavet 880 analyzer (CDC Technologies, Oxford, CT). Cell differentials were determined by manual counting. Serum creatinine and alanine aminotransferase (ALT) levels were measured using an Endocheck Plus Chemistry Analyzer (Hemagen Diagnostics, Columbia, MD) to monitor for possible renal- or hepato-toxicity, respectively. All experiments were approved by The University of Arizona IACUC and conformed to the Guide for the Care and Use of Experimental Animals.
Assessment of bone mineral density by DXA
Serial assessments of bone mineral density (BMD) of the distal 25% of the femur were determined in vivo using a Piximus densitometer (General Electric Medical Systems, Milwaukee, WI) in anesthetized rats one week prior to OVX and bi-monthly for the duration of treatment. The distal femur was selected to assess the effect of botanical treatment on bone loss as this trabeculae-rich site undergoes a rapid loss in bone mass in hypogonadal rats (16).
Evaluation of bone microarchitecture by μCT
Two months following OVX surgery (day 56; 5 months of age), animals were sacrificed and femurs from each animal were fixed in 10% phosphate-buffered formalin (4°C, 48hrs), then transferred to 70% ethanol and stored at 4°C. A subset of samples were randomly selected from each group (n=4–5/group) and sent to the Endocrine Research Unit at San Francisco VA Medical Center for analysis by micro-computerized tomography (μCT). Scans of the distal femur were performed using Scanco vivaCT 40 instrument (Scanco Medical, Basserdorf, Switzerland), as previously described (17). To assess trabecular bone in the distal femoral metaphysis, 300 serial cross-sectional scans (10.5-μm voxel size) of the secondary spongiosa were obtained from the end of the primary spongiosa, extending proximally for ≈3.1 mm with 55-kV x-ray energy. For analysis of μCT images, a threshold (350 mg hydroxyapatite/cm3) was applied to segment or separate the mineralized bone matrix from soft tissue. Linear attenuation was calibrated with hydroxyapatite as the standard. Image analysis and 3D reconstructions were performed using the manufacturer's software (SCANCO Medical AG, Bassersdorf, Switzerland). For trabecular bone morphology the following variables were assessed: bone volume fraction (BV/TV, %), trabecular thickness (Tb.Th., mm), trabecular separation (Tb.Sp., mm), trabecular number (Tb.N., mm−1), and connectivity density (Conn-Dens., 1/mm3).
Serum markers for bone turnover
Biochemical markers of bone turnover were evaluated in serum obtained from the tail vein of fasted, anesthetized rats on days 14 and 56. C-telopeptide fragments of type I collagen (CTX-1) were measured by commercial enzyme-linked immunosorbent assay (RatLaps, Nordic Bioscience Diagnostics, Denmark, DK-2730), and serum levels of osteocalcin were assessed by rat-specific immunoradiometric assay (Immunotopics, Inc., San Clemente, CA, 50–1500).
Statistical Methods
Values are presented as mean ± SEM with differences considered significant at p < 0.05 determined by ANOVA with Student-Newman-Keuls post hoc testing using Instat software (Graphpad, San Diego, CA).
Results
Temporal changes in bone mineral density (BMD)
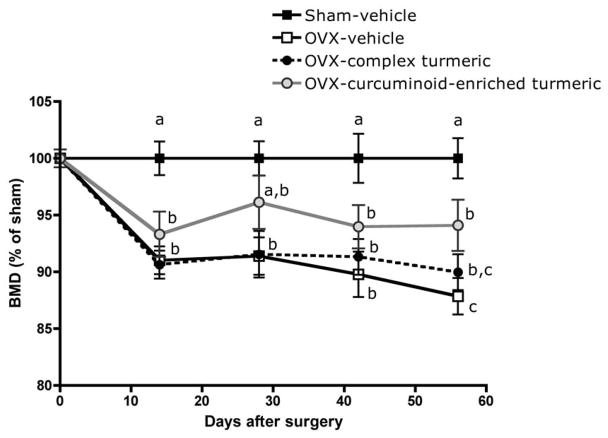
As anticipated, beginning at d 14 and continuing for two months (56 days), untreated OVX animals had significantly lower BMD at the distal femur relative to sham-operated animals (9–12% decrease, Figure 2). Complex turmeric treatment offered no significant BMD protection in OVX animals throughout the course of the experiment (Figure 2). In contrast, treatment with curcuminoid-enriched turmeric prevented up to 50% loss in BMD in OVX animals, reaching statistical significance after two months of treatment relative to vehicle-treated OVX (Figure 2).
FIGURE 2.
Effect of curcuminoid-containing turmeric extracts on bone mineral density (BMD) was assessed every two weeks by dual-energy x-ray absorptiometry (DXA). Three-month old female Sprague Dawley rats (n = 9–11 animals/group) were ovariectomized (OVX) and treated ip for two months (56 days) with vehicle, a complex turmeric fraction (41 % curcuminoids by weight), or a curcuminoid-enriched extract of turmeric (94% curcuminoids by weight). Both extract doses were normalized to curcuminoid content (60 mg/kg, three times per week). Values are expressed as mean % of sham ± SEM and statistical significance was determined by ANOVA with Student-Newman-Keuls post hoc test. Values that do not share the same superscript are significantly different at p < 0.05 for each time point.
Trabecular bone volume and microarchitecture
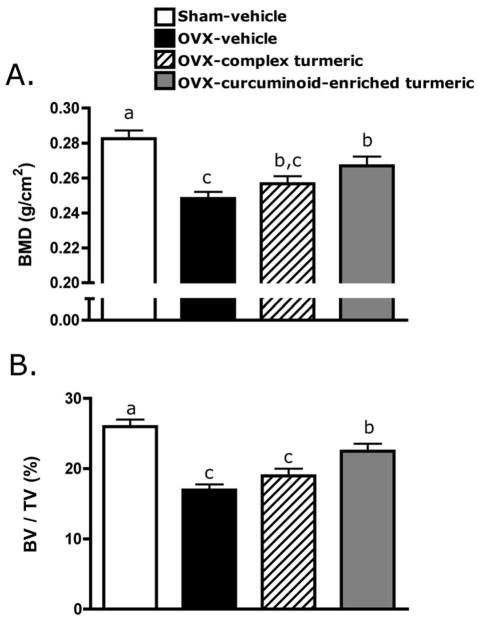
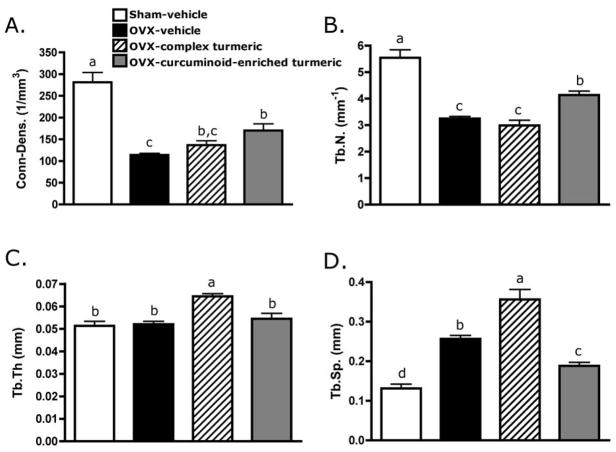
Observed effects of two months (56 days) of turmeric extract treatment on areal BMD of the trabeculae-enriched distal femur in OVX animals (Figure 3A) were confirmed and extended by μCT analysis of the three-dimensional architecture of purely trabecular bone in the femoral metaphysis (Figure 3B, Figure 4). As expected and consistent with documented changes in BMD (Figure 3A), in vehicle-treated OVX animals, trabecular bone volume fraction (BV/TV) decreased by 35% relative to sham (p < 0.001; Figure 3B). Complex turmeric treatment of OVX animals offered no bone protection as assessed by either areal BMD (Figure 3A) or BV/TV (Figure 3B). In contrast, curcuminoid-enriched turmeric treatment, analogous to its 50% inhibition of BMD loss (Figure 3A), prevented 63% of the decrease in trabecular BV/TV occurring in OVX animals (p < 0.05; Figure 3B). Changes in microarchitectural parameters, as assessed by μCT, were consistent with observed effects on BV/TV. Vehicle-treated OVX animals, consistent with expectations, experienced a significant decline in connectivity density (Conn-dens.; p < 0.001, Figure 5A) and trabecular number (Tb.N.; p < 0.001, Figure 5B), with an increase in trabecular spacing (Tb.Sp.; p < 0.001, Figure 5D), while trabecular thickness (Figure 5C) remained unchanged relative to vehicle-treated sham animals. Complex turmeric treatment, consistent with its lack of effect on BMD or BV/TV, did not protect against OVX-induced changes in trabecular morphology (Figure 5A,B,D). However, Tb.Th. was increased relative to all groups (p < 0.001, Figure 5C). Trabecular morphology of curcuminoid-enriched turmeric treated animals was favorably enhanced relative to vehicle-treated OVX animals, as OVX-induced changes in Conn-dens. (p < 0.05, Figure 5A) and Tb.N. (p < 0.01, Figure 5B) and Tb.Sp. (p < 0.05, Figure 5D) were all inhibited by 34–55%.
FIGURE 3.
Effect of two months of curcuminoid-containing turmeric extracts on bone mineral density (BMD) and bone volume fraction (BV/TV) was assessed by dual-energy x-ray absorptiometry (DXA), and micro-computerized tomography (μCT), respectively. Three-month old female Sprague Dawley rats (n = 9–11 animals/group) were ovariectomized (OVX) and treated ip for two months with vehicle, a complex turmeric fraction (41% curcuminoids by weight), or a curcuminoid-enriched extract of turmeric (94% curcuminoids by weight). Both extract doses were normalized to curcuminoid content (60 mg/kg, three times per week). Statistical significance was determined by ANOVA with Student-Newman-Keuls post hoc test. Values that do not share the same superscript are significantly different at p < 0.05 for the respective timepoint. (A) After two months of treatment (d 56), BMD was significantly decreased in OVX-vehicle treated animals relative to sham-vehicle. Complex turmeric treatment had no effect on BMD relative to OVX-vehicle treated animals, however, treatment with curcuminoid-enriched turmeric extract significantly protected BMD, preventing 50% loss. (B) Effects of treatment on BMD were confirmed by μCT, a more sensitive measure of three-dimensional content and architecture of trabecular bone. Treatment effects on BV/TV mirrored BMD results on d 56, confirming a lack of effect of treatment with complex turmeric, and approximately 50% protection of BV/TV conferred by treatment with curcuminoid-enriched turmeric.
FIGURE 4.
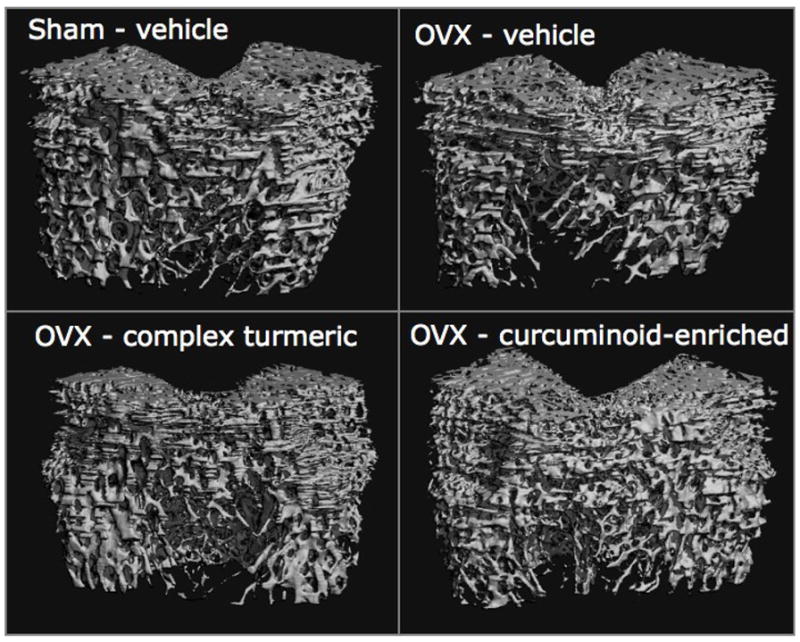
Representative microcomputed tomography images of the trabecular architecture of the distal femur in five-month female Sprague Dawley rats after two months (56 days) of treatment with complex turmeric, curcuminoid-enriched turmeric, or vehicle alone are presented.
FIGURE 5.
Parameters of trabecular morphology, (A) connectivity-density (Conn-Dens.), (B) trabecular number (Tb.N), (C) trabecular thickness (Tb.Th), and (D) trabecular spacing (Tb.Sp.) were measured by micro-computerized tomography (μCT). Three-month old female Sprague Dawley rats were ovariectomized (OVX) and treated ip for two months with vehicle, a complex turmeric fraction (41 % curcuminoids by weight), or a curcuminoid-enriched extract of turmeric (94% curcuminoids by weight). Both extract doses were normalized to curcuminoid content (60 mg/kg, three times per week). Statistical significance was determined by ANOVA with Student-Newman-Keuls post hoc test. Values that do not share the same superscript are significantly different at p < 0.05.
Serum markers of bone turnover
Acutely (two weeks post-OVX), in vehicle-treated OVX animals (relative to shams), the biomarker for bone formation (osteocalcin) increased significantly, while a trend toward an increase in the resorption biomarker (CTX-I) was not statistically significant (Table 2). At this same time point, after two weeks of treatment, turmeric extracts had no effect on bone turnover biomarkers in OVX animals (Table 2). At the end of the experimental period (two months or 56 days), resorption and formation biomarkers in vehicle-treated OVX animals were not statistically different than sham animals, and were also unaltered by curcuminoid-enriched turmeric treatment (Table 2).
TABLE 2.
Biochemical markers for bone turnover in female Sprague Dawley rats treated with curcuminoid-containing turmeric for 2 months1
| CTX-I (resorption) ng/ml |
Osteocalcin (formation) ng/ml |
|||
|---|---|---|---|---|
| 2 weeks | 2 months | 2 weeks | 2 months | |
| Sham - vehicle | 32.7 ±2.3 | 20.0 ±2.2 | 29.5 ±0.7 | 8.47 ±0.7 |
| OVX - vehicle | 37.7 ±1.4 | 26.4 ±2.3 | 37.4 ±1.2a | 9.62 ±0.3 |
| OVX - complex turmeric | 43.0 ±4.0 | not tested | 37.0 ±2.6a | not tested |
| OVX - curcuminoid-enriched | 40.7 ±4.0 | 29.1 ±3.4 | 33.9 ±2.5a | 10.8 ±3.4 |
Values are mean ±SEM, n = 9–11. Differences were determined by ANOVA for each column,
p < 0.01 relative to vehicle-treated sham.
Extra-skeletal effects of turmeric treatment
Body weight, consistent with previous reports, increased 16% in OVX relative to sham despite pair-feeding (18); this effect was unchanged by treatment with either extract (Table 3). Uterine atrophy was evident in OVX animals and was unaltered by complex turmeric or curcuminoid-enriched turmeric treatment (Table 3). Liver weights were slightly elevated in botanical-treated OVX animals, an effect that may be indicative of drug metabolism (19). However, this effect was only statistically significant with complex turmeric treatment (Table 3). Hematocrit, platelet number, white blood cell counts, serum creatinine, and ALT levels were not different between groups (Table 3).
TABLE 3.
Non-skeletal effects of turmeric treatment on female Sprague Dawley rats
| Sham - vehicle | OVX - vehicle | OVX - complex turmeric | OVX - curcuminoid enriched | |
|---|---|---|---|---|
| Body Weight (g) | 230 ± 2.8a | 267 ± 4.7 | 268 ± 4.3 | 269 ± 7.0 |
| Uterus (g) | 0.65 ± 0.06a | 0.16 ± 0.01 | 0.2 ± 0.03 | 0.18 ± 0.02 |
| Spleen (g) | 0.82 ± 0.06 | 0.92 ± 0.06 | 0.95 ± 0.07 | 1.00 ± 0.05 |
| Liver (g) | 6.01 ± 0.13 | 5.85 ± 0.19 | 6.89± 0.20a | 6.48 ± 0.24 |
| ALT (U/L) | 12.6 ± 1.1 | 14.5 ± 1.8 | 14.6 ± 2.5 | 15.9 ± 2.6 |
| Creatinine (mg/dL) | 0.30 ± 0.02 | 0.32 ± 0.02 | 0.27 ± 0.02 | 0.32 ± 0.03 |
| WBC (K/μL) | 10.1 ± 1.2 | 12.5 ± 0.9 | 12.3 ± 1.5 | 12.5 ± 1.2 |
| Hematocrit (%) | 48.2 ± 0.81 | 47.9 ± 0.95 | 41.7 ± 4.4 | 45.0 ± 3.1 |
| Platelet (K/μL) | 1033 ± 59 | 950.8 ± 69 | 1069 ± 47 | 903.4± 51 |
Values are presented as Mean ± SEM for n = 9–13 rats, with differences determined by ANOVA,
p<0.01 relative to OVX-vehicle controls.
Discussion
Turmeric is widely consumed as a spice, and mixtures of polyphenolic curcuminoids extracted in various degrees of purity from the rhizome of turmeric are available for use as dietary supplements (9). As most medical research has focused on pure curcumin, our studies are unique in that they evaluate turmeric extracts more analogous to the composition of currently available dietary supplements (9). Of the two turmeric extracts tested, the curcuminoid-enriched turmeric extract preserved BMD, as well as the microarchitectural structure and trabecular connectivity of bone in OVX rats. Key architectural parameters assessed by μCT that are known to be tightly correlated to mechanical strength and that suffer adversely with OVX, including bone volume fraction, trabecular number, connectivity density, and trabecular spacing (20,21), were significantly improved (34–55%) by curcuminoid treatment. Thus, these improvements in the architectural parameters of trabecular structure in OVX animals may translate into a reduction in fracture risk, which is the fundamental aim of all treatments targeting osteoporosis. This preservation of mass and microarchitecture of bone by curcuminoid-enriched turmeric did not appear to be an estrogenic effect, as uterine atrophy and weight gain in OVX animals were unaffected by curcuminoid treatment, results that are also consistent with prior studies documenting an lack of curcuminoid binding to ER-alpha or ER-beta (22–25). Although not directly tested here, since abrogation of NF-κB activation is thought to be a major biological effect of curcuminoids (11,26), it remains possible that NF-κB may be the central target in the process of protecting OVX-induced bone loss. Turmeric may thus have utility as a non-estrogen receptor (ER) therapeutic in the prevention of osteoporosis, a postulate that deserves further mechanistic investigation.
While the results from this translational study are encouraging, clinical data supporting the use of curcuminoids for osteoporosis is completely lacking. Of interest, however, is one clinical study that examined an oral dose of an uncharacterized curcuminoid product (4 g/day) in patients with monoclonal gammopathy, a potential precursor of multiple myeloma (27). This dose of curcuminoids significantly reduced urinary N-telopeptide type I collagen levels in patients, suggesting that curcuminoids may indeed act in humans as an anti-resorptive agent in diseases characterized by an elevation in bone turnover and generalized bone loss. Unfortunately, in our study the measurement of serum markers for bone turnover after two weeks and two months of OVX (d 14 and d 56, respectively), which were little altered in OVX animals, also did not reveal turmeric treatment effects that could have elucidated whether bone was being favorably altered by an increase in bone formation and/or inhibition of bone resorption. Examination of longitudinal BMD changes in curcuminoid-enriched turmeric treated OVX animals suggests that assay of bone turnover biomarkers after one month of treatment, when OVX-induced BMD loss appears to have stabilized, may have been more informative. Additional pre-clinical and clinical studies will be necessary to determine how curcuminoids act on both bone formation and resorption pathways in vivo under normal and pathological states.
We chose the intraperitoneal route of administration for testing and comparison of the two turmeric extracts as a proof-of-concept approach in an effort to eliminate variables that could alter the oral bioavailability of components in these complex botanicals, such as differential absorption of curcuminoids in the two extracts due to matrix effects of the additional components present in the more complex turmeric extract or the metabolism of non-curcuminoid components by intestinal microflora (28). As with bisphosphonates, oral bioavailability of curcumin is low (29,30). As a result, considerable research efforts are focused on identifying specific formulations to enhance curcuminoid oral bioavailability (e.g. curcuminoid nanoparticles or the use of lipid-based formulation vehicles to enhance absorption; 28,31). Thus, it is possible that currently available turmeric dietary supplements may not be the most ideal agents for osteoporosis prevention. Additionally, because essential oils in the turmeric rhizome have also recently been reported to enhance curcuminoid oral bioavailability (32), currently available turmeric dietary supplements, which, like the extracts tested here, do not contain essential oils, may not be the most optimal formulations for dietary curcuminoid supplementation.
Utilizing distinct and chemically well-characterized turmeric extracts, opposing trends for bone protection in translational models of arthritis vs. osteoporosis have now been identified, as the curcuminoid-enriched turmeric extract was most efficacious in protecting bone in osteoporosis, while we have previously demonstrated that the chemically complex turmeric fraction was most effective in preventing bone loss in arthritis (9). These divergent results illustrate the importance of direct testing of well-characterized botanical extracts in complex disease states (2). Moreover, because extract doses were normalized to curcuminoid content in the studies reported here, these results suggest that the non-curcuminoid constituents of the complex turmeric extract may have accounted for differences seen between the two turmeric treatment groups in altering bone loss in OVX animals. Preliminary studies in our laboratories have found that the non-curcuminoid portion of the complex turmeric extract is primarily composed of polysaccharides. Traditionally, these carbohydrates are thought to have minimal bioactivity by themselves, however, new functional effects of polysaccharides are emerging, particularly when combined with other bioactive molecules (33). This additional component of the complex extract may have had deleterious effects on bone that could not be compensated for by the curcuminoids, or the polysaccharides may have blunted or antagonized the beneficial effects of the curcuminoids on bone in the hypogonadal rat.
Another possibility for the variable efficacies of the two turmeric extracts tested is their differing ratios of the three major curcuminoids, which may be an important determinant of bone protection, as the purified extract contained a higher percentage of curcumin and a lower percentage of demethoxycurcumin and bis-demethoxycurcumin relative to the complex extract. Indeed, differential effects of the three major curcuminoids have been reported in a number of biological systems (31). Since all turmeric dietary supplements are composed of a mixture of the three major curcuminoids (9), this possibility would also have important implications from a consumer standpoint as turmeric supplement labels do not indicate the relative amounts of these 3 compounds present in the product (9). Additionally, our laboratories have identified vast discrepancies between the labeled curcuminoid content and the curcuminoid content detected by HPLC in a random selection of commercially available turmeric dietary supplements (9). Due to the importance of extract composition in bioefficacy, it is clear that the degree of specificity of labeling of turmeric supplements at present is not sufficient to direct usage, should specific turmeric extracts prove to be clinically useful upon further study.
As osteoporosis becomes a widespread health and financial burden to millions of aging Americans, the development of affordable bone-protective therapeutics for the prevention of metabolic bone disorders merits continued study. These results are encouraging, considering the current availability and affordability of turmeric-derived curcuminoid products. Continued characterization of turmeric dietary supplement composition followed by rigorous pre-clinical and clinical testing will be necessary to identify turmeric-derived bone-active constituents, and to optimize their bioefficacy in dietary form.
Acknowledgments
The authors wish to thank Ashley Lukefahr for critical reading of the manuscript.
This work was supported by grant number R21AT003614 (JLF) from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements, and training grant number F31AT004875 (LEW) from NCCAM. The authors claim no conflict of interest.
Literature Cited
- 1.Barnes P, Bloom B, Nahin R. Complementary and alternative medicine use among adults and children. United States: CDC National Health Statistics; 2008. Report #12. [PubMed] [Google Scholar]
- 2.Swanson S. Suggested guidelines for articles about botanical dietary supplements. Am J Clin Nutr. 2002;75:8–10. doi: 10.1093/ajcn/75.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Fabricant D, Farnsworth N. The value of plants used as traditional medicine for drug discovery. Environ Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver C, Martin B, Jackson G, McCabe G, Nolan J, McCabe L, Barnes S, Reinwald S, Boris M, Peacock M. Antiresorptive effects of phytoestrogen supplements compared to estradiol or risedronate in postmenopausal women using 41c methodology. J Clin Endocrinol Metab. 2009;94:3798–805. doi: 10.1210/jc.2009-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alekel D, Van Loan M, Koehler K, Hanson L, Stewart J, Hanson K, Kurzer M, Peterson C. Soy Isoflavones for Reducing Bone Loss (SIRBL) study: a 3-y randomized controlled trial in postmenopausal women. Am J Clin Nutr. 2010;91:218–30. doi: 10.3945/ajcn.2009.28306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Li Q, Wan H, Helferich W, Wong M. Genistein and a soy extract differentially affect three-dimensional bone parameters and bone-specific gene expression in ovariectomized mice. J Nutr. 2009;139:2230–6. doi: 10.3945/jn.109.108399. [DOI] [PubMed] [Google Scholar]
- 7.Wright L, Frye J, Timmermann B, Funk J. Effects of medicinal Zingiberaceae on bone loss in musculoskeletal disease. Annual Meeting of the NARCCIM; Minneapolis, MN, USA. May 12–15, 2009. [Google Scholar]
- 8.Funk J, Oyarzo J, Frye J, Chen G, Lantz R, Jolad S, Sólyom A, Timmermann B. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–5. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funk J, Frye J, Oyarzo J, Kuscuoglu N, Wilson J, McCaffrey G, Stafford G, Chen G, Lantz R, Jolad S, Sólyom A, Kiela P, Timmermann B. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis and Rheum. 2006;54:3452–64. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- 10.Boyle W, Simonet W, Lacey D. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 11.Bharti A, Takada Y, Aggarwal B. Curcumin (diferuloylmethane) inhibits receptor activator of NF-κB ligand-induced NF-κB activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol. 2004;172:1029–40. doi: 10.4049/jimmunol.172.10.5940. [DOI] [PubMed] [Google Scholar]
- 12.Mankan A, Lawless M, Gray S, Kelleher D, McManus R. NF-κB regulation: the nuclear response. J Cell Mol Med. 2009;13:631–43. doi: 10.1111/j.1582-4934.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzler I, Krebbel H, Kuckelkorn U, Heider U, Jakob C, Kaiser M, Fleissner C, Terpos E, Sezer O. Curcumin diminishes human osteoclastogenesis by inhibition of the signalsome-associated IκB kinase. J Cancer Res Clin Oncol. 2009;135:173–9. doi: 10.1007/s00432-008-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozaki K, Kawata Y, Amano S, Hanazawa S. Stimulatory effect of curcumin on osteoclast apoptosis. Biochem Pharmacol. 2000;59:1577–81. doi: 10.1016/s0006-2952(00)00277-x. [DOI] [PubMed] [Google Scholar]
- 15.Miller P. Denosumab: anti-RANKL antibody. Curr Osteoporos Rep. 2009;7:18–22. doi: 10.1007/s11914-009-0004-5. [DOI] [PubMed] [Google Scholar]
- 16.Sims N, Morris H, Moore R, Durbridge T. Increased bone resorption precedes increased bone formation in the ovariectomized rat. Calcif Tissue Int. 1995;59:121–7. doi: 10.1007/s002239900098. [DOI] [PubMed] [Google Scholar]
- 17.Chang W, Tu C, Chen T, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1(35):ra1, 1–13. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wronski T, Cintron M, Dann L. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int. 1988;43:179–83. doi: 10.1007/BF02571317. [DOI] [PubMed] [Google Scholar]
- 19.Greaves P. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation. 3. Elsevier; New York, NY: 2007. Liver and pancreas; p. 466. [Google Scholar]
- 20.Borah B, Dufresne T, Chmielewski P, Gross G, Prenger M, Phipps R. Risedronate preserves trabecular architecture and increases bone strength in vertebra of ovariectomized minipigs as measured by three-dimensional microcomputed tomography. J Bone Miner Res. 2002;17:1139–47. doi: 10.1359/jbmr.2002.17.7.1139. [DOI] [PubMed] [Google Scholar]
- 21.Borah B, Defresne T, Chmielewski P, Gross G, Gross M, Phipps R. Architecture is one of the determinants of bone strength. J Bone Miner Res. 2003;18:385. [Google Scholar]
- 22.Pfeiffer E, Esch H, Hohle S, Solyom A, Timmermann B, Metzler M. In vitro studies on the estrogenic activity and the metabolism of curcumin. In: Eisenbrand G, editor. Functional Foods: safety aspects. Deutsche Forschungsgemeinschaft, Senate Commission on Food Safety. Wiley-VCH Verlag; Weinheim, Germany: 2003. pp. 325–9. [Google Scholar]
- 23.Calligé M, Kieffer I, Richard-Foy H. CSN5/Jab1 is involved in ligand-dependent degradation of estrogen receptor α by the proteasome. Mol Cell Bio. 2005;25:4349–58. doi: 10.1128/MCB.25.11.4349-4358.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakeling A. The future of new pure antiestrogens in clinical breast cancer. Breast Cancer Res Treat. 1993;25:1–9. doi: 10.1007/BF00662395. [DOI] [PubMed] [Google Scholar]
- 25.Breithofer A, Graumann K, Scicchitano M, Karathanasis S, Butt T, Jungbauer A. Regulation of human estrogen receptor by phytoestrogens in yeast and human cells. J Steroid Biochem. 1999;67:421–9. doi: 10.1016/s0960-0760(98)00139-3. [DOI] [PubMed] [Google Scholar]
- 26.Jurenka J. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:277. [PubMed] [Google Scholar]
- 27.Golombick T, Diamond T, Badmaev V, Manoharan A, Ramakrishna R. The potential role of curcumin in patients with monoclonal gammopathy of undefined significance—Its effect on paraproteinemia and the urinary N-telopeptide of type I collagen bone turnover marker. Clin Cancer Res. 2009;15:5917–22. doi: 10.1158/1078-0432.CCR-08-2217. [DOI] [PubMed] [Google Scholar]
- 28.Manach C, Scalbert A, Morand C, Remesy C, Jinenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 29.Major P, Lipton A, Berenson J, Hortobagyi G. Oral bisphosphonates: a review of clinical use in patients with bone metastases. Cancer. 2000;88:6–14. [PubMed] [Google Scholar]
- 30.Anand P, Kunnumakkara A, Newman R, Aggarwal B. Bioavailability of curcumin: problems and promises. Mol Pharmacol. 2006;4:807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 31.Anand P, Thomas S, Kunnumakkara A, Sundaram C, Harikumar K, Sung B, Tharakan S, Misra K, Priyadarsini I, Rajasekharan K, Aggarwal B. Biological activities of curcumin and its analogues (Congeners) made by man and Mother nature. Biochem Pharmacol. 2008;76:1590–611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Antony B, Merina B, Iyer V, Judy N, Lennertz K, Joyal S. Pilot cross-over study to evaluate human oral bioavailability of BCM-95 CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci. 2008;70:445–9. doi: 10.4103/0250-474X.44591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanson B. A structural lexicon of medicinally important chemical families found in plants. In: Hanson B, editor. Understanding medicinal plants, their chemistry and therapeutic action. The Haworth Press Inc; Binghamton, NY: 2005. pp. 71–3. [Google Scholar]