Abstract
Whole organ engineering would benefit from a three-dimensional scaffold produced from intact organ-specific extracellular matrix (ECM). The microenvironment and architecture provided by such a scaffold would likely support site-appropriate cell differentiation and spatial organization. The methods to produce such scaffolds from intact organs require customized decellularization protocols. In the present study, intact adult porcine hearts were successfully decellularized in less than 10 h using pulsatile retrograde aortic perfusion. Serial perfusion of an enzymatic, nonionic detergent, ionic detergent, and acid solution with hypotonic and hypertonic rinses was used to systematically remove cellular content. The resultant cardiac ECM retained collagen, elastin, and glycosaminoglycans, and mechanical integrity. Cardiac ECM supported the formation of organized chicken cardiomyocyte sarcomere structure in vitro. The intact decellularized porcine heart provides a tissue engineering template that may be beneficial for future preclinical studies and eventual clinical applications.
Introduction
Biologic scaffolds composed of extracellular matrix (ECM) have been shown to promote the constructive remodeling of numerous tissue types in preclinical studies and in clinical practice.1–9 The most widely studied ECM scaffold materials include those derived from small intestine,6,10 urinary bladder,5,7,9 and dermis.11,12 The ECM of each tissue is synthesized by the resident cells and is in a state of dynamic equilibrium in response to environmental cues.13,14 Convincing arguments can be made for the advantages of tissue-specific ECM scaffolds for tissue-specific repair.15–22 Logically, a biologic scaffold derived from the targeted tissue source would possess the ideal three-dimensional (3D) architecture and biochemical composition to support tissue-specific cell phenotype, cell proliferation, and tissue biomechanical properties. If organs can be decellularized and still maintain their 3D integrity, the resulting scaffold would possibly represent the ideal scaffold for all components of the organ, including vascular and lymphatic structures, nerves, and the parenchymal cells.
Most commercially available biologic scaffold materials are manufactured as thin sheets. The source tissues are typically decellularized by immersion and agitation in a combination of salt solutions, detergents, and enzymatic solutions. Due to the density, mass, and 3D architecture of most whole organs such as the heart, liver, and kidney, these approaches are ineffective at removing cellular material.1,23 Recently, Ott et al. described the decellularization of rat hearts by vascular perfusion.19 Decellularization of a porcine heart was shown to be possible, but a comprehensive, reproducible, and time-effective decellularization technique was not provided.
The purpose of this article is to describe a decellularization method for a complex organ, specifically the porcine heart, by pulsatile retrograde aortic perfusion to generate cardiac extracellular matrix (C-ECM). Decellularization was confirmed by immunohistochemical (IHC) methods and DNA quantification. The C-ECM was characterized by immunohistochemical (IHC) analysis, scanning electron microscopy (SEM), and mechanical testing.
Materials and Methods
Preparation of C-ECM
Porcine hearts weighing approximately 300 g were obtained immediately after euthanasia of adult pigs. Excess fat and connective tissue were removed, and the ventricles were rinsed with water to remove coagulated blood. Each heart was frozen at −80°C for at least 16 h for storage and to aid in cell lysis. The hearts were then thawed in type 1 reagent–grade water at room temperature. The aorta was cannulated with a ½″ to ¼″ straight barbed reducer and connected to ¼″ internal diameter (ID) silicone tubing. Each heart was placed in a 4 L beaker containing 3 L of hypotonic type 1 water that was recirculated using a peristaltic pump (L/S® Drive EW-07550-30; Cole-Parmer, Vernon Hills, IL) for 15 min at 1 L/min. The type 1 water was replaced with 2 × phosphate-buffered saline (PBS) at 1 L/min each for 15 min. Three liters of 0.02% trypsin/0.05% ethylenediaminetetraacetic acid/0.05% NaN3 solution was warmed to 37°C using a digital hotplate and then perfused through the myocardial vasculature at 1 L/min for 2 h. A 3% Triton X-100/0.05% ethylenediaminetetraacetic acid/0.05% NaN3 was then used for perfusion followed by a 4% deoxycholic acid solution at 1.3 L/min each for 2 h at room temperature. After each chemical solution was used as a perfusate, type 1 reagent–grade water was perfused through the heart for approximately 5 min with no recirculation followed by recirculating 2 × PBS for 15 min to aid in cell lysis and removal of cellular debris and chemical residues. Disinfection was accomplished by perfusion of 0.1% peracetic acid/4% ethanol at 1.7 L/min for 1 h. The acid was neutralized and removed from the ECM by perfusing the intact matrix with PBS (pH 7.4) two times and type 1 water three times for 15 min each at 1.7 L/min. Fluid pressure was measured at the aorta during the entire decellularization process. The free walls of the left ventricle (LV) and right ventricle (RV) were excised, and were either used immediately for mechanical properties testing in the hydrated state or laid flat on nonstick aluminum foil, frozen at −80°C for at least 2 h, and then lyophilized until dry for biochemical analysis.
Immunohistochemistry and immunofluorescence studies
Full-thickness samples of C-ECM and native (nondecellularized) ventricles were fixed in 10% formalin and then paraffin embedded. Eight-micron-thick sections were cut and deparaffinized. Hematoxylin and eosin and 4′,6-diamidino-2-phenylindole were used to evaluate the presence of nuclear material. Movat's Pentachrome stain was used to allow observation of the distribution of nuclei, elastic fibers, collagen, glycosaminoglycans (GAGs), fibrin/fibrous structures, and muscle. Herovici stain was used to discriminate and observe collagen I and III in the ECM scaffolds. In addition, collagen IV, a basement membrane component, was observed using a mouse anti-human collagen IV antibody. Slides were imaged using a Nikon™ E600 microscope with 4 × and 20 × objectives and captured using MetaVue™ Software package (Molecular Devices, Sunnyvale, CA).
Scanning electron microscopy
C-ECM ventricle and native ventricle were fixed with 2.5% glutaraldehyde followed by dehydration by 1% osmium tetroxide. The samples were then dehydrated in a graded series of ethanol concentrations in PBS. The samples were sputter coated with 3.5 μm of gold and observed using a Jeol 9335 field emission gun SEM (Jeol, Tokyo, Japan) to capture standard scanning electron digitized images at 1000 × and 30 ×.
DNA quantification
Approximately 10 mg of native and ventricular C-ECM was digested with 0.1 mg Proteinase K (Sigma-Aldrich, St. Louis, MO) in 1 mL of PBS at 37°C on a rocker overnight. The digest was then purified using phenol/chloroform/isoamyl alcohol.24 The Quant-iT™ PicoGreen® dsDNA assay (Molecular Probes, Eugene, OR) was used for quantification of the amount of DNA using the manufacturer's instructions. Samples were evaluated in triplicate. Equal volumes of digest were separated by gel electrophoresis in a 1% agarose gel with ethidium bromide at 60 V for approximately 1 h, and the gel was observed under ultraviolet transillumination to determine the fragment size of residual DNA.
GAGs and elastin quantification
Total sulfated GAGs, and cross-linked elastin within the C-ECM were determined using the manufacturer's instructions (Biocolor, Carrickfergus, United Kingdom). One sample of three lots each of lyophilized C-ECM LV and RV as well as one sample of LV and RV from native ventricle were digested with papain for the GAG and elastin assay. Samples were evaluated in duplicate, and all values were normalized to 1 mg dry sample for comparison. A two-tailed Student's t-test was performed to determine whether differences existed between the GAG and elastin contents of native ventricle versus C-ECM with the p-value set at 0.05 (Minitab® version 15.1.1.0; Minitab, State College, PA).
Ball burst mechanical testing
An MTS Insight with a 2000 N MTS load cell model 569327-03 (MTS, Eden Prairie, MN) was used with a ball burst compression cage (Instron, Norwood, MA) to measure the biaxial burst strength of native LV and RV and the C-ECM derived from each ventricle. The test was performed in accordance with ASTM D3787-07 Test Method for Bursting Strength of Textiles-Constant-Rate-of-Traverse Ball Burst with deviations for sample geometry. Similar methodology has been used in multiple published studies to describe the biaxial strength of synthetic and biologic scaffolds.23,25–27 The native ventricles were excised and tested the same day as euthanasia. The C-ECM was tested within 48 h of the completion of decellularization. For all groups, the specimen was clamped in the fixture such that the polished ball contacted the endocardium. A 25.4 mm polished steel ball was advanced at a constant rate (25.4 mm/min) through the test material. Each experiment was conducted three separate times for each ventricle in the native and decellularized form. A two-tailed Student's t-test was performed to determine whether differences existed between the maximum force to failure and extension at maximum force for the LV and RV C-ECM compared with the values for native LV and RV with the p-value set at 0.05 (Minitab version 15.1.1.0; Minitab).
Cell seeding
Lypophilized C-ECM sheets were packaged in sterilization pouches and terminally sterilized using ethylene oxide. As previously described, Hamburger-Hamilton Stage 31 (day 7) White leghorn chicken embryonic cardiomyocytes (CMs) were isolated using collagenase and trypsin digests and preplated for 1 h to selectively isolate CMs.28,29 CMs were seeded on the luminal side of the C-ECM sheet. Approximately 500,000 cells/cm2 were cultured on the ECM scaffolds for 4 days in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 1% chick embryo extract, and 1% penicillin and streptomycin. The scaffolds were then fixed in 4% paraformaldehyde for 20 min followed by rinsing with PBS. Monoclonal anti-α-actinin antibody (clone EA-53; Sigma-Aldrich) and anti-β-tubulin antibody (TU-06; Abcam, Cambridge, MA) were used to distinguish CMs from other cell types.28 The scaffolds were placed between two cover slips and imaged using an inverted Olympus Fluoview 1000 confocal microscope (Olympus, Center Valley, PA).
Results
Preparation of C-ECM
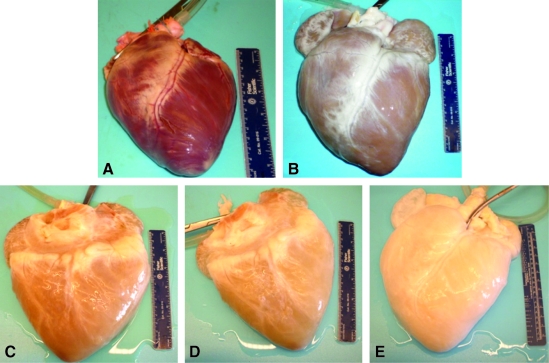
At the beginning of the decellularization process, the mean perfusion pressure was recorded as approximately 150 mm Hg. During the trypsin step, the hearts lost some of their red–brown coloration and became more flaccid, and the reagent became pinkish in appearance. With each subsequent reagent change, the hearts became whitish in appearance and expanded to approximately twice the original volume (Fig. 1). Although the flow rate was increased throughout the decellularization process, the mean perfusion pressure dropped to approximately 50 mm Hg by the time of the final rinses. Sixteen porcine hearts have been successfully decellularized using the method described.
FIG. 1.
Representative images of the gross appearance of intact porcine hearts subjected decellularization by retrograde perfusion. (A) Before decellularization, (B) after 0.02% trypsin, (C) after 3% Triton X-100, (D) after 4% sodium deoxycholate, and (E) after 0.1% peracetic acid.
Immunohistochemistry
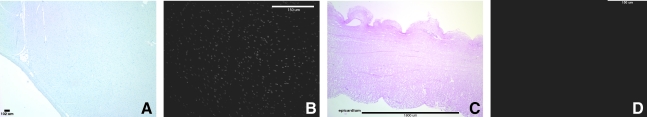
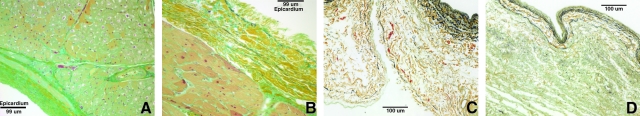
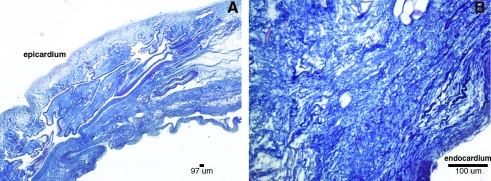
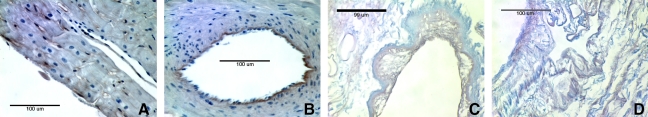
Hematoxylin and eosin and 4′,6-diamidino-2-phenylindole showed no visible cell nuclei or double-stranded DNA at 40 × and 200 × magnification, respectively, whereas the native heart showed dense cellularity (Fig. 2). Movat's Pentachrome staining of the C-ECM showed the absence of muscle cells after decellularization (Fig. 3). Movat's and Herovici's staining showed the presence of collagen type I and III as well as elastin, with particularly dense collagen structure localized at the epicardium and endocardium (Figs. 3 and 4). The basement membrane structures present within native ventricle tissue and C-ECM were identified by the positive staining for collagen IV, a basement membrane component, on the endocardium, myocardium, and coronary arteries (Fig. 5).
FIG. 2.
Representative photomicrographs showing no nuclear staining after perfusion decellularization. (A) Native ventricle hematoxylin and eosin, (B) native ventricle 4′,6-diamidino-2-phenylindole, (C) cardiac extracellular matrix (C-ECM) hematoxylin and eosin, and (D) C-ECM 4′,6-diamidino-2-phenylindole.
FIG. 3.
Movat's Pentachrome photomicrographs of (A) native ventricle epicardial surface, (B) native ventricle endocardial surface, (C) C-ECM endocardial surface with coronary, and (D) C-ECM epicardial surface. All at 200 ×; nuclei, purple/black; elastic fibers, black; collagen, yellow; proteoglycan, green; muscle, light red; fibrin/fibrous structures, vibrant red.
FIG. 4.
Herovici stain of C-ECM. (A) 40 × epicardial surface labeled and (B) 200 × endocardial surface labeled collagen I (blue) and collagen III (pink).
FIG. 5.
Collagen IV staining of (A) native left ventricle (LV) endocardium, (B) native LV coronary artery, (C) C-ECM endocardium, and (D) C-ECM coronary artery. All at 400 ×.
Scanning electron microscopy
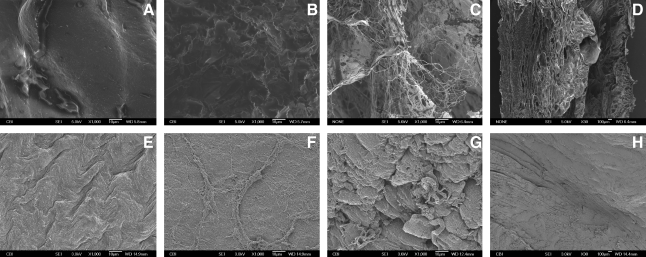
The native and C-ECM epicardium and endocardium both showed a dense collagen layer with topographic variances. The native ventricle has a densely cellular myocardium, whereas the C-ECM shows a more open configuration within the myocardium. Intact vascular matrix was evident throughout the C-ECM. Figure 6 shows a 0.5-mm-diameter coronary artery in the cross-sectional view. No cells were visible in any of the SEM samples for C-ECM, but they were apparent in the native samples (Fig. 6).
FIG. 6.
Scanning electron microscopy of native and lyophilized C-ECM from the LV. (A) C-ECM epicardium 1000 ×, (B) C-ECM endocardium 1000 ×, (C) cross section of C-ECM 30 ×, (D) cross section of C-ECM 1000 ×, (E) native epicardium 1000 ×, (F) native endocardium 1000 ×, (G) cross section of native 30 ×, and (H) cross section of native 1000 ×.
DNA quantification
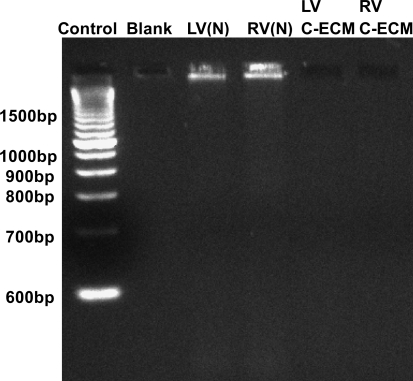
Quantitative analysis of DNA content within the C-ECM with the Pico Green assay showed a significant decrease in the amount of DNA compared to the DNA present in the native ventricles (0.66 ng DNA/mg sample vs. 8.48 ng DNA/mg sample [p = 0.014]). This value represents a 92% decrease in the amount of the double-stranded DNA found in the tissue as a result of decellularization (Table 1). The ethidium bromide gel showed no DNA bands associated with the decellularized C-ECM, whereas the native ventricle showed a large band above 1500 bp (Fig. 7).
Table 1.
Extracellular Matrix Component Quantification
| Sample | DNA (ng) | GAG (μg) | Elastin (μg) |
|---|---|---|---|
| Native heart | 8.48 | 4.72 | 38.74 |
| STDV | 0.45 | 0.39 | 38.23 |
| C-ECM | 0.66 | 5.43 | 19.72 |
| STDV | 0.20 | 0.24 | 3.40 |
Values are normalized to 1 mg lyophilized sample.
GAG, glycosaminoglycan; C-ECM, cardiac extracellular matrix; STDV, standard deviation.
FIG. 7.
DNA fragment size as determined by ethidium bromide gel. (N), native. RV, right ventricle.
GAG and elastin quantification
The amount of GAGs and elastin in C-ECM was not different from that measured in the native ventricle tissue (p < 0.05) (Table 1).
Ball burst mechanical testing
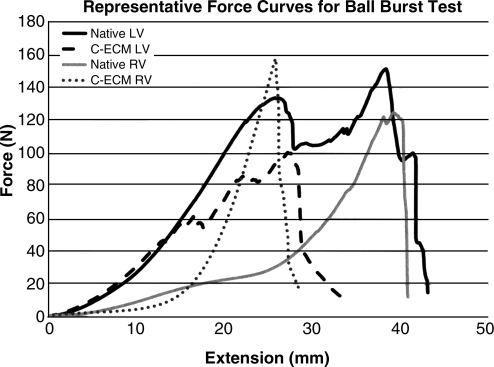
The maximum force or extension at maximum force of C-ECM for the respective ventricle was not different from the native ventricle (p < 0.05), although the average extension at maximum force was greater for the native ventricles (Table 2). The native ventricles showed multiple subfailure peaks that were associated with failure of layers within the tissue, whereas the C-ECM showed a smoother curve consistent with rotation and extension of fibers that likely occurred as a result of dilation of the heart during decellularization (Fig. 8). Failure was observed as the ball penetrated through the epicardium in all groups (Fig. 8).
Table 2.
Maximum Force and Extension at Maximum Force with Standard Error and p-Value of t-Test Comparing Native Ventricle to Cardiac Extracellular Matrix Ventricle
| Native LV | C-ECM LV | Native RV | C-ECM RV | |
|---|---|---|---|---|
| Average maximum force (N) | 130.77 | 113.99 | 132.49 | 125.00 |
| Standard error | 14.68 | 22.80 | 8.98 | 19.86 |
| t-Test between native and C-ECM | 0.58 | 0.76 | ||
| Average extension at maximum force (mm) | 30.26 | 26.30 | 34.72 | 26.10 |
| Standard error | 5.52 | 0.60 | 6.46 | 1.27 |
| t-Test between native and C-ECM | 0.55 | 0.32 |
LV, left ventricle; RV, right ventricle.
FIG. 8.
Graph of representative force curves for ball burst test.
In vitro cell seeding
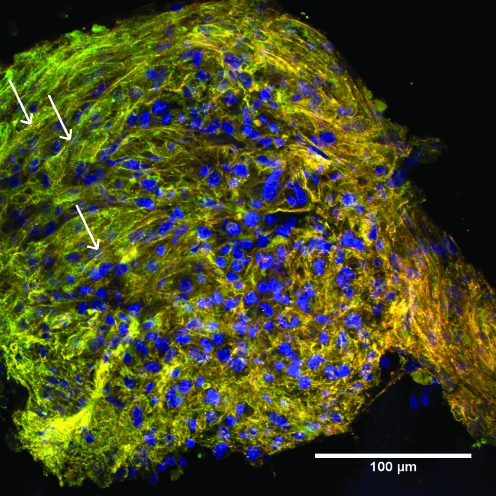
C-ECM sheet supported the formation of organized CM sarcomere structure, as indicated by striations of the α-actinin fibers.30 The α-actinin fibers showed no global preferred orientation on the scaffold. CMs were the primary population of cells observed on the C-ECM, as only a few cells solely stained positive for β-tubulin (Fig. 9).
FIG. 9.
Cardiomyocytes (CMs) cultured on ECM for 4 days on endocardial surface of C-ECM (400 ×). CMs are identified by positive α-actinin staining and positive β-tubulin staining (green–yellow). Non-CMs are recognized by negative α-actinin staining and positive β-tubulin staining (red). Arrows denote example of striations.
Discussion
The present study described a reproducible and time-efficient decellularization technique for the intact porcine heart. The technique utilized retrograde aortic perfusion with successive hypertonic, hypotonic, enzymatic, acid, and detergent solutions to maximize the distribution of chemicals throughout the tissue, maximize the disruption of cells, and minimize the damage to the ECM. The protocol took less than 10 h to complete, and effectively removed DNA from the tissue to levels comparable to other commercially available ECM products.24,31
It is important to limit the tissue exposure time and concentration of trypsin, Triton® X-100, deoxycholic acid, sodium dodecyl sulfate, peracetic acid, or other commonly used decellularizing agents, as each solution can have a disruptive effect on the ECM structure while removing the cellular components.32 By appropriate utilization of the series of reagents described in the present method, the decellularization time was significantly reduced from the time reported for decellularization of porcine heart valves, sliced porcine heart, and intact rat hearts.19,33–35 A previous study on the decellularization of heart valves with trypsin and Triton X-100 found that collagen and elastin structure were severely disrupted and that stable suture lines for anastomosis could not be formed.36 In the present study, the IHC, SEM, and mechanical data showed intact collagen structure. The complex 3D architecture of the heart was maintained, including vasculature and basement membrane structures. The biaxial rupture strength of the C-ECM was not different from the native tissue; this result is especially impressive as the dry weight of the C-ECM samples tested was approximately 1/3 of the native samples (data not shown).
The composition of the resulting C-ECM was largely preserved, with similar GAG (essential for water retention) and elastin content compared to native ventricles. The C-ECM was shown to be a suitable substrate for cardiac cell attachment with maintenance of CM phenotype. Although the method described is specific to cardiac tissue, the technique could be systematically modified for decellularization of other tissues and organs.
As shown by Ott et al. on a smaller scale, an intact decellularized porcine heart could serve as a scaffold for cardiac organ engineering.19 The remnant vasculature serves as a conduit for distribution of select cells throughout the tissue and for delivery of cell culture media to support cell viability and differentiation. The presence of an organ-specific ECM may also provide signals to seeded cells to enhance their differentiation and function.37,38 Additional work will be required to determine the optimal culture conditions for recellularization of a porcine heart with appropriate cellular diversity and restored tissue function before transplantation of a tissue-engineered heart becomes feasible.
Summary and Conclusion
The porcine heart can be efficiently decellularized using aortic retrograde perfusion. The resultant natural biodegradable scaffold maintains much of its complex structure and composition. While there is much work to be done, the methodology described herein provides a useful step to fully realizing an engineered complex organ. With slight modification, it is conceivable that the perfusion decellularization method described herein could be used to decellularize other organs, such as the liver, kidney, or lung.
Acknowledgment
Partially supported by the NIH-NHLBI training grant (T32-HL76124) entitled “Cardiovascular Bioengineering Training Program.”
Disclosure Statement
No competing financial interests exist.
References
- 1.Badylak S.F. Freytes D.O. Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Badylak S.F. Kochupura P.V. Cohen I.S. Doronin S.V. Saltman A.E. Gilbert T.W. Kelly D.J. Ignotz R. Gaudette G.R. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15(Suppl 1):S29. doi: 10.3727/000000006783982368. [DOI] [PubMed] [Google Scholar]
- 3.Badylak S.F. Vorp D.A. Spievack A.R. Simmons-Byrd A. Hanke J. Freytes D.O. Thapa A. Gilbert T.W. Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert T.W. Gilbert S. Madden M. Reynolds S.D. Badylak S.F. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann Thorac Surg. 2008;86:967. doi: 10.1016/j.athoracsur.2008.04.071. ; discussion 967–974. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert T.W. Nieponice A. Spievack A.R. Holcomb J. Gilbert S. Badylak S.F. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res. 2008;147:61. doi: 10.1016/j.jss.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert T.W. Stewart-Akers A.M. Simmons-Byrd A. Badylak S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 7.Nieponice A. Gilbert T.W. Badylak S.F. Reinforcement of esophageal anastomoses with an extracellular matrix scaffold in a canine model. Ann Thorac Surg. 2006;82:2050. doi: 10.1016/j.athoracsur.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Ota T. Gilbert T.W. Badylak S.F. Schwartzman D. Zenati M.A. Electromechanical characterization of a tissue-engineered myocardial patch derived from extracellular matrix. J Thorac Cardiovasc Surg. 2007;133:979. doi: 10.1016/j.jtcvs.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 9.Zantop T. Gilbert T.W. Yoder M.C. Badylak S.F. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of Achilles tendon reconstruction. J Orthop Res. 2006;24:1299. doi: 10.1002/jor.20071. [DOI] [PubMed] [Google Scholar]
- 10.Dejardin L.M. Arnoczky S.P. Ewers B.J. Haut R.C. Clarke R.B. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 11.MacLeod T.M. Sarathchandra P. Williams G. Sanders R. Green C.J. Evaluation of a porcine origin acellular dermal matrix and small intestinal submucosa as dermal replacements in preventing secondary skin graft contraction. Burns. 2004;30:431. doi: 10.1016/j.burns.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Wainwright D.J. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 13.Bissell M.J. Hall H.G. Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 14.Nelson C.M. Bissell M.J. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson T.W. Liu S.Y. Schmidt C.E. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10:1346. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 16.Macchiarini P. Jungebluth P. Go T. Asnaghi M.A. Rees L.E. Cogan T.A. Dodson A. Martorell J. Bellini S. Parnigotto P.P. Dickinson S.C. Hollander A.P. Mantero S. Conconi M.T. Birchall M.A. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 17.Montoya C.V. McFetridge P.S. Preparation of ex vivo-based biomaterials using convective flow decellularization. Tissue Eng Part C Methods. 2009;15:191. doi: 10.1089/ten.tec.2008.0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omae H. Zhao C. Sun Y.L. An K.N. Amadio P.C. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J Orthop Res. 2009;27:937. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott H.C. Matthiesen T.S. Goh S.K. Black L.D. Kren S.M. Netoff T.I. Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 20.Ozeki M. Narita Y. Kagami H. Ohmiya N. Itoh A. Hirooka Y. Niwa Y. Ueda M. Goto H. Evaluation of decellularized esophagus as a scaffold for cultured esophageal epithelial cells. J Biomed Mater Res A. 2006;79:771. doi: 10.1002/jbm.a.30885. [DOI] [PubMed] [Google Scholar]
- 21.Sellaro T.L. Ravindra A.K. Stolz D.B. Badylak S.F. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13:2301. doi: 10.1089/ten.2006.0437. [DOI] [PubMed] [Google Scholar]
- 22.Xu C.C. Chan R.W. Tirunagari N. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 2007;13:551. doi: 10.1089/ten.2006.0169. [DOI] [PubMed] [Google Scholar]
- 23.Freytes D. Badylak S. Webster T. Geddes L. Rundell A. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials. 2004;25:2353. doi: 10.1016/j.biomaterials.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert T.W. Freund J.M. Badylak S.F. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freytes D.O. Stoner R.M. Badylak S.F. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84:408. doi: 10.1002/jbm.b.30885. [DOI] [PubMed] [Google Scholar]
- 26.Horan R.L. Bramono D.S. Stanley J.R. Simmons Q. Chen J. Boepple H.E. Altman G.H. Biological and biomechanical assessment of a long-term bioresorbable silk-derived surgical mesh in an abdominal body wall defect model. Hernia. 2009;13:189. doi: 10.1007/s10029-008-0459-9. [DOI] [PubMed] [Google Scholar]
- 27.Coburn J.C. Brody S. Billiar K.L. Pandit A. Biaxial mechanical evaluation of cholecyst-derived extracellular matrix: a weakly anisotropic potential tissue engineered biomaterial. J Biomed Mater Res A. 2007;81:250. doi: 10.1002/jbm.a.30943. [DOI] [PubMed] [Google Scholar]
- 28.Tobita K. Liu L.J. Janczewski A.M. Tinney J.P. Nonemaker J.M. Augustine S. Stolz D.B. Shroff S.G. Keller B.B. Engineered early embryonic cardiac tissue retains proliferative and contractile properties of developing embryonic myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H1829. doi: 10.1152/ajpheart.00205.2006. [DOI] [PubMed] [Google Scholar]
- 29.Sreejit P. Kumar S. Verma R.S. An improved protocol for primary culture of cardiomyocyte from neonatal mice. In Vitro Cell Dev Biol Anim. 2008;44:45. doi: 10.1007/s11626-007-9079-4. [DOI] [PubMed] [Google Scholar]
- 30.Hilenski L.L. Xuehiu M. Vinson N. Terracio L. Borg T.K. The role of beta integrin in spreading and myofibrillogenesis in neonatal rat cardiomyocytes in vitro. Cell Motil Cytoskeleton. 1992;21:87. doi: 10.1002/cm.970210202. [DOI] [PubMed] [Google Scholar]
- 31.Derwin K.A. Baker A.R. Spragg R.K. Leigh D.R. Iannotti J.P. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert T. Sellaro T. Badylak S. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Ota T. Taketani S. Iwai S. Miyagawa S. Furuta M. Hara M. Uchimura E. Okita Y. Sawa Y. Novel method of decellularization of porcine valves using polyethylene glycol and gamma irradiation. Ann Thorac Surg. 2007;83:1501. doi: 10.1016/j.athoracsur.2006.11.083. [DOI] [PubMed] [Google Scholar]
- 34.Grauss R.W. Hazekamp M.G. Oppenhuizen F. van Munsteren C.J. Gittenberger-de Groot A.C. DeRuiter M.C. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. 2005;27:566. doi: 10.1016/j.ejcts.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 35.Singelyn J.M. Dequach J.A. Seif-Naraghi S.B. Littlefield R.B. Schup-Magoffin P.J. Christman K.L. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grauss R.W. Hazekamp M.G. van Vliet S. Gittenberger-de Groot A.C. DeRuiter M.C. Decellularization of rat aortic valve allografts reduces leaflet destruction and extracellular matrix remodeling. J Thorac Cardiovasc Surg. 2003;126:2003. doi: 10.1016/s0022-5223(03)00956-5. [DOI] [PubMed] [Google Scholar]
- 37.Segers V.F.M. Lee R.T. Stem-cell therapy for cardiac disease. Nature. 2008;451:937. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Latif A. Bolli R. Tleyjeh I.M. Montori V.M. Perin E.C. Hornung C.A. Zuba-Surma E.K. Al-Mallah M. Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]