Abstract
Sinusoidal endothelial cells (SECs) comprise the platform where trafficking into and out of the BM occurs and where hematopoietic stem and progenitor cells (HSPC) harbor and receive cues for self-renewal, survival, and differentiation. Therefore, SECs are referred to as a bone marrow vascular niche (BMVN). Hematopoietic regeneration has been shown to occur only with concurrent angiogenic regeneration. However, there are still not sufficient means to identify and isolate SECs, therefore the “niche endothelial cell” remains incompletely characterized. VEGF-receptor-3 (VEGFR3) is expressed exclusively by the SECs, while Sca1 and Tie2 are only expressed on the VEGFR3− arteriolar endothelium. We previously demonstrated the importance of vascular recovery in hematopoietic regeneration from myelosuppression due to cytotoxic agents or whole-body irradiation. Therefore to establish the functional importance of SECs, the mechanisms underlying BMVN regeneration were examined utilizing a 5-fluorouracil (5-FU) myelosuppression model of vascular damage. Injection of antibodies against murine VEGFR-1 and -2 had no significant effect on hemangiogenic recovery. However, when soluble VEGFR-1, a decoy receptor for VEGF-A and PlGF, was injected after 5-FU, both angiogenic remodeling and regeneration of megakaryopoiesis were delayed. In conclusion, we show that the bone marrow vasculature comprises heterogeneous compartments. SECs are distinguished from arterioles by unique immunophenotypes. Regeneration of damaged SECs is the rate-limiting step in hematopoietic regeneration from myelosuppressive therapy. Novel, high-efficiency VEGF-binding drugs in combination with chemotherapeutic agents may lead to cases of prolonged cytopenia.
Keywords: bone marrow vasculature, sinusoids, angiogenesis, regeneration, VEGF-A
Introduction
Hematopoietic stem cells (HSCs) residing in the bone marrow (BM) provide mammalian organisms with a steady supply of mature blood cells. In recent years, the ultrastructural basis for the functional capacity of the BM has been investigated. The current hypothesis holds that hematopoietic stem and progenitor cells (HSPCs) dwell in two specialized niches: the osteoblastic niche (OBN) and the BM vascular niche (BMVN).1–6 However, the BM vasculature is heterogeneous in nature. The 3-dimensional network of arterial vessels spanning the marrow cavity divides into arterioles and thereafter into capillaries, which supply the sinusoidal vessels. The sinusoids are interconnected by intersinusoidal capillaries and collectively drain into the central sinus.7 It is the specialized, discontinuous sinusoidal endothelial cells (SECs) that are the predominant vascular surface of the BM and constitute the functional hematopoietic niche.
In addition to providing a niche for the self-renewal, expansion, and maintenance of HSPCs, SECs play a role both in providing a differentiation platform for hematopoietic cells, such as megakaryocytes, and as a conduit for mobilization and homing of hematopoietic cells into and out of the BM.7,8 In contrast to the OBN, the BMVN is highly dynamic and undergoes profound changes after onset of myelosuppression via cytotoxic agents and/or irradiation. Pioneering work by Tavassoli and colleagues has shown that cyclophosphamide or ionic radiation not only damage the hematopoietic, but also the vascular, compartment of the BM.9,10 Since those early works, the biological effects of angiogenic factors including the family of vascular-endothelial growth factors (VEGFs), fibroblast growth factors (FGFs), angiopoietins (Ang-1, Ang-2), and stromal-derived factor 1 (SDF-1/CXCL-12) have been characterized. However, the role of angiogenic factors in the functional regulation of the BMVN both in the steady state and during stress, such as myelosuppression, is not entirely clear.
The connection between the blood vascular system and hematopoiesis has long been known since the hemangioblast hypothesis was suggested just a century ago.11–13 Since then, studies have shown that hematopoietic recovery occurs only after angiogenic regeneration.14 Recent data corroborated this concept in that the vascular endothelial receptor Tie2 has been shown to link hematopoietic and angiopoietic recovery,15 and the angiogenic phenotype of perisinusoidal megakaryocytes determines the rate of vascular and consecutively hematopoietic regeneration after myelosuppression.16 These findings have important clinical implications: neutropenia and thrombocytopenia are predominant causes of chemotherapy-related morbidity and mortality and often times are the dose-limiting toxicities in the treatment of malignancies with cytotoxic agents.17 Animal experiments have demonstrated that adenoviruses coding for proangiogenic substances like FGF-4 and SDF-1 can shorten aplasia after myelotoxic treatment.8 Whether the application of these and other recombinant proangiogenic cytokines would influence hemangiogenic recovery after myelosuppression has not been established.
Herein, we show that SECs are distinguished from other BM ECs by the expression of VEGFR-3. Moreover, we demonstrate that the combined blockade of vascular endothelial growth factor-A/placental growth factor (VEGF-A/PlGF) with soluble VEGFR-1 is sufficient to significantly delay hemangiogenic recovery after 5-FU.
Materials and Methods
Animals
C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were maintained in air-filtered Thoren units. Animal experiments were performed with the authorization of the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University and of Eberhard-Karls University of Tuebingen. Sex-matched, age-matched 6-to10-week-old animals were used for all studies.
Administration of 5-FU and Angiogenic Factors
Animals received 5-fluorouracil (5-FU) at a dose of 250 mg/kg intravenously (i.v.) at day 0. Some experimental animals were injected with neutralizing antibodies to VEGFR-1, cloneMF1 at 400 µg every 3 days intraperitoneal (i.p.)18 and/or VEGFR-2, clone DC101, at 800 µg i.p. every 3 days.19 MF1 and DC101 were kindly provided by ImClone Systems Incorporated (New York, NY). Other mice received a single i.v. dose of adenoviral vectors expressing soluble VEGFR-1 (AdsVEGFR) or in 150 µL phosphate-buffered saline (PBS) or vehicle control, 24 hours after injection of 5-FU.
Peripheral Blood Analysis
Retro-orbital peripheral blood (PB) was collected on days specified in the text and legends using micro-hematocrit capillary tubes (Fisher Scientific, Hampton, NH). White blood cell counts with differential, hemoglobin, calculated hematocrit, and platelets were obtained using Bayer Advia 120 Multi-Species Hematology Analyzer (Bayer HealthCare, Tarrytown, NY) with multi-species software (Bayer) on days −2, +4, +7, +10, +14, +18, +22, and +28 with capillary pipettes (Fisher Scientific). Peripheral blood counts are depicted as average values plus or minus SEM.
Bone Marrow Histology, Immunohistochemistry, and Immunofluorescence
Mice from each treatment group were sacrificed on the specified days after myelosuppression. Femurs were harvested and processed for either hematoxylin and eosin (H&E) staining or immunohistochemistry, essentially as previously described.15,16,20 Briefly, femurs were fixed in 2–4% paraformaldehyde (PFA), decalcified using Decalcifying Solution (Thermo Scientific, Waltham, MA, USA), and embedded in paraffin (Histoserv, Germantown, MD, USA). Paraffin sections were stained with H&E (Histoserv). For detection of mouse pan-endothelial cell antigen 32 (MECA32), VEGFR3, and thrombospondin-1 (TSP-1), paraffin sections were retrieved using Target Retrieval Solution (DAKO, Carpinteria, CA). After endogenous peroxidase and nonspecific protein block, primary antibodies were incubated overnight at 4°C as follows: anti–pan EC antigen mAb (clone MECA32, BD), anti-VEGFR3 mAb (clone AFL4, BD), or biotinylated mouse antihuman/mouse TSP-1 mAb (LabVision/Neomarkers, Fremont, CA). Polyclonal secondary antibodies (pAb, Jackson ImmunoResearch [IR], West Grove, PA) were incubated, followed by incubation in streptavidin horseradish peroxidase (HRP, Jackson IR). Staining was developed with DAB+ per manufacturer’s instructions (DAKO) and briefly counterstained in Meyer’s hematoxylin (DAKO) before coverslipping in Cytoseal permanent mounting media.
Histologic Image Acquisition and Analysis
Histological and IHC images of BM sections were captured with AxioCam and AxioVision software (Zeiss, Thornwood, NY) mounted on an Olympus BX51 microscope (Olympus America, Center Valley, VA).
Statistical Analysis
Results were statistically analyzed using a two-tailed Student’s t-test. The results are expressed as mean value plus or minus standard error of the mean (SEM). P less than 0.05 was considered significant.
Results
Phenotypic Heterogeneity of the Bone Marrow Vasculature
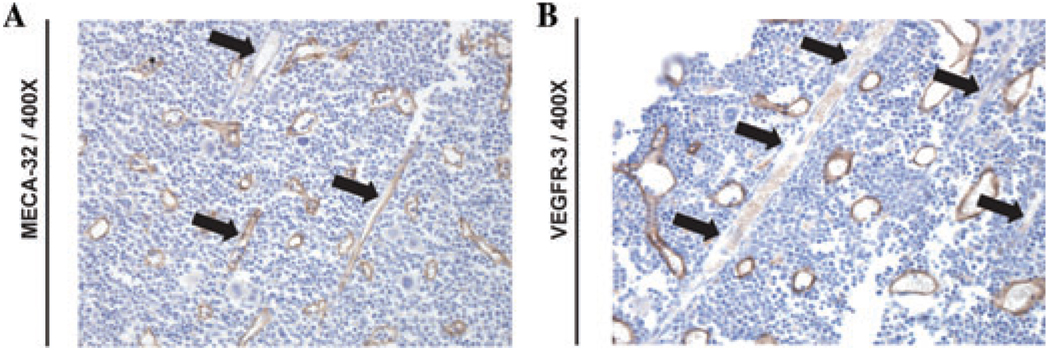
Utilizing modified standard immunohistochemical (IHC) and immunofluorescence (IF) protocols,20 we sought to immunophenotype BM SECs both at steady-state and during hemangiogenic regeneration in C57BL/6 mice. At steady state, the BM vasculature consists of small arterioles and capillaries supplying the radially and regularly distributed SECs. As we have shown previously, SECs are decorated by thrombospondin (TSP)+ megakaryocytes.16 As we have previously shown,20 SECs are positive for VEGFR3, whereas both arterioles and SECs were immunopositive for MECA32 (Fig. 1A, B). All endothelial cells stained positive for VE-cadherin, VEGFR2, and CD31 (data not shown). Moreover, while SECs are VEGFR3+ and Sca1−, the arteriolar endothelium was VEGFR3− and Sca1+ (data not shown).20
Figure 1.
BM SECs are VEGFR3+. WT C57BL/6 mice were stained with anti–pan endothelial cell antigen (clone MECA-32) and anti-VEGFR-3 (clone AFL4). Note that SECs are VEGFR3+ while MECA32+ arterioles are VEGFR3− (black arrows)
Based on these results, we propose a specific immunophenotypic signature for steady state BM SECs as VE-cadherin+MECA32+CD31+VEGFR2+VEGFR3+Sca1− while BM arterioles were identified as VE-cadherin+ MECA32+CD31+VEGFR2+VEGFR3− Sca1+.20
Dynamic Changes in the Sinusoidal Compartment after Myelosuppression
While it has long been known that myelosuppressive therapy damages not only hematopoietic cells, but also the vascular compartment, the effect of myelosuppression on the SECs has not been specifically examined. Although we have shown previously that 5-FU induces some damage to the BMECs, we sought to further assess the specific contribution of the SECs to recovery after myelosuppression.15 Utilizing VEGFR3as a specific immunomarker of SECs, we analyzed the injury to the vascular niche as a consequence of 5-FU treatment. C57BL/6 mice were injected i.v. with 5-FU at a myelosuppressive dose of 250 mg/kg and were allowed to recover. Femurs were harvested and analyzed at various time points after 5-FU. We found that recovery occurs differentially within anatomically defined regions of the BM. The distal femur showed the most prominent changes in both the degree of destruction of vascular structures and hypocellularity. Indeed, hemangiogenic recovery was delayed in the distal femur, and regeneration commenced in the femoral head, traveling down the femoral diaphysis towards the distal metaphysis, indicating that the functional BMVN in the proximal epiphysis/metaphysis is a significant regulator of regional hematopoietic recovery after myeloablation. The processes we observed in the myelosuppressed femora after 5-FU essentially resemble changes typical for the aging marrow in humans, where fatty metaplasia occurs distally, while hematopoietically active marrow remains confined to the proximal femur bone.21
Anti-VEGFR1 and/or Anti-VEGFR2 Neutralizing Antibodies Are not Sufficient to Modulate Hemangiogenic Recovery after 5-FU Myelosuppression
VEGFR-1 and -2 are critical vasculoendothelial receptors for proliferation, stabilization, and maintenance in early postnatal life. VEGF-signaling through these receptors is responsible for processes dependent on neoangiogenesis in the adult, such as angiogenic recovery after destructive events.22 Targeted anti-angiogenic therapeutic approaches, including anti-VEGF antibodies, have been introduced into clinical treatment of metastasized malignant disease, and typically these agents are delivered in combination with cytotoxic agents. We therefore sought to examine the influence of antibodies directed against VEGFR-1 and -2 during recovery from 5-FU myelosuppression. Mice (n = 16) received 5-FU at a dose of 250 mg/kg at day 0. Neutralizing monoclonal antibodies to VEGFR-1 (clone MF1, 400 µg i.p.), VEGFR-2 (clone DC101, 800 µg i.p.), or both in combination were injected i.p. on days 1, 4, 7, 10, and 13. The control group received vehicle alone. Retro-orbital blood collection for differential blood counts was performed on days 4, 7, 10, 14, 18, 22, and 25. The control and treatment groups did not differ in the extent or duration of cytopenia after myelosuppression (data not shown).
Reconstitution of the BMVN Is Dependent on VEGF-A/VEGFR Pathway
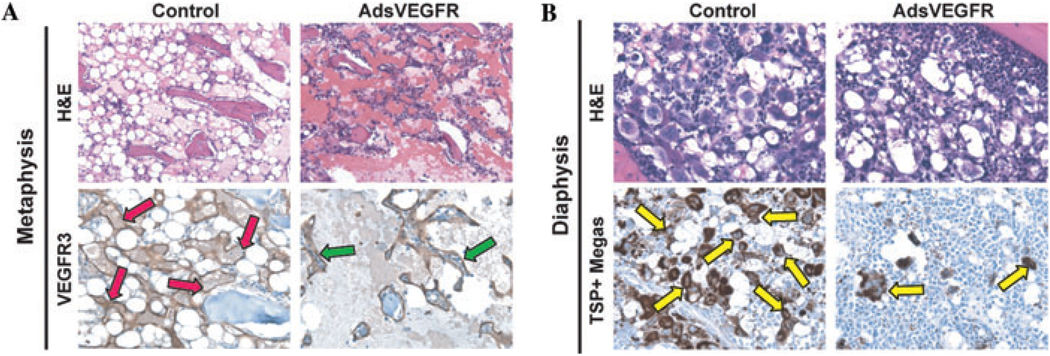
In order to test whether vascular reconstitution of the BM after myeloablation is dependent on VEGF/PlGF-mediated signaling, C57BL/6 mice were treated with 5-FU on day 0, followed by i.v. administration of adenoviral vectors encoding sVEGFR-1 (AdsVEGFR) on Day 1. This construct is designed to “trap” VEGF-A and PlGF and thereby prevent binding to the receptors VEGFR-1 and VEGFR-2. BM sections from the 5FU/AdsVEGFR-treated mice were examined at day 10 by immunostaining SECs for VEGFR3 and megakaryocytes for TSP (Fig. 2A,B). At Day 10, treatment with AdsVEGFR interfered with regeneration of VEGFR3+ SECs in the BM, particularly at the metaphyseal/epiphyseal distal region (Fig. 2A). In the distal metaphysis, the vasculature was highly disrupted and the residual SECs were dilated and dysfunctional (Fig. 2A, green arrows). Examination of the proximal diaphyseal region of the bone at day 10 demonstrated a dearth of megakaryocytes in the treated group as compared with control, which was undergoing robust megakaryopoiesis (Fig. 2B).
Figure 2.
Blockade of VEGF signaling in BM leads to vascular disruption and regional impairment of recovery after myelosuppression. C57Bl/6 mice were treated with 250 mg/kg 5-FU followed by administration of adenoviral vectors encoding for soluble VEGFR (AdsVEGFR) or vehicle at 24 h after 5-FU. Femurs were harvested at Day 10 and then stained for H&E, VEGFR-3, or TSP-1 as described in text. (A) H&E and VEGFR-3 immunostaining of metaphyseal region in control versus. AdsVEGFR group (red and green arrows, respectively). Note reduction in vascular integrity of VEGFR3+ SECs in treated animals. 200×. (B) H&E and anti-TSP immunohistochemistry of diaphyseal region in treated versus control. Note reduction in thrombopoiesis in treated group as measured by TSP immunoreactivity (yellow arrows). 200×.
Discussion
Although the sinusoidal vasculature of the BM has been found to harbor HSPCs, to govern hemangiogenic recovery after myelosuppression, and to provide a platform for differentiation of hematopoietic progenitor cells as well as a conduit for mobilization from and homing to the BM, the specific immunophenotype, molecular regulation, and therapeutic modulation capabilities of SECs have not been sufficiently examined.
Our group has previously shown that the BMVN is highly dynamic in that it undergoes regressive changes after myelosuppression followed by angiogenesis and remodeling, which contribute to hematopoietic regeneration.7,15,16 Interestingly, the BM vasculature is a heterogeneous pool of different types of vessels each with unique endothelia characterized by distinct expression patterns of EC markers that allow for the specific staining of these highly specialized cells.20 Herein, we provide an overview of immunohistochemical markers allowing for the specific identification of different BM ECs. We have shown that the differential expression of VEGFR3 can differentiate SECs from arterioles. Moreover, we describe regional changes of the femoral BM microarchitecture after myelosuppression that resemble age-related involution as the distal femur undergoes fatty metaplasia. Indeed, hemangiogenic regeneration after chemotherapy in mice represents a reversal of these age-related changes, with fatty metaplasia regressing in the opposite direction as the hematopoietic compartment regenerates. The molecular mechanisms of these changes remain unknown. However, the elucidation of the signaling pathways responsible for regeneration after myelosuppression may help us to reverse changes brought about by age and reestablish a functionally more active blood cell–producing marrow in elderly patients displaying prolonged cytopenia after chemotherapy.
The discontinuous BM SECs depend on maintenance signaling through receptors for VEGF-A, FGF-2, CXCR-4, and PDGFs.8,23 Cotreatment of cancer patients with the antihuman VEGF antibody bevacizumab has not been reported to result in increased hematotoxicity. Because the main receptors for VEGF-A—VEGFR-1 and VEGFR-2—mediate EC proliferation, survival, and migration and are expressed on SECs, we utilized antibodies against these VEGF-receptors to study their inhibitory effects on the functions of the BM vasculature. Mice that received neutralizing antibodies against VEGFR-1 and/or VEGFR-2 after 5-FU administration surprisingly did not display altered hemangiogenic regeneration profiles. However, adenovirally encoded soluble VEGFR-1 (“VEGF-trap”) on day 1 after 5-FU was sufficient to delay hemangiogenic regeneration: at day 10 after 5-FU, the sVEGFR-1-treated mice displayed dilated sinusoids decorated with a lower number of megakaryocytes as compared with control animals. These data suggest that a complete blockade of the VEGF-A/VEGFR1–2 signaling axis is indeed sufficient to delay vascular recovery in the BM after a myelosuppressive dose of 5-FU. Apparently, the blockade achieved by sVEGFR-1 is more pronounced as compared to antibody-based neutralization of VEGFR-1 and/or VEGFR-2.
One reason for the failure of anti-VEGFR-1 and VEGFR-2 antibodies to induce additional toxicity is likely to be the myelosuppression model chosen in the studies herein. In another report, we found that the degree of injury to the SEC population correlates with the degree of myelosuppressive insult.20 Therefore, although SEC injury does occur after 5-FU,15 5-FU is not a robust enough model to cause severe SEC regression, the recovery of which is VEGF-A dependent. In fact, we showed that hemangiogenic recovery from myelosuppression induced by 950 rad can be inhibited using neutralizing antibodies to VEGFR2.20
Another reason for the lack of activity of neutralizing antibodies to VEGFRs in the 5-FU model, may be due to a partially “private,” that is, autocrine, nature of VEGF-signaling maintaining the BMVN. An internal autocrine VEGFR-1 and VEGFR-2 loop mechanism has been postulated to be active in HSCs. Such “private” autocrine signaling is insensitive to extracellular inhibitors like antibodies, while intracellularly active VEGF-receptor tyrosine kinase inhibitors (RTKIs) have been shown to influence HSC survival in vitro.24 Therefore, VEGF-RTKIs may cause hematopoietic cytotoxicity in a dual way: by interfering with HSC and via BMSEC private VEGF-signaling.
We conclude from these studies that specific combinations of different angiogenic agents may be able to protect the BMVN after myelosuppression and accelerate recovery and that the combinations of agents is different depending on the severity of damage to the SECs and the regeneration profile of the BMVN. The determination of effective combination treatment doses could bring about substantial improvements for patients suffering from prolonged cytopenia after HSC transplantation, where cytotoxic drugs and marrow irradiation may have caused widespread destruction of the BM vascular micro-architecture. Alternatively, similar to HSCs, the BMVN may partially depend on an internal or “private” VEGF-signaling pathway, which could not be inhibited with extracellularly acting agents like antibodies, thereby in part explaining the hematotoxicity observed with VEGF-receptor tyrosine kinase inhibitors.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Arai F, Hirao A, Suda T. Regulation of hematopoietic stem cells by the niche. Trends Cardiovasc. Med. 2005;15:75–79. doi: 10.1016/j.tcm.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 3.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 5.Hooper AT, Butler J, Petit I, Rafii S. Does N-cadherin regulate interaction of hematopoietic stem cells with their niches? Cell Stem Cell. 2007;1:127–129. doi: 10.1016/j.stem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 8.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 9.Shirota T, Tavassoli M. Cyclophosphamide-induced alterations of bone marrow endothelium: implications in homing of marrow cells after transplantation. Exp. Hematol. 1991;19:369–373. [PubMed] [Google Scholar]
- 10.Shirota T, Tavassoli M. Alterations of bone marrow sinus endothelium induced by ionizing irradiation: implications in the homing of intravenously transplanted marrow cells. Blood Cells. 1992;18:197–214. [PubMed] [Google Scholar]
- 11.His W. Lecithoblast und Angioblast der Wirbeltiere. Abhandl KS Ges. Wis. Math-Phys. 1900:171. [Google Scholar]
- 12.Murray PDF. The development in vitro of the blood of the early chick embryo. Proc. R. Soc. Lond. B. Biol. Sci. 1932:497–521. [Google Scholar]
- 13.Sabin FR. Studies on the original of blood vessels and of red corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Contrib. Embryol. 1920:213–262. [Google Scholar]
- 14.Fliedner TM, Graessle D, Paulsen C, Reimers K. Structure and function of bone marrow hemopoiesis: mechanisms of response to ionizing radiation exposure. Cancer Biother. Radiopharm. 2002;17:405–426. doi: 10.1089/108497802760363204. [DOI] [PubMed] [Google Scholar]
- 15.Kopp HG, Avecilla ST, Hooper AT, et al. Tie2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood. 2005;106:505–513. doi: 10.1182/blood-2004-11-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopp HG, Hooper AT, Broekman MJ, et al. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J. Clin. Invest. 2006;116:3277–3291. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyman G, Lyman C, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10:427–437. doi: 10.1634/theoncologist.10-6-427. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe H, Mamelak AJ, Wang B, et al. Anti-vascular endothelial growth factor receptor-2 (Flk-1/KDR) antibody suppresses contact hypersensitivity. Exp. Dermatol. 2004;13:671–681. doi: 10.1111/j.0906-6705.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 20.Hooper AT, Butler JM, Nolan DJ, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14:10–19. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 22.Gerber HP, Hillan KJ, Ryan AM, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 23.Mohle R, Green D, Moore MA, et al. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl. Acad. Sci. USA. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerber HP, Malik AK, Solar GP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]