Abstract
Mental rotation, the ability to manipulate three-dimensional objects in space, is a widely-studied and neurally-complex aspect of spatial cognition that has been largely ignored in the HIV literature. The present study reports evidence of a significant interaction between HIV serostatus and performance on mental rotation tasks, whereby individuals with HIV (n = 19) committed a greater number of errors than demographically similar seronegative persons (n = 15) on Luria's {1} hand rotation task (d = 0.74, p < 0.05), but not on the corresponding parallelogram rotation task (p > .10). Hand rotation errors were associated with worse performance on measures of executive functions and working memory, but not with measures of visuoperception. Considered in the context of the preferential frontostriatal neuropathology of HIV-associated neurocognitive disorders, these preliminary findings suggest that the observed deficit in the mental rotation of hands may arise from a disrupted fronto-striato-parietal network.
Keywords: HIV, AIDS, spatial ability, neuropsychological assessment, executive functions
Infection with the human immunodeficiency virus (HIV) often leads to neuropathology, which is diffusely distributed throughout brain parenchyma, but is commonly believed to preferentially disrupt the structure and function of frontostriatal and temporolimbic systems {2}. Understandably, the vast majority of neuropsychological research in HIV has therefore been centered on constructs of direct relevance to these neural systems (e.g., executive functions, working memory, motor skills, and episodic memory). However, very little is currently known about the nature and extent of deficits in spatial cognition in persons living with HIV infection. Spatial cognition refers to the ability to detect, understand, manipulate, and integrate visual stimuli in the context of its environment. Although spatial cognition is primarily linked to the integrity of the posterior parietal cortex (PPC), optimally functioning parieto-striato-cortical pathways are also needed for the integration of visual input {e.g., 3}, as observed in patients with frontostriatal pathology (e.g., Parkinson's disease) {4}. Considering that frontostriatal dysfunction is considered a hallmark of HIV-associated neurocognitive disorders, this overlap suggests that research regarding spatial cognition may in fact be relevant to the neuropsychology of HIV.
Early studies of spatial cognition in HIV included limited batteries and did not find much evidence of impairment {e.g., 5, 6}, leading to a temporary neglect of this thread of research. However, recent meta-analyses have produced mixed results regarding the effect to which spatial abilities are affected by HIV infection {e.g., 7, 8}, suggesting that there may be a signal worthy of further examination. Indeed, a closer review of the HIV literature explores the fronto-striatal components of this construct, indicating that subtler differences may exist in the distinct subcategories of spatial cognition (e.g., visuoconstruction, spatial attention) in this cohort. For instance, Poutiainen and colleagues found significant differences between a group of 13 HIV+ individuals and 10 seronegative controls on measures of visuoconstruction, visusospatial skills, and praxis {9}. Furthermore, Martin {10} suggested that HIV infection was associated with impairment on measures of egocentric spatial ability (i.e., spatial tasks that are built around the participant's frame of reference, as opposed to that of an observer). Measures of spatial attention {e.g., 11, 12} and, more recently, number line orientation {e.g., 13} also may be impaired in HIV infection, perhaps demonstrating the particular sensitivity of visuospatial tasks that draw upon complex attention and working memory to the prefrontostriatal pathologies of HIV disease.
Mental rotation, the ability to manipulate three-dimensional objects in space, is a widely-studied and neurally-complex area of spatial cognition that has been largely ignored in the neuroAIDS literature. These tasks are sensitive to both the functioning of the posterior parietal cortex and fronto-striato-parietal neural networks {14}. While mental rotation has been studied more extensively in other frontostriatal disorders (i.e., Parkinson's disease) {15}, to our knowledge, only one study to date has examined this construct in HIV infection. Olesen and colleagues {16} used a computerized mental rotation task in which two stimuli appear at different degrees of rotation; participants are asked to evaluate whether the objects are identical (same) or mirror images of each other (different). Impairment between groups presented as a disproportionate increase in errors and reaction time in the HIV+ group (n = 14) as the degree of rotation increased. Although the reaction time analyses showed trend-level group differences overall, this effect was primarily seen in the object condition, as explained by a three-way interaction between stimulus, group, and level of rotation. In conjunction with group differences found on a measure of global versus locally-biased processing, the authors concluded that HIV-related deficits in spatial cognition were likely due to parietal lobe pathology.
Although these data support the notion of impaired spatial cognition in HIV infection, the lack of supplemental neuroanatomical and neuropsychological data raise questions about the underlying neural and cognitive mechanisms. Despite relying on the PPC for basic processing of visual input, frontal systems are additionally required to adequately integrate, manipulate, and utilize visual information {e.g., 3}. For instance, the ability to mentally rotate three-dimensional objects requires the external processes of viewing the object as it physically exists pre-rotation, but also simultaneously viewing and manipulating it on the visuospatial scratchpad of the mind's eye {17}. Additionally, the cognitive requirements of the task likely vary based on the relative position of the object to be rotated. In fact, neuroimaging studies have shown that different types of rotations (i.e., object-centered or viewer-centered) rely on discrete neural pathways (i.e., right parietal lobes and intraparietal sulcus, or left parietal and left frontal regions, respectively) {18}. The involvement of frontal regions and the internal manipulation requirements of the task imply that mental rotation not only requires intact visuospatial skills, but also optimally functioning working memory abilities. Considering the overlapping neural networks between mental rotation abilities and HIV-associated neuropathologies, further exploration is needed to determine the neuropsychological correlates of this impairment, thereby elucidating the deficient component processes. In the present study, two tasks of mental rotation were administered within a larger battery containing measures of executive functions, information processing, motor skills, as well as other tasks of spatial cognition. We hypothesized that there would be evidence of HIV-related impairment on tasks of mental rotation, and that poorer performance would be associated with impairment on measures of executive functions and working memory. Furthermore, we hypothesized that mental rotation impairments would be most evident on the hand rotation task, as egocentric spatial tasks tend to rely more heavily on frontal systems {19}, which are particularly affected by HIV infection.
Method
Participants
The study was approved by the University Human Research Protections Program and all participants provided written, informed consent. All participants were recruited from a larger cohort at the HIV Neurobehavioral Research Center (HNRC) who agreed to participate in an additional hour of neuropsychological testing that was funded by a Core Support Program for AIDS Research (CSPAR) developmental grant designed to examine spatial cognition in HIV. Participants included 19 individuals with HIV infection (HIV+) and 15 HIV seronegative comparison subjects (HIV-). HIV serostatus was determined by enzyme linked immunosorbent assays and a Western Blot confirmatory test. Potential participants were pre-screened for histories of exclusionary psychiatric (e.g., mental retardation, psychotic disorders) or neurological (e.g., seizure disorders, traumatic brain injury, cerebrovascular disease) disorders by a subject recruiter. Upon enrollment, participants were subsequently further screened by the study psychometrist using an unpublished, but standardized and semi-structured interview. Additionally, participants who tested positive for illicit drugs (except marijuana) on a urine toxicology screen conducted on the day of testing were excluded. The study groups did not differ statistically in age, education, sex, handedness, and ethnicity (all ps > 0.10; see Table 1 for demographic and disease characteristics). Premorbid verbal IQ, as measured by a demographically-based estimate from the Wechsler Test of Adult Reading {20}, was also comparable between groups (p > 0.10). No between-group differences were observed on the Profile of Mood States (POMS) {21}, which is self-report measure of mood disturbance within the previous week (p > .10).
Table 1. Demographic and HIV disease characteristics of the study samples.
| Variable | HIV+ (n = 19) | HIV- (n = 15) | p |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 43.8 (9.9) | 44.9 (9.2) | 0.731 |
| Education (years) | 13.7 (1.9) | 14.7 (2.6) | 0.249 |
| WTAR estimated verbal IQ | 106.9 (5.6) | 107.9 (7.3) | 0.666 |
| Sex (% men) | 68% | 60% | 0.610 |
| Ethnicity (% Caucasian) | 53% | 67% | 0.409 |
| Handedness (% right) | 79% | 93% | 0.130 |
| HIV Disease Characteristics | |||
| Duration of Infection (years)a | 7.4 (4.7, 14.3) | - | |
| Current CD4 Counta | 588.0 (300.0, 726.0) | - | |
| Nadir CD4 Counta | 269.0 (50.0, 348.0) | - | |
| RNA Viral Load (log10 c/ml)a | 2.6 (2.5, 2.8) | - | |
| % AIDS | 37% | - | |
| % HAARTb | 74% | - | |
| Psychiatric Characteristics | |||
| POMS Mood Disturbance | 43.9 (43.8) | 35.9 (22.1) | 0.507 |
Note. All descriptive statistics are listed as mean (standard deviation) unless otherwise noted. POMS = Profile of Mood States. WTAR = Wechsler Test of Adult Reading. HAART = highly-active antiretroviral therapy.
Median (interquartile range)
All participants on antiretroviral medications were prescribed HAART
Materials and Procedure
Mental Rotation
Participants were administered two tasks of mental rotation (i.e., hands and parallelograms) {1}, as part of an extensive neuropsychological, medical, and psychiatric evaluation. These particular mental rotation tasks were chosen because they were published, paper-and-pencil tasks that were created by the same author (i.e., Luria) to maximize comparability. More importantly and directly related to our a priori hypotheses, together these tasks examine both ego- (i.e., body part) and allo-centric (i.e., object) mental rotation. For the hand rotation task (HIV+: n = 19; HIV-: n = 15), participants were presented with 21 1.5 × 2 cm line drawings of hands in different postures and orientations for which they were asked to indicate whether a right or left hand was depicted. The 10-item parallelogram task (HIV+: n = 19; HIV-: n = 13, due to minor administration errors) required individuals to examine the placement of a circle in a rotated parallelogram and subsequently draw circles onto two empty, mirror-imaged parallelograms so that they would appear identical to the rotated figure (for further details, please see Luria {1}). Performance on both tasks was recorded as total number of errors.
Standard Clinical Tests
The comprehensive battery was administered as part of the standard HNRC protocol to which this developmental grant was linked and included tests tapping standard cognitive domains that are: a) sensitive to HIV infection, and b) implicated in mental rotation performance, including executive functions, working memory, processing speed, and visuoperception. Specifically, individual tests included: 1) Trail Making Test (TMT parts A and B; difference in time to completion [B-A]) {22}, 2) Wisconsin Card Sorting Test (WCST-64; raw perseverative responses) {23}, 3) Paced Auditory Serial Addition Task (PASAT-200; raw total correct) {24}, 4) Grooved Pegboard (time to completion) {25}, 5) Wechsler Adult Intelligence Scale (3rd ed) (WAIS-III) Processing Speed Index (raw total score) {26}, 6) Hooper Visual Organization Test (HVOT; raw total correct) {27}, and 7) Benton Judgment of Line Orientation (JLO; raw total correct) {27}.
Data Analyses
A repeated-measures analysis of variance with HIV serostatus as the between-groups variable and mental rotation task (i.e., hands or parallelograms) as the within-group variable was conducted to evaluate the primary study hypotheses. Follow-up analyses were conducted using independent-samples t-tests and Cohen's d effect size estimates. Note that, although the mental rotation data were non-normally distributed according to Shapiro-Wilk W tests (parallelograms: W = 0.94, p < 0.06, hands: W = 0.93, p < 0.05), follow-up analyses using a nonparametric approach yielded the same results; the parametric approach to testing the statistical interaction is described in addition for ease of interpretation. Nevertheless, correlational analyses regarding the associations between mental rotation and standard clinical tests within the HIV group were conducted using Spearman's rho. Considering the small sample sizes, a critical alpha level of .05 was used throughout to minimize our risk of Type II statistical error. To minimize our risk of Type I error, correlations were analyzed only in the HIV group (rather than in both groups separately) and only when the mental rotation task differed by serostatus. This approach has been shown to: a) provide clear insight of neuropsychological constructs in a clinical sample (cf. convolution of shared variance in mixed clinical samples) and b) reduce the risk of Type I error by running correlational analyses only in the target population (cf. running analyses separately in the clinical and healthy comparison groups, which may then require Bonferroni-like corrections, in turn diluting genuinely significant findings) {28, 29}.
Results
A repeated-measures ANOVA revealed no main effects of HIV status (F [1, 29] = 0.02, p > 0.10; η2 = 0.007) or mental rotation task (i.e., hands or parallelograms) (F [1, 29] = 0.08, p > 0.10, η2 = 0.078). However, the interaction between group and task proved significant (F [1, 29] = 5.47, p = 0.020, η2 = 0.168). Based on the non-normal distribution of the data, these statistics were also conducted using non-parametric analyses (i.e., Wilcoxon rank-sum test), yielding a significant between-groups result on a hands minus parallelograms errors difference score variable (χ2 [1, n = 32] = 7.02, p = 0.008). A planned follow-up univariate analysis indicated that the interaction effect was driven by a greater number of errors committed by the HIV+ group on the hands task (M = 6.5, SD = 4.2) compared with the HIV- group (M = 3.9, SD = 2.4; t [29] = 2.27, p = 0.031), representing a large effect (d = 0.74). There was no significant difference between groups on the parallelograms task (t [27] = -0.89, p > 0.10; d = -0.31).
To examine neuropsychological correlates of our significant primary finding, follow-up analyses were performed in the HIV+ group (n = 19) to obtain Spearman's rho correlation coefficients, as shown in Table 2. Errors on the hands task were moderately associated with measures of working memory and executive functioning, as defined as poorer performance on the PASAT-200 and increased perseverations on WCST-64, respectively (both ps ≤ 0.05; see Figures 2a & 2b). All other neuropsychological constructs examined (i.e., motor skills, information processing, divided attention, and visuospatial functioning) did not relate to mental rotation performance (ps > 0.10). Additionally, performance on the hands task was not associated with HIV disease characteristics, including current and nadir CD4 count, plasma viral load, AIDS status, highly-active antiretroviral therapy (HAART), and estimated duration of infection (ps > 0.10). Total mood disturbance as measured by the POMS also did not relate to hand rotation errors (p > 0.10).
Table 2. Correlations between a task of mental rotation (hands) and select neuropsychological tests in the HIV+ sample (n = 19).
| Test variable | Spearman's rho | p-value |
|---|---|---|
| TMT B-A (difference in time to completion) | -0.040 | 0.869 |
| Benton Judgment of Line Orientation (total correct) | -0.271 | 0.261 |
| Hooper Visual Organization Test (total correct) | -0.325 | 0.175 |
| PASAT-200 (total correct) | -0.457 | 0.050* |
| Grooved Pegboard (dominant; completion time) | 0.036 | 0.884 |
| Grooved Pegboard (non-dominant; completion time) | 0.074 | 0.763 |
| WCST-64 (raw perseverative responses) | 0.488 | 0.034* |
| WAIS-III Processing Speed Index (raw total) | -0.087 | 0.722 |
Note: TMT B-A, Trail Making Test Parts B-A; PASAT-200, Paced Auditory Serial Addition Test; WCST-64, Wisconsin Card Sorting Test; WAIS-III, Wechsler Adult Intelligence Scale (3rd ed.).
p ≤ 0.05.
Figure 2.
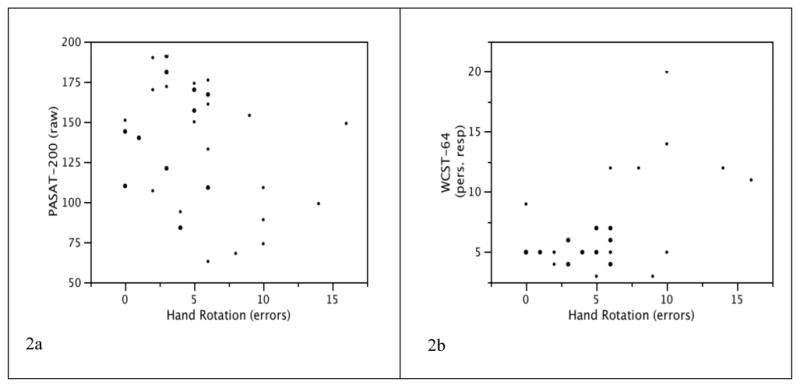
Scatter plots displaying the relationship between mental rotation of hands on the X axis and the PASAT-200 total correct (2a) and WCST-64 perseverative responses (2b) on the Y axes (ps ≤ 0.05).
Discussion
Overall, findings from this brief report revealed that HIV infection is associated with impairment in mental rotation. As predicted, a significant interaction was observed between HIV serostatus and mental rotation task, indicating that individuals with HIV committed a greater number of errors than their seronegative counterparts on Luria's hand rotation task, but not on the corresponding parallelogram rotation task. In other words, persons infected with HIV experienced disproportionately greater difficulties accurately manipulating mental representations of hands. This finding, which was associated with a large effect size, contrasts against the widely-held belief that deficits in spatial cognition are not generally evident in individuals with HIV; in fact, these preliminary data build on a small literature suggesting that HIV infection may lead to impairment in complex spatial cognition {7, 10, 13}, including one prior study on mental rotation deficits {16}.
Although this pilot study contributes to the basic evidence of a mental rotation deficit in HIV, as was first reported by Olesen and colleagues {16}, it also argues for an alternate interpretation of the underlying cognitive (and neural) substrates of this deficit. As noted above, Olesen et al. attributed the HIV-associated mental rotation deficit to an impairment in visuospatial processing related to dysregulation of posterior parietal cortex; however, findings from the current study indicate that fronto-striatal systems may also contribute to the observed deficits. First, we observed a disproportionate impairment in the mental rotation of body parts (i.e., hands) as compared to objects (i.e., parallelograms). Although we did not directly query participants as to their relative strategies for mental rotation, it is posited that the mental rotation of body parts (relative to object rotation) requires greater integration of visuoperceptual and motor neural systems, whereby viewers imagine rotating their own extremities in relation to the stimuli {19}. These viewer-centered mental processes during body part rotation are linked to traditional spatial cognition circuits (e.g., posterior parietal cortex) {30}, as well as fronto-striato-thalamo-cortical loops {e.g., 15, 18, 19}. This interpretation also converges with a prior study by Martin {10}, who found that HIV infection was associated with deficits in egocentric spatial ability.
In further support of the involvement of frontal systems, deficits in the mental rotation of hands were associated with executive and working memory dysfunction in the HIV+ group. More specifically, errors on the hand rotation task demonstrated medium-to-large correlations in the expected directions with performance on the PASAT-200 and perseverative responses on the WCST-64. In contrast, hand rotation errors did not relate to performance on tasks of visuospatial functioning, information processing speed, or motor skills. Although the PASAT-200 also places demands on processing speed, the lack of relationship between mental rotation and the WAIS-III Processing Speed Index supports the hypothesis that the correspondence between the PASAT-200 and hands may be primarily driven by working memory. Taken together, these data provide convergent and divergent evidence that the HIV-associated deficit in mental rotation of hands involves cognitive processes that are strongly linked to frontal systems, which are preferentially affected in HIV disease {e.g., 2, 7}. Nevertheless, it is likely that this deficit arises from dysfunction in a distributed fronto-striato-parietal network that includes the posterior parietal cortex, which is involved in general mental rotation {31}, as well as working memory {e.g., 32} and executive functions {e.g., 33}.
Although our interpretation of the cognitive and neural mechanisms of HIV-associated mental rotation deficits departs from that of Olesen et al. {16}, who emphasized the role of visuoperception and the parietal cortex, a few important methodological differences exist between these two studies. First, Olesen et al. used computerized mental rotation tasks that included reaction time, which was not captured by our paper-and-pencil tasks. Second, their tasks employed a same/different response paradigm in which two hands were shown, whereas the task used in this study required a right/left response to a single stimuli. Additionally, our task of object rotation task used two-dimensional parallelograms, compared to the traditional – and arguably more difficult – three-dimensional blocks in Olesen's study. Indeed, Olesen's findings may be interpreted as a breakdown in spatial working memory, thus supporting the possible involvement of frontal systems, which the authors themselves acknowledged as an alternate interpretation {16}. A more direct evaluation of the role of fronto-striato-parietal systems in HIV-associated mental rotation impairment is needed, perhaps using experimental paradigms to manipulate the cognitive components of rotation in relation to structural (e.g., morphological) and functional (e.g., metabolic and neural) neuroimaging markers.
Results from this study are not confounded by demographic factors or affective distress, as the HIV+ and seronegative groups were well balanced on these characteristics. However, the small sample sizes (total n = 34) and well-controlled disease status of the HIV+ group likely limited our power to detect more modest relationships between hand rotation and other cognitive ability areas (e.g., visuoperception) and disease parameters. Indeed, rotation errors were not significantly associated with any HIV disease or treatment factors (e.g., HAART status). Despite the relatively small sample sizes, the present study was reasonably well-powered to detect at least a medium effect size interaction using a critical alpha of 0.05 (1 - β = 0.78). To minimize risk of Type II error (given our small sample size), we did not use additional corrective measures for multiple comparisons (e.g., Bonferroni corrections), but instead employed other methodological techniques (e.g., conducting analyses only in the HIV sample) to control Type I error while still using the standard critical alpha of 0.05. It is, however, important to note that these results would not be statistically significant had we controlled for multiple comparisons. Moreover, these limitations likely restrict the extent to which our results are generalizable to the broader HIV-infected population. Nevertheless, the presence of a significant HIV-associated impairment in hand rotation and its correspondence to deficits in working memory and executive functions in such a small HIV sample with relatively well-controlled disease speaks to the magnitude of the observed effect. Another limitation of the present findings is that the mental rotation tasks were not specifically constructed for the purpose of dissociation and therefore differed in several respects, including length and response modality (i.e., the parallelogram task required a motor response). As such, studies are needed regarding the psychometric characteristics of these mental rotation tasks (e.g., reliability and demographically-adjusted normative standards). Future studies are needed to examine the relationship between mental rotation and its cognitive mechanisms across a larger group of persons with HIV infection with a broader range of disease characteristics. Further examination of spatial cognition in HIV may also lead to enhancements in the detection of problems in everyday functioning (e.g., driving), as has been shown in older adults {e.g., 34} and clinical populations, including Parkinson's disease {e.g., 35}.
Figure 1.
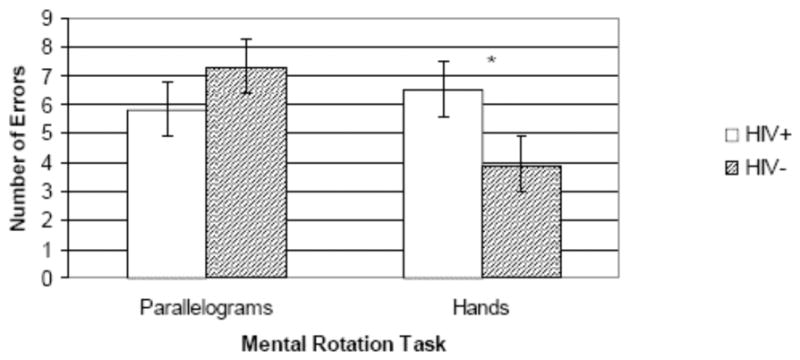
Bar chart displaying the significant interaction between mental rotation tasks (parallelograms and hands) and HIV serostatus.
* p < 0.05
Acknowledgments
Erica Weber, Steven Paul Woods, Marizela V. Cameron, Sarah A. Gibson, and Igor Grant, Department of Psychiatry.
This research was supported by a Core Support Program for AIDS Research developmental grant to Dr. Woods (CSPAR 570) and a National Institute of Mental Health center grant to Dr. Grant (P30-MH62512). The HIV Neurobehavioral Research Center (HNRC) Group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Igor Grant, M.D.; Co-Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and J. Allen McCutchan, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Naval Hospital San Diego: Braden R. Hale, M.D., M.P.H. (P.I.); Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Scott Letendre, M.D., Edmund Capparelli, Pharm.D., Rachel Schrier, Ph.D.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), Mariana Cherner, Ph.D., David J. Moore, Ph.D., Steven Paul Woods, Psy.D.; Neuroimaging Component: Terry Jernigan, Ph.D. (P.I.), Christine Fennema-Notestine, Ph.D., Sarah L., Archibald, M.A., John Hesselink, M.D., Jacopo Annese, Ph.D., Michael J. Taylor, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Ian Everall, FRCPsych., FRCPath., Ph.D., T. Dianne Langford, Ph.D.; Neurovirology Component: Douglas Richman, M.D., (P.I.), David M. Smith, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.); Developmental Component: Ian Everall, FRCPsych., FRCPath., Ph.D. (P.I.), Stuart Lipton, M.D., Ph.D.; Clinical Trials Component: J. Allen McCutchan, M.D., J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., Scott Letendre, M.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Rodney von Jaeger, M.P.H.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman, B.A., (Data Systems Manager), Daniel R. Masys, M.D. (Senior Consultant); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Christopher Ake, Ph.D., Florin Vaida, Ph.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank Drs. Julie D. Rippeth and Brian C. Schweinsburg for their guidance regarding study conceptualization and design and Danielle A. Sires for her assistance with data collection.
References
- 1.Luria AR. Higher cortical functions in man. New York: Basic Books, Inc.; 1966. [Google Scholar]
- 2.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. National Review of Immunology. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence AD, Watkins LHA, Sahakian BJ, Hodges JR, Robbins TW. Visual object and visuospatial cognition in Huntington's disease: Implications for information processing in corticostriatal circuits. Brain. 2000;123:1349–1364. doi: 10.1093/brain/123.7.1349. [DOI] [PubMed] [Google Scholar]
- 4.Boller F, Passafiume D, Keefe NC, Rogers K, Morrow L, Kim Y. Visuospatial impairment in Parkinson's disease: Role of perceptual and motor factors. Archives of Neurology. 1984;41:485–490. doi: 10.1001/archneur.1984.04050170031011. [DOI] [PubMed] [Google Scholar]
- 5.Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson J, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 6.Saykin AJ, Janssen RS, Sprehn GC, Kaplan JE, Spira TJ, Weller P. Neuropsychological dysfunction in HIV-infection: Characterization in a lymphadenopathy cohort. International Journal of Clinical Neuropsychology. 1988;10:81–95. [Google Scholar]
- 7.Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- 8.Cysique LA, Maruff P, Brew BJ. The neuropsychological profile of symptomatic AIDS and ADC patients in the pre-HAART era: A meta-analysis. Journal of the International Neuropsychological Society. 2006;12:368–382. doi: 10.1017/s1355617706060401. [DOI] [PubMed] [Google Scholar]
- 9.Poutiainen E, Iivanainen M, Elovaara I, Valle SL, Lähdevirta J. Cognitive changes as early signs of HIV infection. Acta Neurologica. 1988;78:49–52. doi: 10.1111/j.1600-0404.1988.tb03618.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin A. HIV, cognition, and the basal ganglia. In: Grant I, Martin A, editors. Neuropsychology of HIV infection. New York: Oxford University Press; 1994. [Google Scholar]
- 11.Martin EM, Sorensen DJ, Robertson LC, Edelstein HE, Chirurgi VA. Spatial attention in HIV-1 infection: a preliminary report. Journal of Neuropsychiatry. 1992;4:288–293. doi: 10.1176/jnp.4.3.288. [DOI] [PubMed] [Google Scholar]
- 12.Maruff P, Malone V, McArthur-Jackson C, Mulhall B, Benson E, Currie J. Abnormalities of visual spatial attention in HIV infection and the HIV-associated dementia complex. Journal of Neuropsychiatry. 1995;7:325–333. doi: 10.1176/jnp.7.3.325. [DOI] [PubMed] [Google Scholar]
- 13.Bogdanova Y, Neargarder S, Cronin-Golomb A. Mapping mental number line in physical space: Vertical and horizontal visual number line orientation in asymptomatic individuals with HIV. Neuropsychologia. 2008;46:2914–2923. doi: 10.1016/j.neuropsychologia.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepherd RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171:701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- 15.Amick MM, Schendan HE, Ganis G, Cronin-Golomb A. Frontostriatal circuits are necessary for visuomotor transformation: Mental rotation in Parkinson's disease. Neuropsychologia. 2006;44:339–49. doi: 10.1016/j.neuropsychologia.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Olesen PJ, Schendan HE, Amick MM, Cronin-Golomb A. HIV infection affects parietal-dependent spatial cognition: Evidence from mental rotation and hierarchical pattern perception. Behavioral Neuroscience. 2007;121:1163–73. doi: 10.1037/0735-7044.121.6.1163. [DOI] [PubMed] [Google Scholar]
- 17.Baddeley AD, Hitch GJ. Working memory. In: Bower G, editor. Recent advances in learning and motivation Vol. VIII. New York: Academic Press; 1974. [Google Scholar]
- 18.Kosslyn SM, DiGirolamo GJ, Thompson WL, Alpert NM. Mental rotation of objects versus hands: Neural mechanisms revealed by positron emission tomography. Psychophysiology. 1998;35:151–161. [PubMed] [Google Scholar]
- 19.Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, Jerabek PA, Lancaster JL. Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature. 1995;375:54–8. doi: 10.1038/375054a0. [DOI] [PubMed] [Google Scholar]
- 20.Psychological Corporation. Wechsler test of adult reading. San Antonio, TX: Author; 2001. [Google Scholar]
- 21.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1981. [Google Scholar]
- 22.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 23.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test – 64 card computerized version. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 24.Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): Norms for age, education, and ethnicity. Assessment. 1998;5:375–87. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- 25.Kløve H. Grooved Pegboard. Lafayette, IN: Lafayette Instruments; 1963. [Google Scholar]
- 26.Psychological Corporation. Wechsler Adult Intelligence Scale. 3rd. San Antonio, TX: Author; 1997. [Google Scholar]
- 27.Benton AL, Hamsher KD, Varney NR, Spreen O. Contributions to neuropsychological assessment. New York: Oxford University Press; 1994. [Google Scholar]
- 28.Delis DC, Jacobson M, Bondi MW, Hamilton JM, Salmon DP. The myth of testing construct validity using factor analysis or correlations with normal or mixed clinical populations: Lessons from memory assessment. Journal of the International Neuropsychological Society. 2003;9:936–946. doi: 10.1017/S1355617703960139. [DOI] [PubMed] [Google Scholar]
- 29.Brandt J. 2005 INS presidential address: Neuropsychological crimes and misdemeanors. The Clinical Neuropsychologist. 2007;21:553–568. doi: 10.1080/13854040600770800. [DOI] [PubMed] [Google Scholar]
- 30.Vingerhoets G, de Lange FP, Vandemaele P, Deblaere K, Achten E. Motor imagery in mental rotation: An fMRI study. Neuroimage. 2002;17(3):1623–33. doi: 10.1006/nimg.2002.1290. [DOI] [PubMed] [Google Scholar]
- 31.Zacks JM. Neuroimaging studies of mental rotation: A meta-analysis and review. Journal of Cognitive Neuroscience. 2008;20:1–19. doi: 10.1162/jocn.2008.20013. [DOI] [PubMed] [Google Scholar]
- 32.Lazeron RH, Rombouts SA, de Sonneville L, Barkhof F, Scheltens P. A paced visual serial addition task for fMRI. Journal of Neurological Sciences. 2003;213:29–34. doi: 10.1016/s0022-510x(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 33.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson ED, Nadler JD, Malloy PF. Neuropsychologic prediction of performance measures of daily living skills in geriatric patients. Neuropsychology. 1995;9:565–572. [Google Scholar]
- 35.Amick MM, Grace J, Ott BR. Visual and cognitive predictors of driving safety in Parkinson's disease patients. Archives of Clinical Neuropsychology. 2007;22:957–967. doi: 10.1016/j.acn.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


