Abstract
The severity of Cushing’s Syndrome (CS) depends on the duration and extent of the exposure to excess glucocorticoids. Current measurements of cortisol in serum, saliva and urine reflect systemic cortisol levels at the time of sample collection, but cannot assess past cortisol levels. Hair cortisol levels may be increased in patients with CS, and, as hair grows about 1 cm/month, measurement of hair cortisol may provide historical information on the development of hypercortisolism. We attempted to measure cortisol in hair in relation to clinical course in six female patients with CS and in 32 healthy volunteers in 1 cm hair sections. Hair cortisol content was measured using a commercially available salivary cortisol immune assay with a protocol modified for use with hair. Hair cortisol levels were higher in patients with CS than in controls, the medians (ranges) were 679 (279–2500) and 116 (26–204) ng/g respectively (P <0.001). Segmental hair analysis provided information for up to 18 months before time of sampling. Hair cortisol concentrations appeared to vary in accordance with the clinical course. Based on these data, we suggest that hair cortisol measurement is a novel method for assessing dynamic systemic cortisol exposure and provides unique historical information on variation in cortisol, and that more research is required to fully understand the utility and limits of this technique.
Keywords: glucocorticoids, pituitary adenoma, cancer, adrenal gland, hormones, cushing hair
Introduction
Cushing’s Syndrome (CS) consists of signs and symptoms caused by prolonged exposure to elevated glucocorticoid levels. The severity of the symptoms depends on the degree and duration of glucocorticoid excess (Stuart, 2007). In iatrogenic CS, these factors are usually well documented. In endogenous CS, the duration of hypercortisolism can usually only be estimated based on subjective patient reports detailing the time course of symptom development and progression (Giraldi et al., 2003). The degree of cortisol over production is usually assessed by measuring twenty-four hour urinary free cortisol excretion (Mengden et al., 1992), although serum cortisol levels after dexamethasone suppression and night-time serum and salivary cortisol levels are also used (Nieman et al., 2008). These measurements can currently only be obtained prospectively after first presentation.
Hair analysis is increasingly used to reflect exposure to drugs of abuse and environmental toxins (Villain et al., 2004). Measurement of endogenous hormones in hair is a relatively nascent technique, and has been described for testosterone, estradiol, progesterone and glucocorticoid steroids (Cirimele et al., 2000; Yang et al., 1998). More recently, Davenport and colleagues (Davenport et al., 2006) reported that in male rhesus macaque monkeys, cortisol levels in hair and saliva were increased under stress from relocation, and returned to the pre-stress levels after the animals had adapted to their new environment, suggesting that cortisol levels in hair reflect systemic cortisol levels. For the present pilot study, we hypothesized that cortisol levels in hair are increased in patients with CS. Furthermore, as hair grows about 1 cm a month (Wennig, 2000), we hypothesized that segmental analysis of hair will provide a historical record of the development of hypercortisolism. Therefore, we performed segmental hair cortisol measurements and related the levels to the clinical course in several patients with CS, and compared with levels in control subjects.
Subjects and Methods
We included 5 female patients in whom endogenous CS had been diagnosed based on clinical presentation and cortisol levels in serum and 24 h urine according to consensus guidelines (Arnaldi et al., 2003), and 1 patient with iatrogenic CS. To determine normal values for cortisol in hair, we recruited healthy volunteers. All participants gave written informed consent before inclusion. The Health Sciences Research Ethics Board of the University of Western Ontario approved the study.
The cortisol content of hair samples was determined using a protocol modified from Van Uum et al. (Van Uum et al., 2008). Briefly, a hair sample of approximately 150 strands was collected from the vertex posterior using scissors as close to the scalp as possible. Scalp end of the hair samples was carefully noted. Each hair sample was sectioned into one centimeter long segments, each weighing 10–20 mg. Each hair segment was minced finely with scissors and incubated overnight in 1 ml of 50 ° C methanol. The methanol was removed from the hair and evaporated. The residue was reconstituted in 250 µl PBS buffer (pH 8.0). The cortisol concentration in the resulting buffer solution was determined using a commercially available salivary cortisol EIA kit. (Cat # 11-CORHU-E01-SLV, ALPCO Diagnostics, Salem, NH). The manufacturer of the EIA reports the cross reactivity of other steroids with the kit’s antibodies as follows: prednisolone 14%, corticosterone 8%, 11-deoxycorticosterone 7%, progesterone 7%, cortisone 6%, 11-deoxycortisol 6%, prednisone 6%, and dexamethasone <2 %. The reproducibility of the assay (inter-assay variability) was measured by testing aliquots of the same bulk sample weekly for 5 weeks and was 11%. Based on a minimum hair mass of 10 mg, the upper and lower limits of detection are approximately 2000 and 25 ng/g, respectively.
The cortisol levels in serum and urine were measured by the hospital laboratory using a competitive immunoassay (ADVIA Centaur – COR Lite reagent pack. Siemens Healthcare Diagnostics, Tarrytown, USA).
To determine changes of hair cortisol levels over time, hair samples of the Cushing’s patients and of 9 control subjects (hair samples at least 10 cm long) were analyzed in one centimeter segments. We assumed an average hair growth rate of 1 cm per month for all subjects (Wennig, 2000).
Results
Volunteers
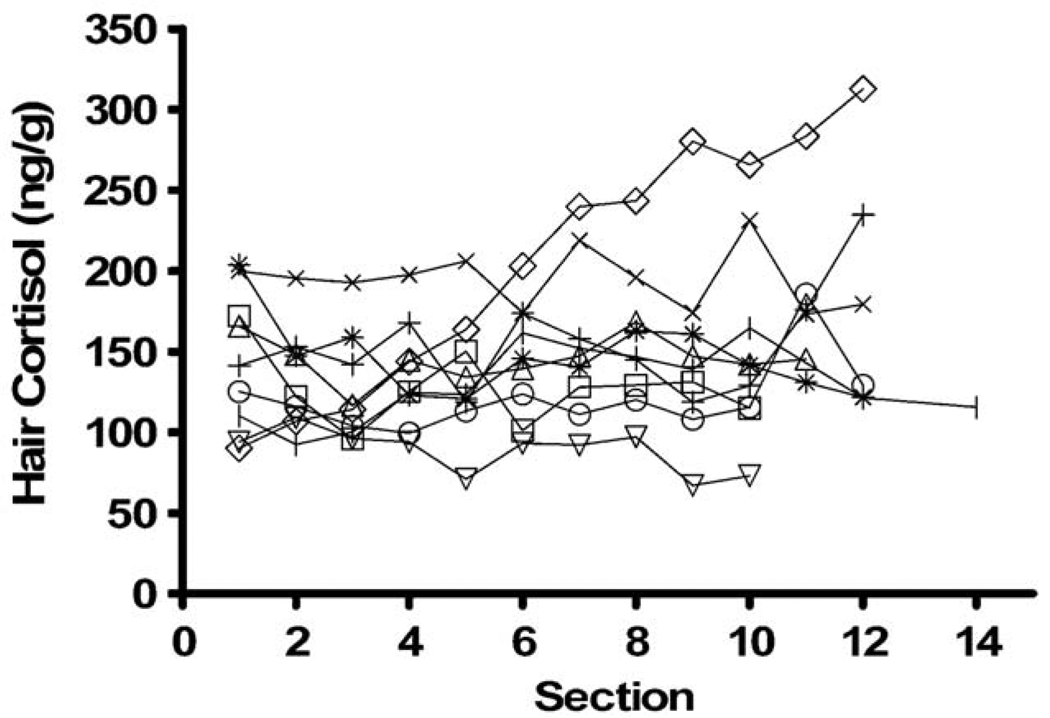
We included 32 healthy control subjects, 21 females and 11 males, all without clinical symptoms of CS, age (mean ± SD) 29 ± 9.5 years (range: 20–51), blood pressure 113 ± 10/74 ± 10 mmHg, BMI 23.3 ± 4.0 kg/m2, and waist circumference 80 ± 12 cm. The hair cortisol level in the most proximal 1 cm segment was 116 ± 54 ng/g (range: 26–204 ng/g) and normally distributed (Shapiro-Wilk). There was no statistically significant difference in hair cortisol concentrations between healthy men and women, the results were 134 ± 40 and 102 ± 58 ng/g (P = 0.16, Mann-Whitney U-test), respectively. Using this control population the upper limit of normal, defined as the mean plus two standard deviations, was 221 ng/g. In 9 healthy female control subjects, selected because they had long hair, we also performed segmental analysis on 1 cm sections. The average hair cortisol level for all segments was 147 ± 46 ng/g (range: 67–312 ng/g). All control participants showed variations in cortisol content over time (Fig 1). There was no change of cortisol levels over time in 8 of the 9 subjects, as the slope of the linear regression line of cortisol versus time was not different from 0 in 8 subjects, while one subject had a significant (P <0.05, linear regression) decrease of hair cortisol over time.
Fig. 1.
Hair cortisol levels in 1 cm segments in 9 healthy control subjects. Section number indicates distance in cm from scalp. Lower numbers indicate sections taken closer to the scalp and thus reflect more recent exposure.
Cases
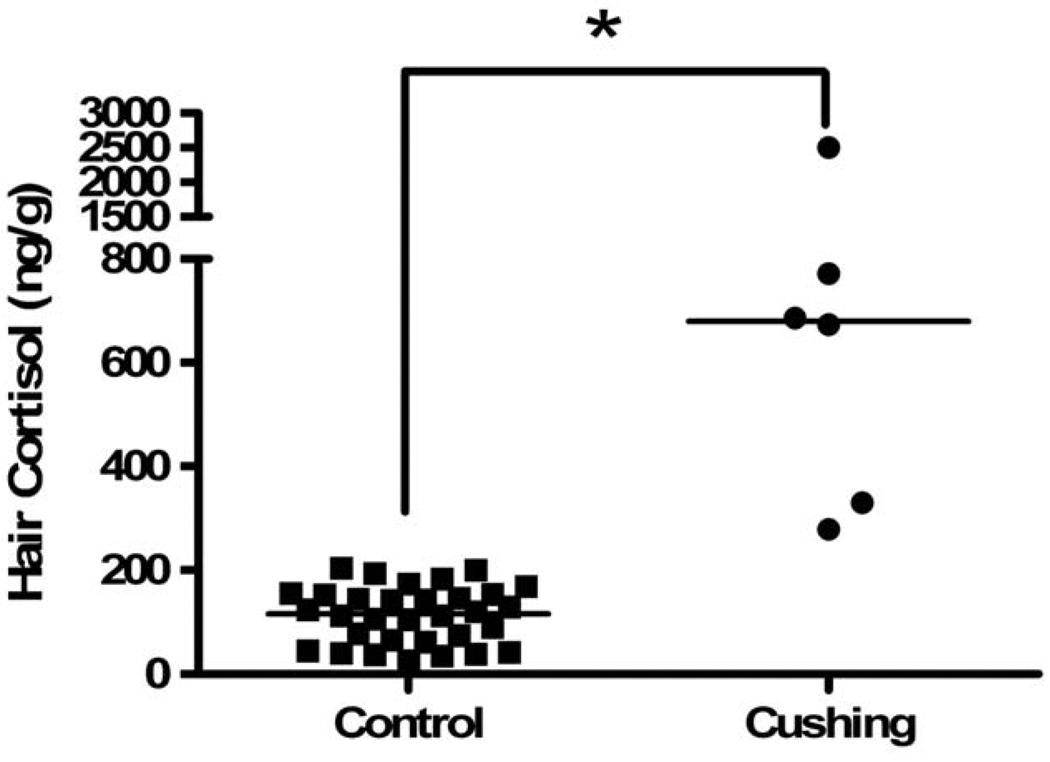
We included 6 patients with confirmed CS. The details of the patients seen in Fig 3 are given in their case histories below. The hair cortisol levels in the most proximal hair segments, obtained at presentation, were higher in the subjects with CS than in the control subjects, the medians and ranges were 679 (279–2500) and 116 (26–204) ng/g, respectively (Fig. 2, P <0.001 Mann-Whitney U).
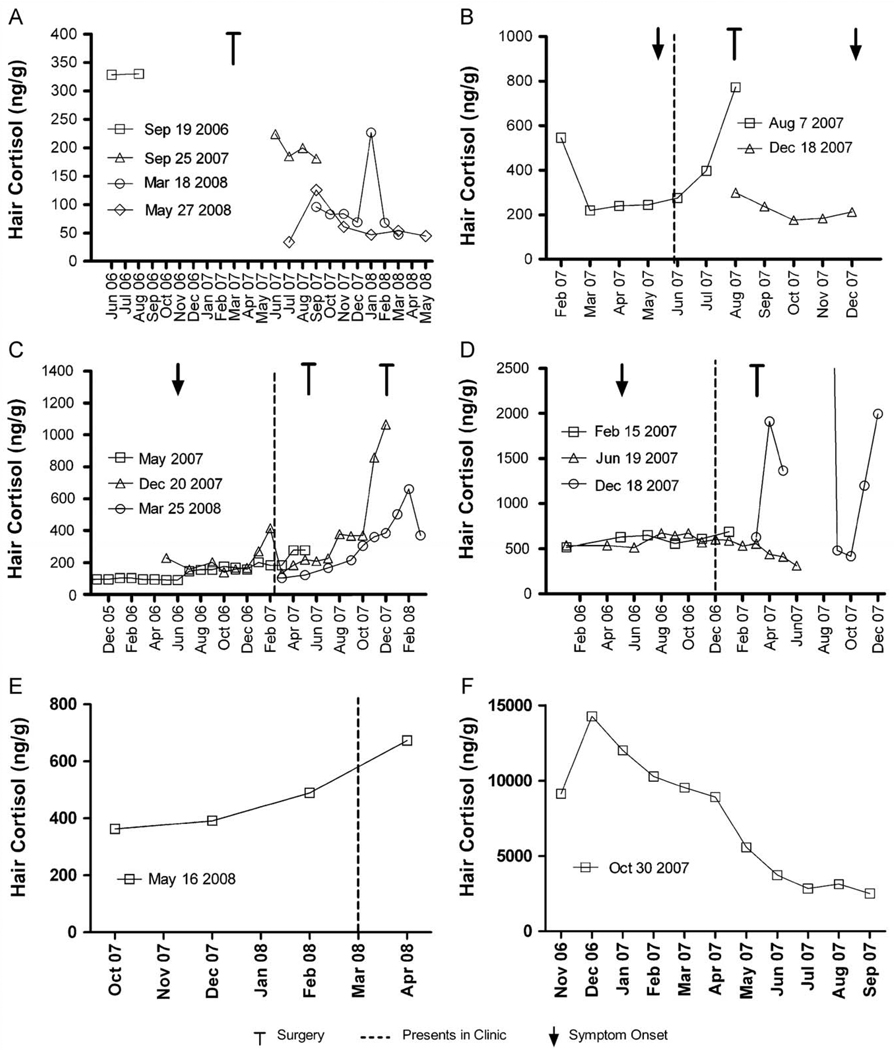
Fig. 3.
Individual hair cortisol levels in 5 patients with endogenous Cushing’s Syndrome and one patient with unconfirmed exogenous Cushing’s Syndrome (see text for detailed case descriptions). Legends indicate the dates the hair samples were collected, the retrospective time course is assigned based on an assumed average hair growth rate of 1 cm/month.
Fig. 2.
Comparison of cortisol levels in the first section of hair (closest to the scalp) from control and Cushing’s syndrome patients (at time of first presentation), respectively. * indicates P <0.01 Mann-Whitney U, horizontal line indicates median.
Patient A, a 51-year old woman, was referred in March 2006 for evaluation of possible hypercortisolism. She had a twenty-year history of hirsutism with gradual increasing severity and acne since 2001. On examination, she had a mild male pattern hirsutism, and proximal myopathy. Her 24-hour urine free cortisol (UFC) excretion was increased 719 and 1 039 (120–384) nmol/D. Following administration of 8 mg dexamethasone at 11 PM, the 8 AM cortisol and plasma ACTH were insufficiently suppressed at 252 (119–618) and 6.6 (2.0–12.5) pmol/L, respectively. Petrosal venous sampling confirmed a pituitary source of ACTH overproduction. In April 2007 she underwent endoscopic transsphenoidal resection of a right-sided ACTH-producing adenoma. Post-operatively, she was started on hydrocortisone 40 mg daily, tapered to 20 mg every other day in January 2008. After a brief increase to 15 mg daily in February and March because of hand joint pain, the hydrocortisone was further tapered and discontinued in July 2008. Her hair cortisol concentration decreased from 325 ng/g at presentation to 50 ng/g after surgery while on tapering hydrocortisone therapy. Two subsequent hair samples showed a downward trend reaching the normal range, with one sample showing a transient increase in early 2008 (Fig 3A).
Patient B, a 36-year old woman, presented in June 2007 after noticing pitting edema and mood changes since May 2007. She had increased facial hair growth, acne, a round face and abdominal distention with increased UFC excretion at 11,022 (120–384) nmol/D and undetectable ACTH. A CT scan of the abdomen demonstrated a 6 cm left adrenal mass that was macroscopically completely removed with an open adrenalectomy. Pathological examination demonstrated an adrenal cortical neoplasm with several features indicative of malignancy. Post-surgery, she received hydrocortisone replacement therapy. In August 2007, her morning fasting cortisol (obtained 48 h after the last hydrocortisone intake) was 29 nmol/L. In December 2007. she noticed recurrence of symptoms. Off steroid therapy, her UFC excretion had increased to 3875 nmol/D. A CT scan of the abdomen demonstrated local recurrence and multiple liver lesions. Her hair cortisol levels increased rapidly until surgery, after which levels decreased the normal range (Fig. 3B). A later hair sample could not be obtained due to hair loss following chemotherapy.
Patient C, a 21-year old female, presented in April 2007. Over the previous year, she had experienced progressive fatigue, acne, facial hair growth, depression, decrease of libido, anorexia and a 16 kg weight gain. On examination she had a moon face, signs of easy bruising, increased abdominal size and proximal myopathy. Her UFC was elevated at 1 165 (< 180) nmol/D from an outside laboratory. A morning plasma ACTH was 14 pmol/L, and serum cortisol was 931 nmol/L, with insufficient suppression to 534 nmol/L after a 2-day low dose dexamethasone suppression test. An MRI scan demonstrated a pituitary macroadenoma. She underwent transsphenoidal surgery; pathological examination confirmed Cushing’s disease. In the months after surgery she remained Cushingoid, and laboratory tests indicated persistent hypercortisolism: her UFC remained elevated at 1044 nmol/D with insufficient suppression of cortisol 458 nmol/L and ACTH 11 nmol/L after a 1 mg overnight dexamethasone suppression test. A repeat MRI scan demonstrated a reduction in size of the pituitary from 12.0 to 8.6 mm, and she underwent subtotal hypophysectomy in December 2007. Clinically there was persistent Cushing’s disease, her urinary cortisol excretions were 2489, 618 and 462 nmol/d in January, February and March 2008, respectively. As this patient had long hair, segmental analysis could be done for about 20 months before first presentation in the clinic, including about 6 months before the subjective onset of symptoms in May 2006. The hair cortisol levels from June 2006 until April 2007 were increased compared to before June 2006 (Fig. 3C). After her first surgery, the hair cortisol levels increased further, corresponding with persistent and clinically progressive Cushing’s disease. After the second surgery, the cortisol in both hair and urine remained elevated, even though both showed a downward trend.
Patient D, a 34-year old woman, presented in December 2006 with a left adrenal mass of 4.8 cm. She had noted a 13 kg weight gain, fatigue, muscle weakness and increased hirsutism beginning in June. Her 24-h UFC was 286 (< 180) nmol/D. The 1-mg overnight dexamethasone suppression test resulted in an insufficiently suppressed serum cortisol of 426 nmol/L and the morning ACTH remained undetectable, confirming autonomous adrenal cortisol overproduction. A 5 cm left adrenal gland was removed laparoscopically in February 2007, and pathological examination revealed an adrenal cortical adenoma. Hydrocortisone was initiated at 50 mg daily and gradually tapered until discontinuation in October. In December, her morning fasting serum cortisol was 362 nmol/L and plasma ACTH was 6.1 pmol/L, confirming cure and indicating normal function of the remaining adrenal gland. Segmental hair analysis demonstrated that cortisol levels were increased in all samples, up to about 10 months before first clinical presentation (Fig. 3D). Following surgery, hair cortisol levels started to decrease during hydrocortisone tapering. A third hair sample showed extremely elevated and sporadic hair cortisol levels. Upon questioning, the patient indicated that she had been applying a cortisol containing cream to her scalp for a dermatological condition until late 2005, when she discontinued. Around November 2007, the skin symptoms had returned and she resumed use of the cream.
Patient E was a 49-year old female who presented in March 2007 with a gradual 50 pound weight gain that occurred during the last year. She had also developed diabetes mellitus type 2 and hypertension that was difficult to control. Her urinary cortisol excretion was elevated at 3150 (reference range: 153–789) nmol/day, further work-up demonstrated she had Cushing’s disease. Her sectional hair analysis shows that her hair cortisol level was already elevated 6 months before presentation and since then had gradually increased to almost double the initial level.
Patient F was a 57-year old female referred in February 2006 for fatigue and unproven adrenal insufficiency for which she took hydrocortisone 20 mg BID. We advised her to taper this to enable evaluation of the adrenal function. When she returned for follow-up in December 2006 she was profoundly Cushingoid, and admitted to taking up to 150 mg hydrocortisone daily because she was feeling unwell. We then advised her to gradually taper the hydrocortisone dose. She started this in January 2007, and managed to decrease the dose to 90 mg daily by October 2007. Her segmental hair cortisol analysis shows a gradual decrease of cortisol during the time that she was tapering the dose, but levels still remain significantly elevated.
Discussion
In this pilot study, we found that hair cortisol levels were significantly higher in patients with CS than in healthy control subjects, and that the levels decreased following successful therapy. This supports our hypothesis that hair cortisol levels reflect endogenous cortisol secretion.
The present study is the first to explore segmental analysis of hair cortisol content in patients with CS. We found that cortisol levels can be measured in all hair segments along the hair shaft. Therefore, variation in hair cortisol concentrations along the length of the hair may reflect variations in cortisol levels over time, indicating that segmental hair analysis can provide a historical record of systemic cortisol exposure. This is supported by the findings in our patients with CS, in whom hair cortisol levels appeared to change in parallel with the clinical course of the disease, and in the patient with exogenous Cushing’s syndrome, in whom hair cortisol levels decreased during tapering of hydrocortisone intake. The notion that hair cortisol levels can provide a retrospective calendar of cortisol production is supported by a recent study by Kirschbaum and coworkers (Kirschbaum et al., 2009) who measured hair cortisol levels in mothers of 3–4 month old children. They found that hair cortisol levels in the segment representing the last trimester of pregnancy were two-fold higher than the segment reflecting the post-partum period. This is consistent with previously reported 3-fold higher endogenous cortisol levels during the third trimester of pregnancy (Sandman et al., 2006). In the same paper this group suggests that hair cortisol segments are only reliable for a period of up to 6 months. This is in contrast with our findings in Cushing’s patients that indicate elevated levels in segments up to 18 months old. It should be kept in mind that these studies use entirely different models. Importantly, Kirschbaum et al. described physiological changes in hair cortisol in pregnancy and post partum, using a limited number of segments of hair, whereas we deal with a totally different clinical scenario – Cushing syndrome, with levels of cortisol in the pathological range. Moreover, the treatment of some of our patients allowed us to validate this method based on the effect of surgery on cortisol levels in hair in parellel with the effect of this intervention on cortisol in blood and urine. While there is debate over the exact historical period over which hair cortisol measurement is reliable, overall both papers support the hypothesis that segmental hair analysis indeed reflects historical systemic cortisol exposure.
In patients with CS, hair cortisol levels may also provide intra-individual changes in segments corresponding to the time period before subjective onset of symptoms, and may thus provide an indication of normal values specific to that individual. This ‘window into the past’ is a unique feature of hair analysis, and may provide clinicians with an objective tool to determine past cortisol exposure, which can currently only be determined by subjective report based on signs and symptoms. Other advantages of measuring hormone levels in hair include non-invasive collection which can be performed by non-health care workers at any time of the day and collected samples can be stored at room temperature and be sent by mail.
In the present study we did not find any difference in hair cortisol concentrations between healthy male and female subjects. Segmental analysis, performed in healthy subjects with long hair, showed variation in hair cortisol concentration over time, but in most subjects there was no significant change in hair cortisol over time. Similarly large variations have been seen in the variation urinary cortisol excretion that is found in healthy subjects (Kuil et al., 1998; Reimondo et al., 2008). Importantly, sectional analysis of hair cortisol levels does not suggest any systematic effect of repeated environmental exposures (which would be expected to be more frequent in the distal hair segments) on hair cortisol levels.
There are several limitations to this study. The timing of the historical record is not precise. The human hair growth rate, here assumed to be 1 cm/month, changes seasonally and varies between individuals and may also be affected by the presence of CS. While data on the hormonal regulation of human hair growth is limited, it has been shown that the scalp hair of women grows faster than men (Myers et al., 1951; Saitoh et al., 1969). In contrast, Loussouarn (Loussouarn, 2001) did not find a difference in hair growth rate between sexes in an African population. Despite this potential difference in hair growth rate between sexes, we did not find a significant difference in hair cortisol concentrations between men and women. Currently, there is no viable technique available to determine the specific hair growth rate in individual hair strands.
Of note, it is important to take all hair samples from the posterior vertex, as we have previously found that the intra-individual variation in hair cortisol concentration is 16% for two samples simultaneously obtained from the posterior vertex, compared to 24% for samples obtained simultaneously from two different scalp sites in the same individuals (Sauve et al., 2007). Previous work has found that while human hair grows in a mosaic fashion, meaning that two adjacent follicles are in different growth phases, the posterior vertex shows the greatest growth cycle synchrony and this is why it has been recommended as the ideal site for sampling (Consensus, 1997). Another consideration for the interpretation of hair cortisol concentrations is the latency between the hair formation in the follicle and the time the hair is accessible for clipping, creating a potential timing error of several weeks (Alonso et al., 2006; Krause et al., 2006; Paus et al., 1999).
In our previous studies, we did not find an effect of natural hair colour or hair dying post sampling on hair cortisol content, but did find that hair cortisol levels were lower in hair that had been artificially dyed before the samples were obtained (Sauve, et al., 2007). The effects of environmental exposure, for example oral contraceptives, shampoos, frequent swimming and bleaching, are not known and will require additional carefully designed experiments. The effect of ethnicity/race on hair cortisol concentration needs to be determined, as some, but not all, studies have found ethnic and/or racial variation in serum or urinary cortisol (Masi et al., 2004; Reynolds et al., 2006). However, this would not affect the results of the present study as all subjects were Caucasian.
Incorporation of hormones into hair is thought to occur mainly via diffusion from blood during the formation of the hair shaft, although some authors have suggested that hair cortisol originates from cortisol excreted in sweat or sebum (Cone, 1996; Henderson, 1993). Further use of hair cortisol analysis for research and/or clinical application requires improved understanding of the mechanism (s) by which cortisol enters the hair and the various factors that affect this. Our observation in patient D indicates that use of hydrocortisone-containing cream is a major confounder and the use of such creams should be specifically documented when analyzing hair samples. Despite this limitation, hair segmental analysis can provide a novel objective tool to assess past exposure to both endogenous cortisol and hydrocortisone treatment.
Our findings suggest that measurement of cortisol in hair may help improve understanding the development of Cushing’s syndrome in individual patients. Further, our results suggest that hair cortisol levels may potentially be helpful in excluding a diagnosis of Cushing’s syndrome. However, the current number of patients is small, and the values in some patients with Cushing’s syndrome are close to the range seen in healthy control subjects. This is not different from the clinical experience in which patients sometimes have only limited elevation of cortisol in urine, serum and saliva (Friedman et al., 2007).
A prospective study evaluating hair cortisol levels in patients referred for possible Cushing’s syndrome is in preparation and will also include patients receiving dexamethasone suppression and other glucocorticoid therapies.
In conclusion, this is the first study to document that hair cortisol levels reflect systemic cortisol exposure in patients with CS, and that segmental hair analysis may provide a more objective tool for assessment of cortisol levels during the past months to years.
Acknowledgments
Funding
This study was supported by financial assistance of the Physicians of Ontario through The P.S.I. Foundation, and the Canadian Institutes of Health Research.
Footnotes
Conflict of interest : None.
References
- 1.Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006;119:391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- 2.Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of cushing’s syndrome: A consensus statement. J Clin Endocrinol Metab. 2003;88:5593–5602. doi: 10.1210/jc.2003-030871. [DOI] [PubMed] [Google Scholar]
- 3.Cirimele V, Kintz P, Dumestre V, et al. Identification of ten corticosteroids in human hair by liquid chromatography-ionspray mass spectrometry. Forensic Sci Intl. 2000;107:381–388. doi: 10.1016/s0379-0738(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 4.Cone EJ. Mechanisms of drug incorporation into hair. Ther Drug Monit. 1996;18:438–443. doi: 10.1097/00007691-199608000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Consensus. Society of hair testing. Forensic Sci Intl. 1997;84:3–6. doi: 10.1016/s0379-0738(96)02057-9. [DOI] [PubMed] [Google Scholar]
- 6.Davenport M, Tiefenbacher S, Lutz C, et al. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 2006;147:255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Friedman TC, Zuckerbraun E, Lee ML, et al. Dynamic pituitary mri has high sensitivity and specificity for the diagnosis of mild Cushing’s syndrome and should be part of the initial workup. Horm Metab Res. 2007;39:451–456. doi: 10.1055/s-2007-980192. [DOI] [PubMed] [Google Scholar]
- 8.Giraldi FP, Moro M, Cavagnini F. Gender-related differences in the presentation and course of cushing’s disease. J Clin Endocrinol Metab. 2003;88:1554–1558. doi: 10.1210/jc.2002-021518. [DOI] [PubMed] [Google Scholar]
- 9.Henderson GL. Mechanisms of drug incorporation into hair. Forensic Sci Intl. 1993;63:19–29. doi: 10.1016/0379-0738(93)90256-a. [DOI] [PubMed] [Google Scholar]
- 10.Kirschbaum C, Tietze A, Skoluda N, et al. Hair as a retrospective calendar of cortisol production - Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinol. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Krause K, Foitzik K. Biology of the hair follicle: The basics. Semin Cutan Med Surg. 2006;25:2–10. doi: 10.1016/j.sder.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Kuil M, Endert E, Fliers E, et al. Establishment of reference values for endocrine tests. I: Cushing’s syndrome. Neth J Med. 1998;53:153–163. doi: 10.1016/s0300-2977(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 13.Loussouarn G. African hair growth parameters. Br J Dermatol. 2001;145:294–297. doi: 10.1046/j.1365-2133.2001.04350.x. [DOI] [PubMed] [Google Scholar]
- 14.Masi CM, Rickett EM, Hawkley LC, et al. Gender and ethnic differences in urinary stress hormones: The population-based chicago health, aging, and social relations study. J Appl Physiol. 2004;97:941–947. doi: 10.1152/japplphysiol.00256.2004. [DOI] [PubMed] [Google Scholar]
- 15.Mengden T, Hubmann P, Muller J, et al. Urinary free cortisol versus 17-hydroxycorticosteroids - a comparative-study of their diagnostic-value in cushings-syndrome. Clin Investig. 1992;70:545–548. doi: 10.1007/BF00184788. [DOI] [PubMed] [Google Scholar]
- 16.Myers RJ, Hamilton JB. Regeneration and rate of growth of hairs in man. Ann N Y Acad Sci. 1951;53:562–568. doi: 10.1111/j.1749-6632.1951.tb31957.x. [DOI] [PubMed] [Google Scholar]
- 17.Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of cushing’s syndrome: An endocrine society clinical practice guideline. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 19.Reimondo G, Pia A, Bovio S, et al. Laboratory differentiation of Cushing’s syndrome. Clin Chim Acta. 2008;388:5–14. doi: 10.1016/j.cca.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds RM, Fischbacher C, Bhopal R, et al. Differences in cortisol concentrations in South Asian and European men living in the united kingdom. Clin Endocrinol (Oxf) 2006;64:530–534. doi: 10.1111/j.1365-2265.2006.02504.x. [DOI] [PubMed] [Google Scholar]
- 21.Saitoh M, Uzuka M, Sakamoto M, et al. Rate of hair growth. In: Montagna M, Dobson RL, editors. Advances in biology of skin and hair growth. Oxford: Pergamon Press; 1969. pp. 183–201. [Google Scholar]
- 22.Sandman CA, Glynn L, Schetter CD, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Sauve B, Koren G, Walsh G, et al. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical and Investigative Medicine. 2007;30:E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 24.Stuart P. In: Williams textbook of endocrinology. 11 ed. Henry Kronenberg SM, Polonsky Kenneth, Larsen Reed, editors. Philadelphia: Saunder/Elsevier; 2007. p. 467. [Google Scholar]
- 25.Van Uum S, Sauvé B, Fraser L-A, et al. Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress. Stress. 2008;11:483–488. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- 26.Villain M, Cirimele V, Kintz P. Hair analysis in toxicology. Clin Chem Lab Med. 2004;42:1265–1272. doi: 10.1515/CCLM.2004.247. [DOI] [PubMed] [Google Scholar]
- 27.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci Intl. 2000;107:5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 28.Yang HZ, Lan J, Meng YJ, et al. A preliminary study of steroid reproductive hormones in human hair. J Steroid Biochem Mol Biol. 1998;67:447–450. doi: 10.1016/s0960-0760(98)00120-4. [DOI] [PubMed] [Google Scholar]